1. INTRODUCTION
Since the beginning of the twentieth century, records show that reinforced concrete has been used in Brazil, but the use of concrete as a standard building material took off in the 1950s. This happened due the industrial revolution that brought modified techniques and new materials.
According to Mehta and Monteiro (2014), concrete is only surpassed by water as the most consumed material on the planet. Due to its strength and versatility, concrete has played a major role in construction, and has been widely used by architects, due to the wide variety of possible formats, the aesthetic appreciation of structures and uses, and its ability be molded to the limit of creativity, especially when used uncoated.
With the passage of time, many constructions have been degraded by diverse pathological manifestations that may even lead to collapse. Therefore, there is a great need for research studies on the subject.
Among the principal pathological manifestations that attack reinforced concrete are the actions of heat and cold, climate and humidity, alkali/aggregate reactions, chemical aggression, corrosion by carbonatation and/or chlorides (Cascudo et al., 2014).
The penetration of chloride ions affects the constructions in coastal or seaside sites that have a high concentration of free chloride ions. They are considered to be the major cause of premature corrosion of structures (Verás Ribeiro et al, 2014 and Medeiros, 2014).
Brazil has a great amount of equipment and constructions that are in direct or semi-direct contact with the sea. Recife, the capital of Pernambuco, is one of the cities with the most urban facilities in contact with sea water. Buildings, bridges, anchorages, catwalks, in other words, projects where concrete structures are placed into a maritime environment: either submerged, partially submerged, or within the tidal zone, splash zone, or mist zone environments. Sea water contains high amounts of chloride ions and exposure to these makes these concrete structures more vulnerable (Pitan et al., 2015).
It is therefore necessary to research and build parameters and relationships that allow sustainable and economic decision making for projects that can increase the useful life of concrete structures.
This study aims to investigate with chemical tests the penetration of chlorides into specimens of different types of concrete (characterized as poor, medium, and rich) partially submerged in sea water. For this, the ISO TC 71/SC 1 will be used as a reference for the chloride penetration procedures and the Mohr method will be used as a reference for the laboratory chemical tests.
1.1 Marine environment
According to NBR 6118: 2014 the marine environment is recognized as aggressive to concrete structures, where it is considered to be in aggressiveness class IV. Its influence on the durability of concrete structures depends on the microclimate in which it is found (Cascudo et al., 2014). The aggressive agents present in the marine environment cause both the corrosion of the steel reinforcement and the corrosion of the concrete (cement matrix) (Lima and Morelli, 2004).
According to Andrade (2001) and Medeiros (2012), the penetration of chlorides into the concrete can happen in different ways, one of which is the incorporation of the chloride into the mass of the concrete, which has become less likely due to the limitations imposed by the current standards. Examples of other mechanisms that occur most frequently are capillary absorption and diffusion, which depend on external factors.
According to Verás Ribeiro (2014), capillary absorption is a mechanism where the chlorides present in a liquid medium penetrate into the concrete from the flow of the liquid, by the effect of surface tension acting on the capillary pores. According to Verás Ribeiro (2014) and Meira (2009), this mechanism depends on the pore diameter, the surface tension of the liquid, its density, and its viscosity.
Diffusion occurs because of chloride concentration gradients. Chlorides in higher concentration regions move to regions of lower concentration (Meira, 2009).
In the 2014 NBR 6118, attention can be drawn to a chapter on this subject, where classes of environmental aggressiveness for concrete constructions in urban and rural areas are identified as weak, moderate, strong, or very strong.
Such regulation also details the type of concrete to use as well as the specifications for the nominal covering to be adopted according to the component or element of reinforced or prestressed concrete. NBR 6118:2014 already recommends that the covering should be respected for construction projects including those with little or no control, since this is equal to the minimum covering necessary plus a tolerance factor.
Most of the constructions in direct contact with the marine environment begin to show pathological manifestations within a short time after construction, with significant decomposition of the concrete and intense corrosion of reinforcement (Verás Ribeiro, 2014). Sea water has high amounts of chloride ions. The classification between aggressive and non-aggressive environments is relative and refers mainly to the amount of H2S, SO2, NOx, SO4, and Cl- present (Helene, 1986).
For Medeiros (2014), these oxides are extremely aggressive and contribute to the acceleration of the process of corrosion of the embedded reinforcements, even in small proportions. As a reference, it is possible for the corrosion rate in a marine atmosphere to be on the order of 30 to 40 times higher than that of a pure rural atmosphere (Verás Ribeiro, (2014).
In cities that are subject to such saline exposure, constructions of concrete or reinforced concrete, whether in contact with sea water or not, always suffer pathological manifestations and mechanical degradation that require permanent maintenance (Cascudo et al., 2014).
Some projects locate concrete structures directly in the marine environment: submerged or partially-submerged in sea water, within the high or low tide zone, the atmosphere zone, the splash zone, or the mist zone (Cascudo et al., 2014).
Sea water is one of the elements of nature most damaging to concrete structures. In its composition, it is possible to find constituent elements of many chemical compounds.
According to Lima and Morelli (2005), oceanographers have identified the elements distributed in ocean waters, as well as their various states and components of chemical compounds. Some compounds are stable, such as those containing sodium and potassium; while others are relatively unstable, such as those containing silicon and magnesium. The magnitude of concentration can be divided into three groups:
Major Inorganic Elements - Cl, Na, S, Mg, Ca, K - found in amounts greater than 100 parts per million (ppm), or 100 mg per liter (mg/L) equivalent to 10% by mass.
Minor elements - Br, C, Sr, B, Si, F - found in amounts greater than 1 and less than 100 mg/L or 0.1% to 10%.
Trace Elements - N, Li, Rb, P, I, Fe, Zn, Mb - found in amounts less than 0.1%).
The composition of sea water, which can vary according to temperature, latitude, depth, and proximity to land, has been researched over a long period of time. Dittmar, in 1870, after analyzing thousands of water samples from all seas, found that, despite varying amounts of salts dissolved, the proportions of the main elements were constant. Salinity varies from 3.3% to 3.7% in the open ocean; with the overall average salinity for all oceans being 3.5%.
1.2 The City of Recife and Contact with the Sea
Recife, the capital of the state of Pernambuco, is popularly known as the “Brazilian Venice” because of its buildings along rivers and by the sea, and its numerous bridges that dominate the urban landscape, being one of the cities with most urban equipment in contact with sea water. Fig. 1 shows a panoramic view of the municipality of Recife.
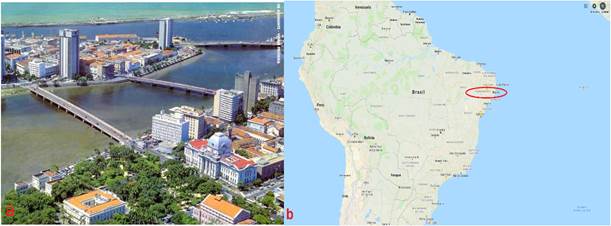
Figure 1 a) Panoramic view of Recife, PE. Source: Google Maps (2017) Available at: <http://embrasil.s3.amazonaws.com/upload/ciudad/81C-37.jpg >. Accessed on: 11/07/2017. b) Map of Brazil with location of Recife, Pernambuco highlighted.
Recife, because it is a coastal city, with a warm climate, high humidity, and predominance of the winds coming from the direction of the Atlantic Ocean, Recife’s concrete structures suffer aggressions from agents of diverse types of pathological manifestations. According to NBR 6118: 2014, Lima and Morelli (2004), Verás Ribeiro (2014) and Medeiros (2014) classified and defined four zones of aggressiveness that fit well with what happens to the existing concrete structures in the city. These regions are:
Marine atmosphere zone: in this region, despite not being in contact with sea water, the structure receives a considerable amount of salts, mainly chloride, that are able to produce salt deposits on its surface, in the form of solid particles or as drops of a saline solution. The quantity of salts present decreases as a function of the distance from the sea, being influenced by the speed and direction of the prevailing winds. The principal mechanism of degradation present in this zone is corrosion of the reinforcement by the action of the chloride ions.
Splash zone: in this region, the structure received direct contact with the action of the sea, due to waves and their splashes. The most significant damages are produced by corrosion of reinforcement by chloride ions and by erosion due to the waves.
Tidal variation zone: this region is bounded by the maximum and minimum levels reached by the tides and, because of this, the concrete is almost always saturated, depending on the climatic conditions and will have a growing concentration of salts. Degradation occurs due to the action of aggressive salts (chemical attack), corrosion of reinforcement (due to the presence of chloride ions), waves action, other substances in suspension (abrasion), and microorganisms.
Submerged zone: the concrete in this region is permanently underwater. The degradation occurs by the action of aggressive salts (sulfate and magnesium ions) and by the action of microorganisms, which in extreme cases, can generate biological corrosion of the reinforcement.
1.3 Mohr method
The need to identify how the chlorides penetrate into reinforced concrete structures is necessary because of the subsequent corrosion that it provokes. Several methods have been developed for this, including the colorimetric silver nitrate spray method, which is a qualitative test to identify free chlorides in concrete, and the Mohr method, which is a laboratory test using titration, also with silver nitrate.
According to Mota (2011), the colorimetric silver nitrate spray method uses a methodology based on the application of a chemical indicator capable of altering the coloration of the concrete in the presence of chlorides. According to studies by Otsuki, Andrade, and Meck, this can vary based on factors such as cement type, water/cement ratio, and type of material used, as shown in Table 1.
Table 1 Summary of variables involved in the colorimetric silver nitrate spraying method.
| Cement Type | Type of
specimen |
w/c | Chloride
content limit for color change |
Year | Country | Researcher |
|---|---|---|---|---|---|---|
| Common Portland Cement |
Plaster | 0.4 0.5 and 0.6 |
0.15% | 1992 | Japan | Otsuki et al. |
| Mortar | ||||||
| Concrete | ||||||
| Common Portland Cement and Portland Cement with additive |
Concrete | 0.4 and
0.7 |
1.13 to 1.4% | 1999 | Spain | Andrade et al. |
| Common Portland Cement and Portland Cement with additive |
Concrete | - | 0.90% | 2003 | Australia | Meck |
Source: Mota (2011).
This method was developed in 1970 by Dr. Mário Collepardi in order to verify the presence or absence of chlorides in concrete samples and, therefore, to be able to determine the penetration front of chlorides into the structures exposed to marine environments. This technique also contributed to the development of the process of fixation of free chlorides in the cement matrix (MOTA, 2011).
To carry out the MOHR method, the 5% K2CrO4 (potassium chromate) solution, which acts as an indicator, is used to stain samples yellow, and then a solution of 0.0141 M silver nitrate (AgNO3) is used until the liquid sample obtains a “brick red” color in titration combining with the free ions in the concrete sample.
1.4 Standardization: ISO TC71/SC 1(26-07-2010) and ISO/WD 1920-11
According to this ISO, reinforced concrete structures exposed to chlorides, whether from marine waters or other sources, must meet the durability criteria for which they have been designed for the entire life of the project. The possibility of corrosion of the reinforcement increases significantly as the chloride content gradually accumulates inside these structures. For this reason, the degree of diffusion or penetrability of the concrete represents important properties to be evaluated, whereas these technical specifications establish a test method that can be applied to samples prepared for the evaluation of the potential properties of resistance to chloride that a given concrete mix will present.
For this reason, the degree of diffusion or penetrability of the concrete represent critically important properties to be evaluated. These technical specifications establish a test method that can be applied to prepared samples in order to evaluate the potential resistance to chloride that a given concrete mix will present.
These technical specifications represent a method for determining the unidirectional chloride penetration parameters in the non-continuous state in preconditioned hardened concrete specimens.
2. MATERIALS AND METHODS
This study aims to determine the quantitative penetration of chlorides in test bodies having different resistances at various depths. For this, tests were carried out at the laboratories of the Catholic University of Pernambuco. In the Materials Laboratory, the specimens were prepared using tests with partial immersion in sea water and with reference to ISO TC 71/SC 1 of 26/07/2010, ISO/WD 1920-1, and ISO TC 71/SC 1/WG SII NBR-9779. Then, the Mohr method was carried out in the Chemistry Laboratory.
To calculate the percentage of chloride absorbed by the samples, equation 1 below was used. Because the aggregate material is considered to be "inert", only the mass of the cement itself is taken into account. Only the chemical components present in the cement react with chlorides, especially the C3A.
Where:
V1 |
= volume of AgNO3 solution used to titrate the sample, in ml |
V2 |
= volume of AgNO3 solution used to titrate the blank control, in ml |
m |
= molar concentration of the AgNO3 solution |
mm |
= molar mass of Cl |
Vamostra |
= sample volume = 0.10 ml |
Dilution factor |
= 1000/250 = 4 |
2.1 Characterization of materials utilized
Cement: CP II-F-32 was used in this study, determined according to the ABNT of Portland Cement compound with filler (NBR 11578/91).
Coarse sand: The fine aggregate used in this research is natural from a riverbed in the city of Pombos, Pernambuco. The sand was tested in the materials laboratory following the standards of granulometric characterization and distribution.
Gravel: The coarse aggregate used was crushed stone of size 25 mm, grade 1 according to NBR 7211 (ABNT, 2009).
Potable water: Water used was from COMPESA, the public water supply network of the city of Recife.
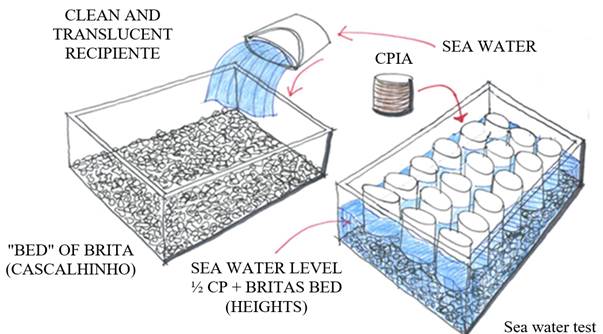
Seawater: The seawater used was from the Boa Viagem beach, Recife, Pernambuco.
2.2 Molding the test specimens
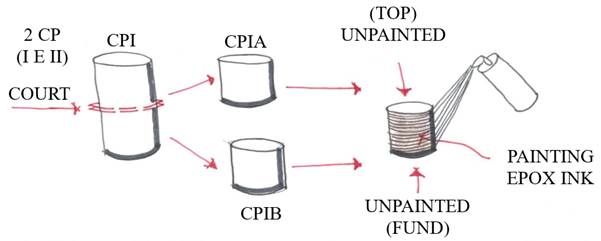
Nine cylindrical concrete specimens (10x20 cm) were molded for three different mechanical strengths> low, medium, and high; with three specimens for each (See Table 2). After being removed from the mold, all specimens were submitted to wet curing for 14 days. One of each type remained in the wet curing until 28 days in order to maintain all types equal and to measure mechanical resistance.
Table 2 Concrete mixes (unitary)
| Type | Ratio | Slump | w/c | Cement | sand | gravel | Average
Resistance (MPa) |
|---|---|---|---|---|---|---|---|
| Poor | 1:3 | 160 ± 20 | 0.516 | 1 | 1.6 | 1.40 | 26.67 |
| Medium | 1:2 | 160 ± 20 | 0.370 | 1 | 0.95 | 1.05 | 32.33 |
| Rich | 1:1 | 160 ± 20 | 0.259 | 1 | 0.3 | 0.70 | 40.87 |
The other six test specimens were divided in half, with their ends aligned and left in a dry environment for another 14 days, resulting in a total of 12 10x10 cm specimens. At 21 days they were waterproofed on all sides except for the top and bottom, as shown in Figure 2. At 28 days of age, they were submerged in fresh water, remaining there until 35 days, when they were removed and placed partially submerged in sea water with one of their ends on a fine bed of crushed stone for another 7 days, according to Figure 3. At 42 days, they were removed from the sea water, allowed to dry for 24 hours in the natural environment, and each identified by type of concrete and the division made.
Table 2 shows the unit ratio of the materials used in relation to the cement, by mass. The slump values were kept fixed at 160 ± 20 (mm), by varying the amount of dry materials.
After completing these processes, holes were drilled in each test piece on the end that was in contact with sea water and were drilled from largest to smallest with depths of 1, 2, and 3 centimeters, respectively. An impact drill was used to make the holes, a ruler for measuring, a bench drill for greater precision and drill bits for concrete of gauges 14, 10, and 8 mm. From each hole, all material (powder) was collected and packaged in individual bags, named and numbered for identification when tested in the chemical laboratory (see Figure 4).
2.3 Performing the Mohr method
The second part of the study was carried out in the chemical laboratory at the Catholic University of Pernambuco, under the guidance of Professor Sérgio Paiva. The purpose of the tests was to use the Mohr method, titration by silver nitrate, to determine the amount of chloride ions that penetrated to the depths drilled in the specimens (1, 2, and 3 cm).
In Table 3, it can be seen that 45 samples were cataloged, 36 of the partially immersed specimens and 9 of the non-immersed specimens.
Table 3 Sample test specimens
| Type | Ratio | Specimens (10x10) |
Drill
holes (3 p/ specimen) |
|---|---|---|---|
| Poor | 1:3 | 4 | 12 |
| Medium | 1:2 | 4 | 12 |
| Rich | 1:1 | 4 | 12 |
| Not submerged | - | 3 (10x20) | 9 |
| Total Samples | 45 | ||
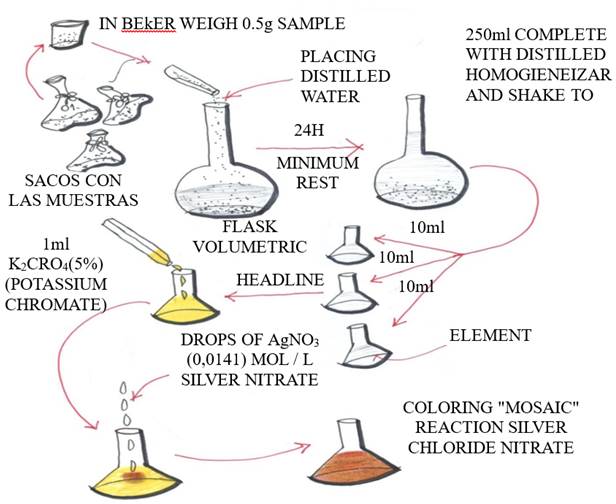
Parts of 45 samples were placed in beakers, weighing 0.5 grams. After this, the drilled powder from each test specimen, parts A and B (two pieces of 10 x 10 cm), was placed in three flasks and numbered from 1 to 18, with a pipette of 50 ml distilled water, and stirred to mix.
Then this heavy "powder" was diluted with distilled water in a 250 ml volumetric flask. The contents of the volumetric flasks were left to rest for at least 24 hours and were then diluted with distilled water to 250 ml and shaken individually to homogenize them.
Of this 250 ml, three samples of 10 ml each were withdrawn and placed in flasks. In each of these samples, 1 ml of potassium chromate (K2CrO4, 5%, 99% pure) was placed, which worked as indicator and left a yellow coloration. After this, each sample was titrated with silver nitrate (AgNO3 - 0.0141 mol/l, 97.8% pure) until reaching a red brick color. This would show the amount of silver nitrate needed to associate with the free chloride ions in the cement mass of each sample.
The results were plotted individually in spreadsheets and the amount of chloride ions in each sample that reacted with the cement mass was calculated according to the ratio (poor, medium and rich) and depth of penetration (1, 2, and 3 centimeters). The process is detailed in Fig. 5.
3. RESULTS
In this section, the results and analyses of the tests performed with silver nitrate titration using the Mohr method will be presented.
First, Tables 4, 5, and 6 will be presented showing the weight, the sample identification, the consumed volume of AgNO3 (silver nitrate) per sample, and the relationship between the three, specifically between the poor and the rich and between the average and the rich.
Table 4 Results of the chemical laboratory tests - Samples of test specimens of poor concrete mix immersed in sea water.
| CP | MIX TYPE |
DEPTH (cm) |
SAMPLE No. |
MASS (g) |
AgNO3 (ml) (Used) | CHLORIDE (%Cl¯) In relation to cement mass |
|||
|---|---|---|---|---|---|---|---|---|---|
| 1st Tit. |
2nd Tit. |
3rd Tit. |
Avg. | ||||||
| POOR CONCRETE MIX | |||||||||
| 1 | CP 1A | 1 | 1 | 0.52 | 0.50 | 0.50 | 0.50 | 0.50 | 0.0234 |
| 2 | 2 | 0.52 | 0.50 | 0.50 | 0.50 | 0.50 | 0.0234 | ||
| 3 | 3 | 0.50 | 0.60 | 0.60 | 0.60 | 0.60 | 0.0292 | ||
| 2 | CP 1B | 1 | 4 | 0.51 | 0.60 | 0.60 | 0.70 | 0.63 | 0.0311 |
| 2 | 5 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.0234 | ||
| 3 | 6 | 0.52 | 0.70 | 0.50 | 0.50 | 0.57 | 0.0273 | ||
| 3 | CP IIC | 1 | 7 | 0.50 | 0.50 | 0.40 | 0.60 | 0.50 | 0.0234 |
| 2 | 8 | 0.50 | 0.50 | 0.40 | 0.40 | 0.43 | 0.0195 | ||
| 3 | 9 | 0.51 | 0.30 | 0.40 | 0.30 | 0.33 | 0.0136 | ||
| 4 | CP IID | 1 | 10 | 0.54 | 0.70 | 0.60 | 0.60 | 0.63 | 0.0311 |
| 2 | 11 | 0.52 | 0.40 | 0.40 | 0.50 | 0.43 | 0.0195 | ||
| 3 | 12 | 0.50 | 0.30 | 0.40 | 0.30 | 0.33 | 0.0136 | ||
*CP IA, CP IB, CP IIC, CP IID - Identification of test specimens of poor concrete mix.
Table 5 Results of the chemical laboratory tests - Samples of test specimens of medium concrete mix immersed in sea water.
| CP | MIX TYPE |
DEPTH (cm) |
SAMPLE No. |
MASS (g) |
AgNO3 (ml) (Used) | CHLORIDE (%Cl¯) In relation to cement mass |
|||
|---|---|---|---|---|---|---|---|---|---|
| 1st Tit. |
2nd Tit. |
3rd Tit. |
Avg. | ||||||
| MEDIUM CONCRETE MIX | |||||||||
| 5 | CP IIIE | 1 | 13 | 0.55 | 0.70 | 0.70 | 0.80 | 0.73 | 0.0272 |
| 2 | 14 | 0.54 | 0.60 | 0.50 | 0.40 | 0.50 | 0.0172 | ||
| 3 | 15 | 0.55 | 0.50 | 0.60 | 0.50 | 0.53 | 0.0186 | ||
| 6 | CP IIIF | 1 | 16 | 0.52 | 0.50 | 0.60 | 0.50 | 0.53 | 0.0186 |
| 2 | 17 | 0.56 | 0.60 | 0.40 | 0.50 | 0.50 | 0.0172 | ||
| 3 | 18 | 0.56 | 0.40 | 0.40 | 0.30 | 0.37 | 0.0114 | ||
| 7 | CP IVG | 1 | 19 | 0.56 | 0.80 | 0.60 | 0.80 | 0.73 | 0.0272 |
| 2 | 20 | 0.58 | 0.60 | 0.60 | 0.60 | 0.60 | 0.0214 | ||
| 3 | 21 | 0.57 | 0.40 | 0.50 | 0.60 | 0.50 | 0.0172 | ||
| 8 | CP IVH | 1 | 22 | 0.63 | 0.80 | 0.70 | 0.80 | 0.77 | 0.0286 |
| 2 | 23 | 0.55 | 0.50 | 0.50 | 0.50 | 0.50 | 0.0172 | ||
| 3 | 24 | 0.56 | 0.30 | 0.40 | 0.40 | 0.37 | 0.0114 | ||
*CP IIIE, CP IIIF, CP IVG, CP IVH - Identification of test specimens of medium concrete mix.
Table 6 Results of the chemical laboratory tests - Samples of test specimens of rich concrete mix immersed in sea water.
| MIX TYPE |
DEPTH (cm) |
SAMPLE No. |
MASS (g) |
AgNO3 (ml) (Used) | CHLORIDE (%Cl¯) In relation to cement mass |
||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Tit. |
2nd Tit. |
3rd Tit. |
Avg. | ||||||
| RICH CONCRETE MIX | |||||||||
| 9 | CP VI | 1 | 25 | 0.53 | 0.80 | 0.70 | 0.50 | 0.67 | 0.0161 |
| 2 | 26 | 0.52 | 0.30 | 0.40 | 0.40 | 0.37 | 0.0076 | ||
| 3 | 27 | 0.55 | 0.30 | 0.40 | 0.40 | 0.37 | 0.0076 | ||
| 10 | CP VJ | 1 | 28 | 0.52 | 0.70 | 0.60 | 0.70 | 0.67 | 0.0161 |
| 2 | 29 | 0.50 | 0.50 | 0.60 | 0.60 | 0.57 | 0.0132 | ||
| 3 | 30 | 0.52 | 0.50 | 0.60 | 0.60 | 0.57 | 0.0132 | ||
| 11 | CP VIL | 1 | 31 | 0.50 | 0.70 | 0.70 | 0.50 | 0.63 | 0.0151 |
| 2 | 32 | 0.56 | 0.60 | 0.50 | 0.50 | 0.53 | 0.0123 | ||
| 3 | 33 | 0.53 | 0.60 | 0.60 | 0.70 | 0.63 | 0.0151 | ||
| 12 |
CP VIM |
1 | 34 | 0.52 | 0.70 | 0.50 | 0.50 | 0.57 | 0.0132 |
| 2 | 35 | 0.54 | 0.40 | 0.50 | 0.50 | 0.47 | 0.0104 | ||
| 3 | 36 | 0.53 | 0.30 | 0.30 | 0.40 | 0.33 | 0.0066 | ||
*CP VI, CP VJ, CP VIL, CP VIM - Identification of test specimens of rich concrete mix.
Tables 6, 7, and 8 show the results obtained from the powder samples taken from the drilling of each test specimen, the laboratory results of titration by silver nitrate, and the percentage of chlorides in the cement by mass.
Table 7 Results of the chemical laboratory tests - Samples of rich, médium, and poor concrete test specimens without immersion in sea water.
| SP. | MIX TYPE |
DEPTH (cm) |
SAMPLE No. |
MASS (g) |
AgNO3 (ml) (Used) | (%Cl¯) In rel. to cement mass |
(%Cl¯) In rel. to cement mass |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Tit. |
2nd Tit. |
3rd Tit. |
Avg. | |||||||
| POOR CONCRETE MIX | W/O IMMERSION | |||||||||
| 13 | CP VII | 1 | 37 | 0.50 | 0.40 | 0.30 | 0.40 | 0.37 | 0.0033 | 0.0156 |
| 2 | 38 | 0.50 | 0.30 | 0.30 | 0.40 | 0.33 | 0.0029 | 0.0136 | ||
| 3 | 39 | 0.54 | 0.40 | 0.30 | 0.40 | 0.37 | 0.0033 | 0.0156 | ||
| MEDIUM CONCRETE MIX | W/O IMMERSION | |||||||||
| 14 | CP VIII | 1 | 40 | 0.55 | 0.40 | 0.50 | 0.30 | 0.40 | 0.0037 | 0.0129 |
| 2 | 41 | 0.54 | 0.40 | 0.40 | 0.50 | 0.43 | 0.0042 | 0.0143 | ||
| 3 | 42 | 0.56 | 0.40 | 0.50 | 0.40 | 0.43 | 0.0042 | 0.0143 | ||
| RICH CONCRETE MIX | W/O IMMERSION | |||||||||
| 15 | CP IX | 1 | 43 | 0.53 | 0.30 | 0.30 | 0.40 | 0.33 | 0.0029 | 0.0066 |
| 2 | 44 | 0.53 | 0.40 | 0.40 | 0.40 | 0.40 | 0.0037 | 0.0085 | ||
| 3 | 45 | 0.54 | 0.40 | 0.40 | 0.40 | 0.40 | 0.0037 | 0.0085 | ||
| SEAWATER - ATLANTIC OCEAN | ||||||||||
| 16 | SAMPLE | 46 | -0.0012 | |||||||
Table 8 Chloride by mass (%Cl-) in test specimens
| Mix | Depth | |||||
|---|---|---|---|---|---|---|
| Immersed | Not immersed | |||||
| 1 cm | 2 cm | 3 cm | 1 cm | 2 cm | 3 cm | |
| Poor | 0.0273 | 0.0214 | 0.0209 | 0.0156 | 0.0136 | 0.0156 |
| Medium | 0.0254 | 0.0182 | 0.0147 | 0.0129 | 0.0143 | 0.0143 |
| Rich | 0.0151 | 0.0109 | 0.0106 | 0.0066 | 0.0085 | 0.0085 |
Because the sample weights varied, three titrations were made with silver nitrate and the arithmetic mean of these values was calculated so that the margin of error would be smaller.
In all of the samples, it can be seen that the one-centimeter depth had the highest concentration of chlorides, due to being closer to the surface.
After applying the formula, the percentage of chloride by mass was found. The cement mass used for the chloride penetration analysis was found from the cement consumption of the samples, where the poor mix consumes 503 kg/m³ of concrete, the medium mix consumes 685 kg/m³, and the specific mass of the concrete is 2,350 kg/m³, making it possible to obtain the percentage of chloride. An arithmetic mean was obtained and is shown in Table 8.
According to the laboratory results obtained through the aforementioned experiment, the following considerations should be highlighted: the technique used is a qualitative technique, with low cost, and one that quickly determines the presence of free chlorides, giving support to the application of other more quantitatively-refined techniques. On the other hand, it is important to note that there are limitations to the technique. For example, when the structure is carbonated, producing confusing results.
4. CONCLUSIONS
The results show that chloride penetration is lower in the partially-immersed concrete test specimens in the following order:
For all mixes that were partially immersed, the penetration of chlorides was greatest at the depth of 1 cm. The specimens not immersed in sea water presented, on average, 50% less chloride than those partially immersed.
It can therefore be concluded that concrete structures that are partially immersed in sea water require special care in their design (mix, façade covering, protective paint) as well as periodic maintenance for their entire lifetime to avoid potential collapses.
From the survey, results were obtained on the depth and the penetration of chlorides for rich, medium, and poor concrete mixes, which should assist professionals to designing parameters so that the useful life of constructions in contact with this aggressive environment can be increased. Care is important with regard to the materials used, the type of cement, and concrete resistance when developing activities in partial contact with sea water in the metropolitan area of Recife or in any other coastal city.
From the tests carried out, it can be concluded that the greater the concrete resistance, the lower the level of chloride ion penetration, when maintaining the same materials in the mix.
It is known that the chemical compounds in the cement and their respective phases will have a significant influence on the ability to combine chemically (to bond) with the chlorides. The aluminate phases (C3A and C4AF) are those that bond chemically with chlorides to form the chloroaluminates. On the other hand, the principal phases responsible for generating the C-S-H gel are C3S and C2S, with their hydrations consequently generating resistance to compression. Therefore, there is a greater influence on the ability of cement compounds to chemically combine with chlorides than on their compressive strength.
This result was expected because smaller pores generally indicate greater resistance by increasing the difficulty of penetration by chloride ions. This case study sought to compare concrete samples having different cement contents in their composition with regard to the penetration of chloride ions when immersed or not in seawater.
With the results, it was concluded that concretes with higher strength (smaller water/cement ratio) have a greater resistance to penetration by chloride ions, as concrete with greater resistance tends to have pores of considerably reduced size, thereby hindering the entry of chloride ions into the structure. It is important to highlight that the concrete studied in this research can be used in structural reinforcements.











 nueva página del texto (beta)
nueva página del texto (beta)