Introduction
The novel beta-coronavirus SARS-CoV-2 infection (COVID-19), that emerged in December 2019, has generated a global crisis. As of April 2021, over 140 million people have been infected with SARS-CoV-2, of which nearly 3 million people have died1. In Mexico over 2.3 million people have been infected and more than 216,000 deaths have been reported, being one of the countries with the highest COVID-19 death tolls worldwide, behind Brazil and The United States of America2.
Severe cases in susceptible patients have been associated with the development of a condition called “cytokine storm” in response to SARS-CoV-2 antigens3. Cytokine storm consists of a deregulated immune response with excessive production and release of interleukin (IL)-1, IL-8 and IFN-γ, leading to hemodynamic compromise and multiple organ failure4,5. The reason why some patients are more susceptible to developing severe disease has not been fully elucidated. According to clinical and basic research, factors such as older age, being male, and comorbidities like type 2 diabetes, obesity, hypertension, asthma, chronic obstructive pulmonary disease (COPD), or cancer have been associated with more severe disease and increased mortality in patients with COVID-196,7,8
It is necessary to find prognosis factors for patients hospitalized with SARS-CoV-2 to identify patients with increased mortality-risk and help manage hospital occupancy more efficiently. Furthermore, the knowledge of these factors could help to understand the mechanisms that lead to severity observed in patients with common characteristics. Besides the factors mentioned above, there has been an increasing interest in the relationship between the ABO blood group system and COVID-19 severity. Several studies have shown that polymorphisms within the ABO gene are associated with different traits, including risk factors for COVID-19 mortality. For instance, variants of ABO groups have been linked to diseases like gastric cancer, myocardial infarction, type 2 diabetes, and venous thromboembolism9.
The relation between ABO groups and morbidity and mortality in viral infections has been well documented. The findings of Liao (2020) suggest that individuals with blood group O may be more susceptible to norovirus infection, although these individuals appear to be less susceptible to SARS-CoV-1 and Norwalk infection10,11. Increased susceptibility to rotavirus gastroenteritis and smallpox have been observed for blood group A and groups A and AB, respectively12. Furthermore, there is evidence suggesting an increased risk of developing severe viral illness according to an individual’s blood group; for instance, group AB has been associated with 2.5 greater risk of developing severe dengue infection11.
There is enough evidence to support the hypothesis that patients with blood group A are more likely to have SARS- CoV-2 infection compared with group O. Gollineli (2020) found through a meta-analysis that COVID-19-positive individuals are more likely to have blood group A (pooled OR 1.23, 95% IC 1.09-1.4) compared with blood group O (pooled OR 0.77 95% IC 0.67-0.88). Some claims have been made about the possible explanation of this relationship, including the modulation of angiotensin-converting enzyme 2 (ACE2) expression by the ABO blood group locus and the possible cross-reactivity of SARS-CoV-2 antigens with blood group O natural anti-A and anti-B antibodies13,14.
Regarding the association of ABO blood groups with COVID-19 mortality, early reports from Wuhan found that group O was linked with a higher risk of COVID-19 severity and mortality compared with group A. In a meta-analysis by Wu (2020) including predominantly Chinese populations, no association between ABO blood groups and COVID-19 severity and mortality was found15. However, studies carried out in other countries have shown contradictory results, proposing a more complex relation between ABO blood groups and COVID-19 mortality. For example, a study from the United States with 14,112 confirmed SARS-CoV-2 patients found a decreased risk of death and intubation in patients with group A relative to group O9.
Latin-American studies of ABO blood groups and COVID-19 are scarce, based on small samples and not adjusted for other variables; thus, the relationship between COVID-19 and in-hospital mortality has not been reported in Mexican populations. The aim of this paper is to explore the association between ABO blood groups and in-hospital mortality in Mexican patients admitted with COVID-19.
Materials and methods
We performed a retrospective study with 2,369 hospitalized patients with confirmed SARS-CoV-2 infection in a tertiary referral hospital in Mexico City, Mexico (Central Military Hospital [CMH], from the National Secretariat of Defense [SEDENA, for its acronym in Spanish]). SARS-CoV-2 infection was confirmed using real-time reverse transcription polymerase chain reaction (RT-PCR) from a nasopharyngeal swab. Epidemiological data including age, sex, blood group and comorbidities were obtained from electronic health records (Digital Health System) of the CMH. We included all patients with confirmed SARS-CoV-2 infection who either died or were discharged between March 27 and December 10, 2020. ABO groups and Rh types were determined through agglutination techniques by a clinical laboratory affiliated to SEDENA when patients were first included in the Digital Heath System. Patients with incomplete data were excluded.
Data are presented as mean (standard deviation [SD]) and counts (percentage) for numerical and categorical variables, respectively. Two groups were formed according to one of two outcomes: death or discharge. Categorical variables were compared using the Chi-square test or Fisher’s exact test, and continuous variables were compared using an unpaired t-test. Survival curves stratified according to ABO blood group were estimated using the Kaplan-Meier method, and curves were compared using the Breslow test. A multivariate Cox proportional hazard regression was performed to identify variables independently associated with COVID-19 death during hospitalization. Hazard Ratios (HR) were estimated as a measure of effect size of the variables included in the Cox regression. We fitted the following saturated model:
Where ln(λt ) is the natural logarithm of the hazard at time t, and ln(λ0t) is the natural logarithm of the baseline hazard. We checked the proportional hazard assumption for categorical variables by using the graph of the log(-log(survival)) versus survival time. We determined adjusted and unadjusted odds ratios (OR) from logistic regression models to predict COVID-19 mortality. We checked the goodness of fit of logistic models with the Hosmer-Lemeshow test. The saturated logistic model included the same variables as the saturated Cox model. P values <0.05 were considered as statistically significant. All analyses were performed with IBM Statistics SPSS 21, whereas bar graphs and forest plots were elaborated in GraphPad Prism 8.4.0.
This study was approved by the research committee at the Central Military Hospital with registration number 066/2020. The present study complied with the basic principles of human research following the Helsinki Declaration of the Medical Association. The data collected was handled confidentially.
Results
A total 2,369 patients were evaluated; 1,685 (71.1%) patients were discharged, and 684 (28.8%) patients died during hospitalization. Most patients were male 1478 (62.3%). The mean age was 53.5 years (SD 16). The most frequent comorbidities were type 2 diabetes (DM) (14.8%), hypertension (14.5%) and obesity (11.4%). Non-survivor patients were older with a mean age of 63 (SD 14.1). Most patients were male, compared with the survivor group (67% vs 60%) (P = 0.003). The most frequent comorbidities among non-survivor patients were hypertension (22.1%), DM (14.8%), and obesity (17.3%). Of the total population, 574 (24.2%) were active military personnel.
Group O was the most prevalent group in 1,999 patients (84.3%); the distribution of ABO groups is shown in Figure 1. Distribution of ABO blood groups in discharged and deceased patients are presented in Figure 2. A total 2,330 (98.3%) patients were Rh-positive among the total population. Epidemiological features of these groups are listed in Table 1. Compared with discharged patients, the percentage of blood group O was significantly higher in patients who died (83.3% vs 87%) (P < 0.001), whereas the percentage of patients with blood group A was significantly lower (12.3% vs 8.88%) (P < 0.001). Kaplan-Meier curves are shown in Figure 3; survival for different ABO blood groups were significantly different (P = 0.02).
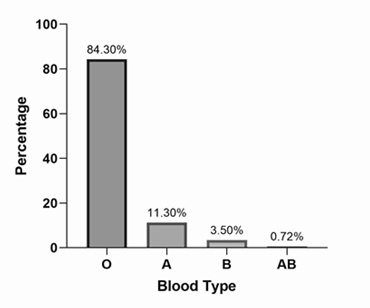
Figure 1 Overall ABO blood group distribution in 2,369 hospitalized patients with COVID-19. Source: Own data.

Figure 2 Comparison of ABO blood group distribution between hospitalized patients with COVID-19 who died or were discharged. Source: Own data.
Table 1 Analysis of risk factors associated with mortality in patients with COVID-19.
| Characteristic | Outcome | Cox proportional-hazards models | |||||
|---|---|---|---|---|---|---|---|
| Death n = 686 | Discharged n = 1683 | P-value | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | |
| Age | 63 (14.1)* | 50 (15.2)* | <0.001 | 1.045 (1.04 - 1.05) | <0.001 | 1.046 (1.04 - 1.05) | <0.001 |
| Sex (Male) | 460 (67%)** | 1.018 (60.4%) | 0.003 | 1.19 (1.01 - 1.39) | 0.03 | 1.4 (1.19 - 1.65) | <0.001 |
| Blood type O | 597 (87%) | 1402 (83.3%) | 0.097*** | 1 Reference | 1 Reference | ||
| Blood type A | 61 (8.8%) | 208 (12.3%) | 0.71 (0.54 - 0.92) | 0.01 | 0.72 (0.55 - 0.95) | 0.02 | |
| Blood type B | 24 (3.5%) | 60 (3.5%) | 0.92 (0.61 - 1.32) | 0.7 | 0.99 (0.65 - 1.49) | 0.9 | |
| Blood type AB | 4 (0.5%) | 13 (0.7%) | 0.95 (0.35 - 2.54) | 0.9 | 0.88 (0.33 - 2.37) | 0.8 | |
| Rh | 677 (98.6%) | 1.653 (98.2%) | 0.4 | 0.78 (0.4 - 1.5) | 0.4 | 0.63 (0.32 - 1.23) | 0.1 |
| Diabetes type 2 | 166 (24.2%) | 185 (10.9%) | <0.001 | 2.06 (1.73 - 2.46) | <0.001 | 1.54 (1.27 - 1.88) | <0.001 |
| Hypertension | 152 (22.1%) | 193 (11.4%) | <0.001 | 1.76 (1.47 - 2.11) | <0.001 | 0.87 (0.7 - 1.06) | 0.8 |
| Obesity | 121 (17.6%) | 150 (8.9%) | <0.001 | 1.77 (1.45 - 2.16) | <0.001 | 1.95 (1.6 - 2.39) | <0.001 |
| *Mean SD: **n, (%): SD, Standard Deviation : HR, Hazard Ratio: CI, Confidence Interval: **Fisher’s exact test | |||||||
Source: Own data

Figure 3 Kaplan-Meier survival curves for 2,369 hospitalized patients with COVID-19 stratified by ABO blood group (Breslow test P = 0.02). Source: Own data.
Adjusted and unadjusted Cox model analyses are presented in Table 1. Unadjusted and adjusted odds ratios of risk factors for COVID-19 mortality are shown in Table 2. A Forest Plot of the hazard ratios of death for ABO blood groups is presented in Figure 4.
Table 2 Odds ratios (OR) from logistic regression models to predict COVID-19 mortality.
| Characteristic | Logistics models | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Age (>59 years) | 3.961 (3.29 - 4.77) | <0.001 | 4.014 (3.29 - 4.89) | <0.001 |
| Sex (Male) | 1.33 (1.10 - 1,60) | 0.003 | 1.65 (1.35 - 2.03) | <0.001 |
| Boold type O | 1 Reference | 1 Reference | ||
| Boold type A | 0.69 (0.51 - 0.93) | 0.015 | 0.68 (0.49 - 0.95) | 0.02 |
| Boold type B | 0.94 (0.58 - 1.52) | 0.8 | 1.02 (0.60 - 1.71) | 0.95 |
| Boold type AB | 0.72 (0.24 - 2.23) | 0.57 | 0.54 (0.16 - 1.82) | 0.32 |
| Rh | 0.73 (0.35 - 1.55) | 0.41 | 0.61 (0.27 - 1.37) | 0.23 |
| Diabetes type 2 | 2.59 (2.05 - 3.26) | <0.001 | 1.97 (1.50 - 2.60) | <0.001 |
| Hypertension | 2.20 (1.74 - 2.78) | <0.001 | 1.08 (0.82 - 1.44) | 0.58 |
| Obesity | 2.19 (1.69 - 2.83) | <0.001 | 2.41 (1.82 - 3.20) | <0.001 |
| OR, Odds Ratio: CI, Confidence Interval | ||||
Source: Own data

Figure 4 Forest Plot showing Hazard Ratios of death for ABO blood types during the period between March 23 and December 10, 2020. 1. Unadjusted Cox Proportional-Hazard model. 2. Adjusted Cox Proportional-Hazard model, considering age, sex, type 2 diabetes, hypertension, and obesity. Source: Own data.
In summary, blood group A had a significant lower risk of death compared with group O (adjusted HR = 0.72, 95% IC 0.55-0.95, P = 0.02). Groups B, AB and Rh-positive were not significantly associated with the outcome. Moreover, type 2 diabetes (adjusted HR 1.54, 95% IC 1.27-1.88, P < 0.001) and obesity (adjusted HR 1.95 95% IC 1.6-2.39 P <0.001) were statistically significantly associated with an increased risk of death by COVID-19. Hypertension showed not statistical significance with respect to mortality in the adjusted model (adjusted HR 0.87, 95% IC 0.7-1.06 P <0.8), despite being associated with mortality in the unadjusted model (unadjusted HR 1.76 1.95% IC 1.47-2.11 P <0.001).
Discussion
Mexico has been one of the most affected countries by COVID-19 worldwide as reflected by the death-toll in hospitalized patients. Therefore, identifying in-patient mortality-associated factors in the Mexican population is of utter importance1. The main results of this study include a statistically significant difference in mortality of COVID-19 patients according to their ABO blood type. Additionally, obesity was the comorbidity with the greatest impact in mortality of the studied population.
In the Central Military Hospital, the three most frequent comorbidities in non-survivors were the same as those reported in other studies of Mexican patients, however, their order of prevalence differed 16,17.Vera-Zertuche (2021) reported that the three main comorbidities present in non-survivors were hypertension (43%), type 2 diabetes (38%), and obesity (30.5%); the lower prevalence of these comorbidities in our study could be attributable to a lower risk of metabolic diseases in 33% of our population who were active military personnel with an active lifestyle due to their military enadeavors18. Thus, comorbidities do not fully explain the high risk of developing severe COVID-19 and death in apparently healthy patients in whom other characteristics could be responsible for favoring disease progression and death.
Several reports have identified genetic mechanisms which are strongly heritable that increase susceptibility to severe SARS-CoV-2 infection. These mechanisms are related to innate antiviral defenses and host inflammatory lung response19. Innate antiviral defense activation is important early during disease and signaling by interferons is essential for response to viral infection. Damage in the interferon receptor subunit 2 (IFNAR2) is associated with severe COVID-1919,20. Furthermore, decreased expression of the interferon-inducible oligoadenylate synthase gene cluster (OAS) has been implicated in susceptibility to SARS-CoV-221. The mechanisms involved in lung inflammation are linked to the late complications of the disease. One of the most important mechanisms involved is the over-expression of CC-chemokine-receptor 2, which promotes chemotaxis of monocytes-macrophages to sites of inflammation, thereby increasing damage due to the inflammatory response22.
After multivariate adjustment, obesity was the comorbidity with the highest impact on mortality in our study; type 2 diabetes, older age, and male gender were also associated with mortality, similar toother observational studies19,23,24. Mechanisms of susceptibility to COVID-19 in obesity have not been fully characterized; preliminary studies mention the role of chronic inflammation, metabolic dysfunction, deficiency of vitamin D, gut microbiota dysbiosis and over- expression of ACE2 protein as factors that could explain increased mortality25. Regarding patients with diabetes, impaired phagocyte cell capabilities, over-expression of Furin and ACE-2 receptor and altered function of T-cells could be involved26. The worst prognosis reported in elderly patients could be due to the many chronic diseases seen in this population and the dysregulation of lymphoid organs; it has also been found that DPP4 enzymatic expression is higher in elderly people. SARS-CoV-2 uses DPP4 to enter cells and its upregulation has been reported in patients with severe disease27. The lower risk of developing severe COVID-19 women could be attributed to their ability to mount more robust innate antiviral responses than males28.
We found evidence that blood group A was associated with a lower hazard of death among COVID-19 patients (Unadjusted HR = 0.71 IC 0.54-0.92), whilst a higher risk of death was found for group O. Moreover, the association between mortality and blood group in the univariate analysis was quite similar when the Cox model was adjusted for the main risk factors that influence COVID-19 mortality in the Mexican16,17,18. In the adjusted model, blood group A kept the lower hazard of death compared with group O (Adjusted HR = 0.72, 95% IC 0.55-0.95). Groups B and AB do not appear to be associated with the studied clinical outcomes. In both the univariate and multivariate analyses, the measures of effect size OR and HR showed the same associations between risk factors and COVID-19 mortality. Interestingly, we obtained very similar adjusted HR and adjusted OR estimates for risk factors when a subgroup analysis was performed excluding active military personnel (Data not shown).
There are only few studies comparable to ours performed in hospitalized populations. Yet, our findings match those of studies carried in Saudi Arabia and Egypt29 (Group O was associated with higher intubation rates compared with group A) and USA (patients with blood Group A had a lower risk of intubation and death compared with groups AB and O)9. Nonetheless, our results contradict those of studies carried out in France, Iraq, and Canada in which group A conferred a higher mortality risk, and those from Denmark and China in which no association was found between ABO groups and death29,15.
Severe and critical COVID-19 have been associated with a pathological state characterized by rapid and prolonged systemic elevation of cytokines and chemokines named “cytokine storm”. Several studies have linked the cytokine storm with tissue injury, multiple organ failure and death30. The increased mortality risk of patients with blood type O observed in our study could be explained by the evidence that suggests that these patients have increased IL-6 and IL-10 levels compared with patients with other blood types. High levels of IL-6 have been reported in patients infected with Helicobacter pylori and a genome-wide association study reported increased susceptibility of group O cells to release more IL-1031. Moreover, another study reported that patients with blood group O had higher levels of IL-10 in response to acute coronary síndrome32. Even though IL-10 was thought to suppress inflammation, new evidence suggests that early dramatic elevation of IL-10 may play a pathological role in COVID-1933.
The controversy across reports could be explained by genetic variations among patients with the same blood type and the traits associated with different blood types which could be developed only under certain lifestyle circumstances such as hypertension in people with blood group O34. Furthermore, the relationship between ABO blood groups and mortality could be more complex, and certain lifestyles or genetic conditions in distinct populations could synergize with certain blood groups to favor a deregulated immune system with greater risk of developing a life-threatening COVID-19 scenario.
Limitations of this study are those inherent to retrospective studies. Secondly, the population consisted only of Mexicans receiving medical care in Mexico City; therefore, our results cannot be generalized to other populations. Thirdly, we had insufficient data to estimate indicators of COVID-19 severity rates.
In conclusion, we found evidence of a significant association between ABO blood type and COVID-19 in-patient death. Blood group A may be less likely to experience death during hospitalization compared with group O. There were no significant associations between blood groups AB and B with the risk of COVID-19 death.











 nueva página del texto (beta)
nueva página del texto (beta)



