Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ecosistemas y recursos agropecuarios
versión On-line ISSN 2007-901Xversión impresa ISSN 2007-9028
Ecosistemas y recur. agropecuarios vol.2 no.5 Villahermosa may./ago. 2015
Artículo científico
Effect of three fodder trees on Haemonchus contortus control and weight variations in kids
Efecto de tres árboles forrajeros en el control de Haemonchus contortus y cambios de peso en cabritos
1Yesenia León-Castro, 1*Jaime Olivares-Pérez, 1Saúl Rojas-Hernández, 2Abel Villa-Mancera, 1Ma. Trinidad Valencia-Almazán, 1Elías Hernández Castro, 3Alejandro Córdova-lzquierdo, 4Régulo Jiménez- Guillén
1 Unidad Académica de Medicina Veterinaria y Zootecnia-Universidad Autónoma de Guerrero, México
*olivaares@hotmail.com
2 Laboratorio de Genética y Reproducción, Facultad de Medicina Veterinaria y Zootecnia, Benemérita Universidad Autónoma de Puebla, México
3 Departamento de Producción Agrícola y Animal, Universidad Autónoma Metropolitana-Xochimilco, México
4 Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias- Campus Iguala Guerrero, México
Recibido el 27 de julio de 2014
Aceptado el 6 de octubre de 2014
ABSTRACT
The objective was to evaluate the activity against Haemonchus contortus by foliage taken from three tanniferous tree species and fed to Creole male kids experimentally infected with a dose of 350 larvae of H. contortus (L3) kg-1 body weight (BW). Twenty Creole kids weighing 12 ± 2.0 kg were randomly distributed in four treatments: T1 = control without foliage, and the addition of fresh foliage (10 % of the dry matter of the total diet) of Guazuma ulmifolia (T2), Pithecellobium dulce (T3) and Acacia cochliacantha (T4). For sixty days, the activity of the foliage against H. contortus was evaluated by quantifying the reduction in the excretion of eggs per gram of feces; also, the excretion of eggs was associated with the concentration of the blood cell pack, dry matter intake and weight change in animals. The data of the evaluated variables were analyzed in a completely randomized design and a Tukey test was carried out for comparison of means (p < 0.05). The secondary compounds of P. dulce and A. cochliacanta foliage were more effective (p < 0.04) in the control of H. contortus with minor excretion of eggs (1.48 and 1.18 Log10 g-1 of feces), respectively. The hematocrit was different (p < 0.005) mainly in hemoglobin, erythrocytes, lymphocytes and eosinophils; dry matter intake (p < 0.05) and changes in total body weight (p < 0.01) were higher in kids which received P. dulce foliage (T3) with 621.5 g d-1 and 2.4 kg, respectively. It was concluded that the secondary compounds of fresh P. dulce and A. cochliacantha foliage have the potential to control H. contortus without affecting the health and productive response in kids.
Key words: H. contortus, Guazuma ulmifolia, Phitecellobium dulce, Acacia cochliacantha.
RESUMEN
El objetivo fue evaluar la actividad contra Haemonchus contortus del follaje de tres árboles taniníferos en cabritos criollos infestados experimentalmente con una dosis de 350 larvas de H. contortus (L3) Kg-1 peso vivo. Veinte cabritos criollos de 12 ± 2.0 kg de peso vivo (PV), fueron distribuidos al azar en cuatro tratamientos: Tl= testigos sin follaje, y la adición de follaje fresco (10 % de la materia seca de la dieta total) de Guazuma ulmifolia (T2), Phitecellobium dulce (T3) y Acacia cochliacantha (T4). Durante 60 d se evaluó la actividad de los follajes contra H. contortus mediante la reducción en la excreción de huevos por gramo de heces (hpgh); además, la excreción de huevecillos se asoció con la concentración del paquete celular sanguíneo y el cambio de peso de los animales. Los datos de las variables evaluadas se analizaron en un diseño completamente al azar y la prueba de Tukey para la comparación de medias (p < 0.05). Los compuestos secundarios del follaje de P. dulce y A. cochliacanta resultaron más eficaces (p < 0.04) en el control de H. contortus, con menor excreción de hpgh (1.48 y 1.18 Log10 g-1 de heces), respectivamente. El hematocrito fue diferente (p < 0.005) principalmente en hemoglobina, eritrocitos, linfocitos y eosinófilos; el consumo de materia seca (p < 0.05) y el cambio de peso vivo total (p < 0.01) fue mayor en los cabritos que recibieron el follaje de P. dulce con 621.5 g d-1 y 2.4 kg de peso, respectivamente. Se concluye que los follajes de P. dulce y A. cochliacanta, por su contenido de taninos, tienen potencial para el control de H. contortus, lo que amerita mayor investigación.
Palabras clave: H. contortus, Guazuma ulmifolia, Phitecellobium dulce, Acacia cochliacantha.
INTRODUCTION
One of the biggest health problems faced by goats is their frequent exposure to parasites. Tropical studies have determined the presence of several genera of parasites, including the parasite Haemonchus contortus, which is one of the most important epidemiologically due to its abundance and severity of infestations (Olivares et al. 2012). The use of chemicals has played an important role in the control and treatment of severe parasite infestations in small ruminants; however, it is a costly and inappropriate method, and excessive use can result in the development of parasite resistance to the chemical formula of the product (Olivares-Pérez et al. 2011).
The development of resistance and the presence of chemical residues in animal products have prompted the search for substances that are less toxic to animals and man, but that are still effective in controlling parasites (Abbas et al. 2014). Fruit tree foliage and extracts have shown some activity against parasites (Calderón-Quintal et al. 2010, Patra and Saxena 2010, Zaman et al. 2012a) and positive effects on weight gain, dry matter intake, milk production and ruminal fermentation parameters (Salem 2011, Olivares et al. 2013a, Olivares et al. 2013b). They can also modify the acetate-propionate relationship and defaunation effects, as well as protect protein in the rumen and promote its absorption in the duodenum (Hart et al. 2008, Jiménez et al. 2011).
The use of tree foliage for feeding goats is most common in tropical regions where P. dulce, A. cochliacantha and G. ulmifolia have been identified as forage species (Rojas et al. 2010, Olivares et al. 2011). Studies have shown that tannins can improve goat resistance to gastrointestinal nematode infestations (Torres et al. 2008, Alemán et al. 2011). The aim of this study is to comparatively determine the chemical composition of A. cochliacantha, P. dulce and G. ulmifolia foliage, its total phenolic and condensed tannin contents, its effect against H. contortus in experimentally infected male Creole kids and its relationship to the animals' productive response.
MATERIALS AND METHODS
The study was conducted on the premises of the Faculty of Animal Husbandry and Veterinary Medicine at the Autonomous University of Guerrero, located 2.5 km from the Altamirano-lguala road in Pungarabato Municipality, situated in the Tierra Caliente region of the State of Guerrero, Mexico.
Tree foliage used. Samples of fresh foliage (leaves, tender stems) of A. cochliacantha, P. dulce and G. ulmifolia of the same phenology were collected, and their chemical composition (AOAC 2000), condensed tannin and total phenolic content (Table 1) determined (Waterman and Mole 1994).
Animals and management
Twenty five-month-old Creole kids with body weights of 12.0 ± 2.0 kg were used. The animals were housed in metabolic cages, with a height of 110 cm from the floor (with free access to water and food for their adaptation), where they were subcutaneously impregnated with 0.2 mg kg-1 body weight of ivermectin (Sumano and Ocampo 2006). The excretion of eggs at days zero, seven, fourteen and twenty-one were monitored by McMaster tests to verify that the animals were at a very low or nil parasite load before initiating the study (MAFF 1986).
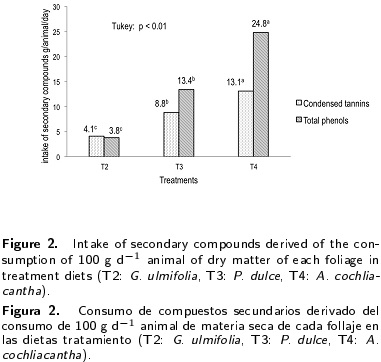
Subsequently the animals were experimentally infected with 350 L3 larvae of H. contortus per kilogram of body weight. Four weeks after infection, the animals were divided randomly into four groups of five animals, balanced according to the level of egg excretion and body weight, and fed with 10 % total dry matter with fresh foliage of the three tree species in the following four treatments: T1= control without foliage, T2= Guazuma ulmifolia, T3= Phitecellobium dulce, T4= Acacia cochliacantha. All animals were fed a maintenance diet according to the requirements of CP and Meal of ME with oat hay, wheat bran and mineral premix (Table 2) (NRC 2007) prepared for the growing stage.
Variables measured
Egg excretion: Fecal samples were collected every seven days to measure the excretion of nematode eggs. Egg counts were performed according to the modified McMaster technique (MAFF 1986).
Red blood cell count: Blood samples were collected in vacutainer tubes with EDTA added at days 0, 15, 30, 45 and 60 of the study by puncturing the jugular vein. Blood samples were analyzed for hematocrit, hemoglobin, erythrocytes and leucocytes (neutrophils, lymphocytes, eosinophils and monocytes). Hemoglobin concentration was determined using the cyanmethaemoglobin method. Hematocrit was determined by the micro hematocrit technique. Erythrocyte and differential leucocyte counts were determined using the haemocytometer method (Archer and Jeffcott 1977).
Productive response of male kids: Dry matter intake was measured in kids by the difference between the dry matter offered daily and the amount rejected. Also, secondary compound intake was calculated with the total phenol and condensed tannin content in the foliage multiplied by the dry matter intake of male kids fed with the foliage (Table 1), and it was associated with the excretion of eggs in the feces and the blood cell concentration in the animals. Body weight was measured at 0, 15, 30, 45 and 60 d of the experiment. With body weight data, the total weight change was calculated by the difference in final body weight minus the initial body weight, and feed conversion was calculated by dividing the dry matter intake between the weight changes observed in the animals (Olivares et al. 2013a).
Statistical analysis
The chemical composition and content of phenols and condensed tannins in foliage and excretion in feces of H. contortus eggs, the hematocrit, hemoglobin, erythrocytes, neutrophils, lymphocytes, eosinophils and monocytes, as well as the variables of animal response (dry matter intake, weight change and secondary compound intake) in male kids, were analyzed in a completely randomized design (SAS 2002). Statistical model: ; where, = is the response variable due to the treatment (= 1, 2, 3 G. ulmifolia, P. dulce and A. cochliacantha foliage in chemical composition and phenolic and condensed tannin content; and = treatment diets 1: control without foliage, 2: G. ulmifolia, 3: P. dulce and 4: A. cochliacantha foliage in hematological and productive response of male kid variables); µ= is the overall mean and = random error. The significance between means was compared by the Tukey test (alpha, p < 0.05) (SAS 2002).
RESULTS
Chemical composition of foliage
In the chemical composition of the three types of foliage used, it is observed that the dry and organic matter content was higher in A. cochilacantha foliage (p < 0.001) with 96.6 and 90.0 % respectively (Table 1). The crude protein content was higher in the P. dulce foliage (p < 0.05) with 25.7 %. The contents of neutral (p < 0.001) and acid (p < 0.01) detergent fibers, condensed tannins (p < 0.01) and total phenols (p < 0.01) were higher in the A. cochliacantha foliage with 78.3, 50.3, 13.1 and 24.8 %, respectively, compared to P. dulce and G. ulmifolia foliage (Table 1).
Fecal egg counts
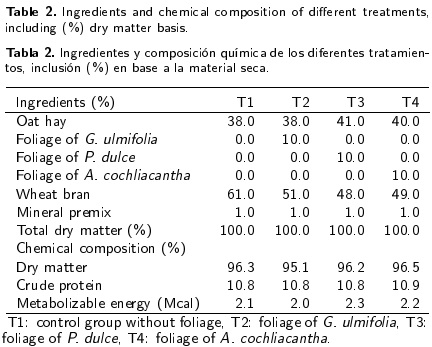
It was observed that the P. dulce and A. cochliacantha foliage decreased (p < 0.01) the excretion of H. contortus eggs in the feces of male kids, compared to control animals (Tl) and those fed with G. ulmifolia foliage (T2) (Figure 1). The excretion of H. contortus eggs in the feces of male kids of T3 and T4 was of 1.18 Log10 g-1 and 1.48 Log10 g-1, respectively, and in male kids of T1 and T2 was of 2.18 Log10 g-1 and 3.08 Log10 g-1, respectively (Figure 1).
Red blood cell count
The male kids of Tl and T2 had low hematocrit (p < 0.005) with 19.60 and 19.48 %, hemoglobin (p < 0.001) with 6.53 and 6.49 g / 100 mL, erythrocytes (p < 0.001) with 3.26 x 1 012 L-1 and 3.24 x 1012 L-1 and lymphocytes (p < 0.001) with 71.36 and 64.56 %, respectively, compared to animals of T3 and T4 (Table 3). The eosinophils showed the highest concentration (p < 0.01) in kids of T1 (1.84 %) and T2 (2.08 %), compared to animals of T3 and T4 (Table 3). The level of neutrophils and monocytes was not different among animals under treatments (p > 0.05) (Table 3).
Productive response of male kids
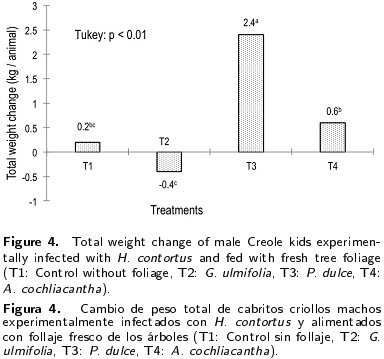
Higher dry matter intake (p < 0.05) was observed in male kids which received T3 (621.5 g d-1 animal) and T4 (634.4 g d-1 animal) compared to animals of T2 (Figure 3). The total weight gain was higher (p < 0.01) in kids which received T3 with 2.4 kg animal compared to animals of other treatments (Tl, T2 and T4) (Figure 4). Also, differences in total weight gain between male kids of T4 with 0.6 kg and those of T2 with -0.4 kg were observed (p < 0.01) (Figure 4).
DISCUSSION
Fecal egg counts
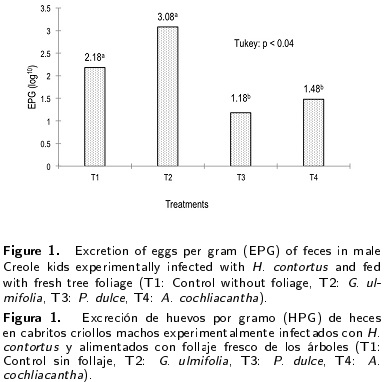
The results in fecal egg counts can be attributed to the consumption of secondary compounds (condensed tannins between 8.8 to 13.1 g d-1 animal and total phenols between 13.4 to 24.8 g d-1 animal) by kids of T3 and T4 (Figure 2), which received the foliages of P. dulce and A. cochliacantha that affected the reproductive cycle of the parasite and the consequent excretion of eggs in the feces of animals (Lange et al 2006, Villalba et al. 2010, Hamad et al 2013). Reports indicate that the use of plants with a certain content of condensed tannins decreases the viability of larvae and adult worms of H. contortus (Terrill et al. 2007, Ademola and Eloff 2011, Zaman et al. 2012b).
Red blood cell count
Differences in the blood cell count in animals were attributed to the degree of parasitic infestation of the male kids (Figure 1). The decrease in hematocrit, hemoglobin, erythrocytes and lymphocytes in male kids of T1 and T2 can be attributed to gastritis caused by injury to the mucosa of the digestive tract and blood suction caused by H. contortus as a hematophagous parasite (Table 3) (Schwarz et al. 2013, Blackie 2014). Moreover, the effect of intake of secondary compounds (condensed tannins and total phenols) of fresh P. dulce (T3) and A. cochliacantha (T4) foliage (Figure 2) for reducing the excretion of H. contortus eggs (Figure 1) favored the concentration of hematocrit, hemoglobin, erythrocytes and lymphocytes in male kids under treatments T3 and T4 (Table 3). The results indicate that H. contortus affects the concentration of white blood cells responsible for the innate immune response in the animals (Provenza and Villalba 2010). However, it is also important to mention that feeding kids (10 % of dietary DM basis) with the fresh foliage of P. dulce and A. cochliacantha with tannin content between 8.8 to 13.1 % and phenols between 13.4 to 24.8 % (Table 1) decreased the excretion of H. contortus eggs in their feces due to increased consumption of secondary compounds (Figure 2) without affecting the concentration of white blood cells, similar to that reported by Paolini et al. (2003), Lange et al. (2006) and Rivero et al. (2012). What has been discussed is important because it has been determined that the tannins and saponins have significant negative effects on hematocrit and hemolytic activity that causes loss of hemoglobin and may result in death of the animal (Wang et al. 2007, Mahgoub et al. 2008). The higher concentration of eosinophils in the blood of male kids of T1 and T2 was attributed to the immune response of animals against the strong parasitic infestation developed by gastritis (Table 3 and Figure 1), similar to that reported by Schwarz et al. (2013) and Blackie (2014).
Productive response of male kids
The higher dry matter intake and weight gain in animals of T3 and T4 (Figures 3 and 4) was attributed to the decrease in parasite load (H. contortus) (Figure 1) that prevented the development of gastritis caused by damage of the parasite to the mucosa of the digestive tract, which favored food digestion and nutrient absorption and increased dry matter intake caused by supplying 10 % (dry matter basis of the diet) Pithecellobium dulce and A. cochliacantha fresh foliage (Table 2), similar to that reported by Hoste et al. (2005). In vivo studies in small ruminants suggest at least 30 - 40 g of condensed tannins per kilogram of dry matter (DM) to observe anti-parasitical activity (Hoste et al. 2006). Waghorn (2008) and Patra and Saxena (2010) reported that tannins in low concentrations (3-6 %) show a positive effect on diet digestion and animal growth. Olivares et al. (2013a) observed that the use of P. dulce and G. sepium foliage increased dry matter intake and weight gain in male kids.
CONCLUSIONS
The secondary compound content of foliage of P. dulce and A. cochliacantha had higher activity for H. contortus control in kids, without affecting the health of the animals, and indirectly increased their dry matter intake and total weight gain. Animals under treatments T1 and T2 were the most parasitized, reducing the blood cell concentration and productive response in kids.
ACKNOWLEDGMENTS
The authors thank PROMEP-SEP for funding this research project which enabled a Master's student to graduate.
LITERATURE CITED
Abbas A, Abbas RZ, Khan JA, Iqbal Z, Bhatti MMH, Sindhu ZuD, et al. (2014) Formulation against gastrointestinal nematodes of sheep. Pakistan Veterinary Journal 32: 117-121. [ Links ]
Ademóla IO, Eloff JN (2011) Anthelminthic activity of acetone extract and fractions of Vernonia amygdalina against Haemonchus contortus eggs and larvae. Tropical Animal Health and Production 43: 221-247. [ Links ]
Alemán Y, Sánchez LM, Pérez T, Rodríguez Y, Olivares JL, Rodríguez JG (2011) Actividad larvicida de extractos de Rhizophora mangle L. contra estrongílidos gastrointestinales de ovinos. Larvicide activity from extracts of Rhizophora mangle L. versus gastrointestinal strongylid parasites in ovines. Salud Animal Magazine 33: 111-115. [ Links ]
AOAC (2000) In: Official Methods of Analysis17th ed. Association of Official Analytical Chemist, Arlington, VA, USA. 74 p. [ Links ]
Archer RK, Jeffcott LB (1977) Comparative Clinical Haemotology. In: Technical methods (ed) Blackwell Scientific Publications. Oxford, pp: 537-586. [ Links ]
Blackie S (2014) A Review of the Epidemiology of Gastrointestinal Nematode Infections in Sheep and Goats in Ghana. Journal of Agricultural Science 6: 109-118. [ Links ]
Calderón-Quintal JA, Torres-Acosta JFJ, Sandoval-Castro CA (2010) Adaptation of Haemonchus contortus to condensed tannins: can it be possible?. Archivos de Medicina Veterinaria 42: 165-171. [ Links ]
Hamad KK, Iqbal Z, Sindhu ZuD, Muhammad G (2013) Antinematicidal activity of integrated strategies for the control and prevention of dengue vectors with particular reference to Aedes aegypti. Pakistan Veterinary Journal 34: 1-10. [ Links ]
Hart KJ, Yáñez-Ruiz DR, Duval SM, McEwan NR, Newbold CJ (2008) Plant extracts to manipulate rumen fermentation. Animal Feed Science and Technology 147: 8-35. [ Links ]
Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoski SO (2006) Effects of tannin-rich plants on parasitic nematodes in ruminants. Trends in Parasitology 22: 253 - 261. [ Links ]
Hoste H, Torres-Acosta JF, Paolini V, Aguilar-Caballero AJ, Etter E, Lefrileux Y, et al. (2005) Interactions between nutrition and gastrointestinal infections with parasitic nematodes in goats. Small Ruminant Research 60: 141-151. [ Links ]
Jiménez P, Salem AZM, Mejia HP, González RM, Albarrán PB, Rojo RR, et al. (2011) Influence of individual and mixed extracts of two tree species on in vitro gas production kinetics of a high concentrate diet fed to growing lambs. Livestock Science 136: 192-200. [ Links ]
Lange KC, Olcott DD, Miller JE, Mosjidis JA, Terrill TH, Burke JM, et al. (2006) Effect of Sericea lespedeza (Lespedeza cuneata) fed as hay, on natural and experimental Haemonchus contortus infections in lambs. Veterinary Parasitology 141: 273-278. [ Links ]
MAFF (1977) Ministry of Agriculture, Fisheries and Food. Manual of Veterinary Parasitological Laboratory Techniques. Tech. Bull., No.18, Her Majesty's Stationery Office, London. 129 p. [ Links ]
Mahgoub O, Kadim IT, Tageldin MH, Al Marzooqi WS, Khalaf SQ, Amnbu Ali A (2008) Clinical Profile of sheep fed non-conventional feeds containing phenols and condensed tannins. Small Ruminant Research 78: 115-122. [ Links ]
NRC (2007) Nutrient Requirements of Goats. National Research Council. National Academy Press, Washington, DC. pp: 271-299. [ Links ]
Olivares PJ, Avilés NF, Albarrán PB, Castelán OOA, Rojas HS (2013a) Use of three fodder trees in the feeding of goats in the sub-humid tropics in México. Tropical Animal Health and Production 45: 821-828. [ Links ]
Olivares JF, Avilés NF, Albarrán PB, Castelán OA, Rojas HS (2013b) Nutritional quality of Pithecellobium dulce and Acacia cochliacantha fruits, and its evaluation in goats. Livestock Science 154: 74-81. [ Links ]
Olivares PJ, Avilés NF, Albarrán PB, Rojas HS, Castelán OOA (2011) Identificación, usos y medición de leguminosas arbóreas forrajeras en ranchos ganaderos del sur del estado de México. Tropical and Subtropical Agroecosystems 14: 739 -748. [ Links ]
Olivares PJ, Gutiérrez SI, Rojas HS, Valencia AMT, Míreles MEJ, Cordova IA (2012) Seasonal prevalence of Strongyle in Creole goats of the Tierra Caliente region, State of Guerrero, México. Research Opinions in Animal and Veterinary Sciences 2: 216-220. [ Links ]
Olivares-Pérez J, Rojas- Hernández S, Valencia- Almazán MT, Gutiérrez-Segura I, Míreles-Martínez EJ (2011) Prevalence of resistant strains of Rhipicephalus microplus to acaricides in cattle ranch in the tropical region of Tecpan of Galeana, Guerrero, México. Pakistan Veterinary Journal 31: 366-368. [ Links ]
Paolini V, Bergeaud JP, Grisez C, Prevot F, Dorchies Ph, Hoste H (2003) Effects of condensed tannins on goats experimentally infected with Haemonchus contortus. Veterinary Parasitology 113: 253-261. [ Links ]
Patra AK, Saxena J (2010) A new perspective on the use of plant secondary metabolites to inhibit methano-genesis in rumen. Phytochemistry 71: 1198-1222. [ Links ]
Provenza FD, Villalba JJ (2010) Role of natural plant products in modulating the immune system: an adaptable approach for combating disease in grazing animals. Small Ruminant Research 89: 131-139. [ Links ]
Rivero N, Salem AZM, Gado HM, González-Ronquillo M, Pliego AB, Peñuelas CG, et al. (2012) Effect of exogenous enzymes and Salix babylonica extract or their combination on haematological parameters in growing lambs. Journal of Animal and Feed Sciences 21: 577-586. [ Links ]
Rojas HS, Avilés NF, Castelán OO, García MA, Olivares PJ, Valencia AMT (2012) Chemical Composition, in vitro digestibility of foliage Guazuma ulmifolia and Crescentia alata and its use in feeding lambs. Pakistan Journal of Nutrition 11: 1139-1145. [ Links ]
Salem AZM, Olivares M, López S, González-Ronquillo M, Rojo R, Camacho LM, et al. (2011) Effect of natural extracts of Salix babylonica and Leucaena leucocephala on nutrient digestibility and growth performance of lambs. Animal Feed Science and Technology 170: 27-34. [ Links ]
SAS (2002) Statistical Analysis System, SAS/STAT. In: Guide for Personal Computers Version Ver 9.0. Institute Inc., Cary, NC, USA. 956 p. [ Links ]
Schwarz EM, Korhonen PK, Campbell BE, Young ND, Jex AR, Jabbar A, et al. (2013) The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biology 14: 1-18. [ Links ]
Sumano LHS, Ocampo CL (2006) Farmacología Veterinaria. 3ra Edición. Mc Graw Hill Interamericana. pp: 451- 526. [ Links ]
Terrill TH, Mosjidis JA, Moore DA, Shaik SA, Miller JE, Burke JM, et al. (2007) Effect of pelleting on efficacy of Sericea lespedeza hay as a natural de-wormer in goats. Veterinary Parasitology 146: 117-122. [ Links ]
Torres AJFJ, Alonso DMA. Hoste H, Sandoval CCA, Aguilar CAJ (2008) Efectos negativos y positivos del consumo de forrajes ricos en taninos en la producción de caprinos. Tropical and Subtropical Agroecosystems 9: 83-90. [ Links ]
Villalba JJ, Provenza FD, Hall JO, Lisonbee LD (2010) Selection of tannins by sheep in response to gastrointestinal nematode infection. Journal of Animal Science 88: 2189-2198. [ Links ]
Waghorn G (2008) Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Animal Feed Science and Technology 147: 116-139. [ Links ]
Wang Y, Zhang Y, Zhu Z, Zhu S, Li Y, Li M, et al. (2007) Exploration of the correlation between the structure, hemolytic activity, and cytotoxicity of steroid saponins. Bioorganic and Medical Chemistry 15: 2528-2532. [ Links ]
Waterman PG, Mole S (1994) Analysis of Phenolic Plant Metabolites. Methods in Ecology. Blackwell Scientific Publications, London. Ed. Oxford. 238 p. [ Links ]
Zaman MA, Iqbal Z, Abbas RZ, Khan MN, Muhammad G, Younus M, et al. (2012a) In vitro and in vivo acaricidal activity of an herbal extract. Veterinary Parasitology 186: 431-436. [ Links ]
Zaman MA, Iqbal Z, Khan MN, Muhammad G (2012b) Anthelmintic activity of an herbal Nicotiana tabacum L. leaf extracts to control benzimidazole-resistant Haemonchus contortus in sheep. Pakistan Veterinary Journal 33: 85-90. [ Links ]