Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista ALCONPAT
On-line version ISSN 2007-6835
Rev. ALCONPAT vol.5 n.2 Mérida May./Aug. 2015
Review papers
AgNO3 spray method for measurement of chloride penetration: the state of art
1 Departamento de Construcción Civil, Universidad Federal de Paraná (UFPR), Brasil.
The durability of the buildings has been evaluated through visual inspections associated with field and laboratory tests. Nowadays, for analysis of the carbonation colorimetric method by spraying phenolphthalein is widely used, due to the ease and high reliability. However, when there is the presence of chlorides, the tests are long and expensive. As an alternative, there is a colorimetric method (AgNO3). The method is easy to use, low cost and allows for on-site reviews. But when there is presence of carbonation, the analysis becomes more complex, since reducing the pH and leads to discoloration of the concrete. This paper presents studies on the use of the colorimetric method for evaluation of the depth of chloride penetration in concrete. There is no consensus in academic circles as to eliminate this influence and to determine the turning point, however there is research demonstrating the influence of cement type on the colorimetric method.
Keywords: concrete; corrosion; chloride penetration; silver nitrate; colorimetric method
La durabilidad de las construcciones se ha evaluado mediante inspecciones visuales asociados a los ensayos de campo y de laboratorio. Hoy, para el análisis de carbonatación método colorimétrico por pulverización fenolftaleína se utiliza ampliamente debido a la facilidad y alta fiabilidad. Sin embargo, en la presencia de cloruros, los ensayos no son expeditos y tienen un alto costo. En su lugar, se utiliza la pulverización de nitrato de plata (AgNO3). El uso del método es simple, de bajo costo y permite evaluaciones in situ. Pero cuando, hay presencia de carbonatación, el análisis se hace más compleja debido a que la reducción del pH y los cambios en la coloración de hormigón. Esta investigación presenta estudios sobre el uso del método colorimétrico para evaluar la profundidad de penetración de cloruro en el hormigón. No hay todavía uno consenso sobre la forma de eliminar esa influencia o determinar el momento decisivo, sin embargo, hay investigaciones que muestran la influencia del tipo de cemento en el método colorimétrico.
Palabras clave: Hormigón; corrosión; penetración por cloruros; nitrato de plata; método colorimétrico
A durabilidade das construções tem sido avaliada através de inspeções visuais associadas a ensaios de campo e laboratório. Hoje, para análise da carbonatação o método colorimétrico por aspersão de fenolftaleína é amplamente utilizado, devido à facilidade e alta confiabilidade. Porém, na presença de cloretos, os ensaios não são expeditos e possuem alto custo. Como alternativa, há a aspersão de nitrato de prata (AgNO3). O método é de fácil aplicação, baixo custo e permite avaliações in loco. Mas quando há presença de carbonatação, a análise torna-se mais complexa, devido a redução do pH e alteração da coloração do concreto. Esta pesquisa apresenta estudos sobre o uso do método colorimétrico para avaliação da profundidade de penetração de cloretos no concreto. Ainda não há consenso de como eliminar essa influência ou determinar o ponto de viragem, entretanto há pesquisas que demonstram a influência do tipo de cimento no método colorimétrico.
Palavras chaves: concreto; corrosão; penetração de cloretos; nitrato de prata; método colorimétrico
1. INTRODUCTION
It is well known that highly alkaline environment provided by the cement matrix maintains this steel in reinforced concrete liability to corrosion. However, the corrosion occurs when chloride ions reach the armature.
The phenomenon of depassivation occurs mainly by two main reasons: First, due to the reduction of the concrete alkalinity caused by carbonation. Second, due the presence of chlorides, even if the concrete has a high pH, the reinforcement depassivation occurs, causing pitting corrosion which reduces the cross section of the bar and reduces its bearing capacity (FRANCE, 2011).
The chloride ions can be found in cementitious matrix in two forms: free (dissolved in pore water) or combined with the hydrated C3A and C4AF (from the cement hydration reaction) forming cloroalumintos (Friedel's salt). The chlorides that are really harmful to the steel in concrete are the free. The combined chlorides can become free due the carbonation of concrete or due to the rise of the concrete temperature (HELENE, 1993; PEREIRA & CINCOTTO, 2001; CAVALCANTI & CAVALCANTI, 2010).
In the context of corrosion induced by chlorides, as well as by carbonation, it is reasonable to consider the lifetime of the concrete structures in two stages: the first is when the critical chloride content reaches the surface of the steel inside the concrete (being this the useful lifetime of the structures) and the second is the subsequent spread of corrosion, in which the structure is damaged by steel corrosion (HE et al., 2011).
The durability results from the interaction of concrete structures, the environment, conditions of use, operation and even maintenance. Thus, to assess the performance of the buildings, it is often used visual inspections combined with field and laboratory tests, making it possible to identify the causes of pathological manifestations and choose the most appropriate recovery techniques and protection and the most cost-effective to maintain building (MOTA, 2011).
In order to assess the condition of concrete structures, the useful life of the construction can be estimated from the diffusion coefficient chlorides in concrete. The most representative current method to determine current status is based on the second Fick's Law. But this is a time consuming procedure and to improve this situation, accelerated methods, as proposed by ASTM C 1202/05, have being used in association with the identification of chloride penetration depth (KIM et al., 2013).
There are several methods to identify and quantify free and total chlorides along the depth of the concrete (chlorides profile), such as gravity (PEREIRA & CINCOTTO, 2001; SILVA, 2006). For the sake of determination of the chloride profile - which requires cutting or drilling, milling and chemical analysis of specific samples - once held towards various equipment and analysis timetable (He et al, 2011). In contrast, the colorimetric method based on AgNO3 for measuring penetration depth of chlorides in the cement matrix is practical and fast (JUCÁ, 2002; MECK & SIRIVIVATNANON, 2003; YUAN et al, 2008; FRANCE, 2011; HE et al. 2011; MOTA, 2011; KIM et al, 2013). Yet, its efficiency and application conditions must be well driven and self- understood to perform the method and to provide the possible application of advantages of such technique.
Silver nitrate spray has been used in association with accelerated test chlorides migration prescribed by ASTM C 1202/05. It consists on sprinkling aqueous solutions of AgNO3 0.1 M on the slices of fractured concrete after the migration assay. This procedure leads to the formation of two well-defined regions (Figure 1): a whitish, with AgCl precipitation, indicating the presence of chlorides and the other is brown, corresponding to region free of chlorides (MEDEIROS, 2008; TRINITY, 2011; MARRIAGA & CLAISSE, 2011; MARCONDES, 2012).
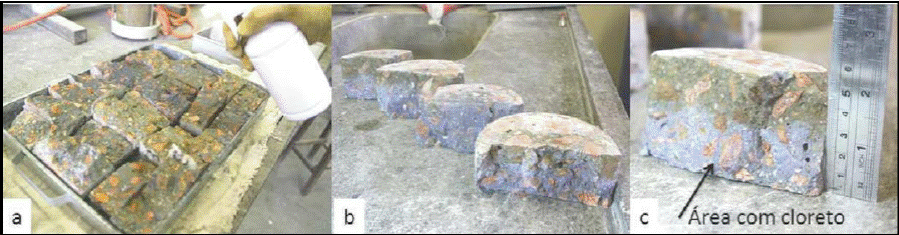
Figure 1 a) Spray silver nitrate solution; b) Comparison of the specimens; c) Measurement of the chloride penetration depth (MARCONDES, 2012)
In 2010, Cavalcanti & Cavalcanti applied the colorimetric method upon a pier located on the beach of Tambaú in João Pessoa/PB/BR. The authors were able to prove the depassivation and reinforcement corrosion occurred because the chloride ions exceeded the thickness of the cover. Regardless of, despite the simplicity of the method, the chemical reaction that triggers the color to change is affected by the concentration of silver nitrate solution, the pH of the concrete, the presence of carbonates and the chloride content of concrete. Consequently, the method is affected by the presence of carbonation (which leads to reduction of the pH) and the level of contamination at which the material is subjected to (OTSUKI et al., 1993; ANDRADE et al., 1999; MECK & SIRIVIVATNANON, 2003; JUCA, 2002; BOUNY et al, 2007;. HE et al, 2012;. FRANCE, 2011; KIM et al, 2013). In this context, the purpose of this article is to evaluate the applicability of the colorimetric method towards silver nitrate spraying once analyzing and comparing researches conducted and published.
2. COLORIMETRIC METHOD
The development of the colorimetric method for silver nitrate spraying began in Italy in 1970 by Collepardi. It is a qualitative method for identifying the presence of free chlorides in cementitious materials (FRANCE, 2011; MOTA, 2011). The method became standard in this country, however, according to Colombo (2001) apud Juca (2002), it did not present reliable results. So the standard "UNI 7928" was removed from service with no foreseeable replacement.
The main application of colorimetric method is to measure the depth of chloride penetration. When the silver nitrate solution is applied on the concrete surface, a photochemical reaction occurs (Figure 2). The reaction with free chlorides forms a white precipitate silver chloride. In the region of combined chlorides, it is formed a brown precipitate of silver oxide (MECK & SIRIVIVATNANON, 2003; FRANÇA, 2011; MOTA, 2011).

Figure 2 Potential precipitation of free chlorides (white) and combined chlorides (brown) (Medeiros et al, 2009).
As the chloride penetration is not uniform, NT Build 492 (2000) recommends performing seven measurements in every 10 mm. The result is the average of all of them (Figure 3). If some reading failure due the presence of aggregates, it should be changed to the next point or this measurement should be ignored if five other are valid.
The chemical reaction occurs with the free chloride ions (1). Although, in the presence of carbonates, the reaction also leads to the formation of a white precipitate, as indicated by the reaction (2). Therefore, Jucá (2002) recommends the use of realkalisation technique, once a carbonated concrete without contamination of chlorides may result in false positive.
3. INFLUENCE OF CEMENT TYPE
The colorimetric method only indicates the presence of free chlorides, therefore, the result could also be influenced by the ability of the cement to react with the chlorides (JUCÁ, 2002). As described above, the chlorides combine to C3A and C4AF, products of hydration of the Portland Cement. The lower the aluminate content, the less is the ability to immobilize chloride ions. Althoug, Pereira & Cincotto (2001) evaluated the ability of combination of chlorides in concrete with different types of Portland cement (Brazilians types of Portland Cement: CP I S, CP II F, CP III, CP IV e CP V ARI) and no significant differences in the content of chlorides combined where found.
In contrast, Jucá (2002) tested concretes with the same five types of cement (CP I S, CP II F, CP III, CP IV e CP V ARI), incorporating 1% and 2% of chloride on the cement mass into the specimens. After spraying the silver nitrate solution on the samples, the results indicated that there is a period of chlorides combination and that the aluminate content of the cement is an important factor on chemical combination process.
France (2011) evaluated the combination of chlorides using the colorimetric method with 0.4 and 2% of chloride on the cement mass for the types CP II F, CP IV e CP V - ARI, and so as to Jucá (2002), the results evidenced there was influence of cement type into the amount of free chlorides. The results of Mota (2011) also indicated the fixing of chlorides throughout the time. For a mortar produced with 2% of chloride on the mass of cement, the white region of the samples (which indicates the free chlorides) has changed. Although there was not a front of chloride penetration, since contamination in this study had internal chlorides, it can be seen in Figure 4 that the area of chloride contamination, as indicated by the whitish discoloration when spraying for silver nitrate, decreased over time. This probably means the chlorides combined with C3A. It is remarkable that the clear edge in Figure 4 are related to the effect of carbonation.
4. TURNING POINT AND pH INFLUENCE
When applying the colorimetric method, there is a turning point staining appearance. That means that a certain concentration of chloride and silver nitrate solution cause a color change (the boundary formation; border-color change), in order to determine the depth of penetration in opposition of free chlorides. According to Otsuki et al. (1993), the concentration of AgNO3 solution suitable for colorimetric method is equal to 0.1N. This value has been a general consensus among the various authors of the area (ANDRADE et al., 1999; MECK & SIRIVIVATNANON, 2003; JUCÁ, 2004; FRANCE, 2011; MOTA, 2011).
Additionally, in accordance to Otsuki et al. (1993), for concentration of 0.1N AgNO3 equal to the minimum content of free chlorides it may change the color is equal to 0.15% relative to the cement mass. On the other hand, Collepardi (1997) argues that this minimum level is equal to 0.01% (JUCA, 2004; FRANCE, 2011; MOTA, 2011). Andrade et al. (1999) found, with 95% reliability, the turning point is 1.14%±1,4 on the mass of cement. This value is in compliance with the argument leverage by Meck & Sirivivatnanon (2003), which is equal to 0.9% chloride on the mass of cement. In 2011, He et al. found the critical chloride content between 0.011 and 2.27% on the cement weight. It should be noted that there is no consensus on the free chloride content which causes a color change in AgNO3 0.1N solution, since available data in the studies cited are so disparate.
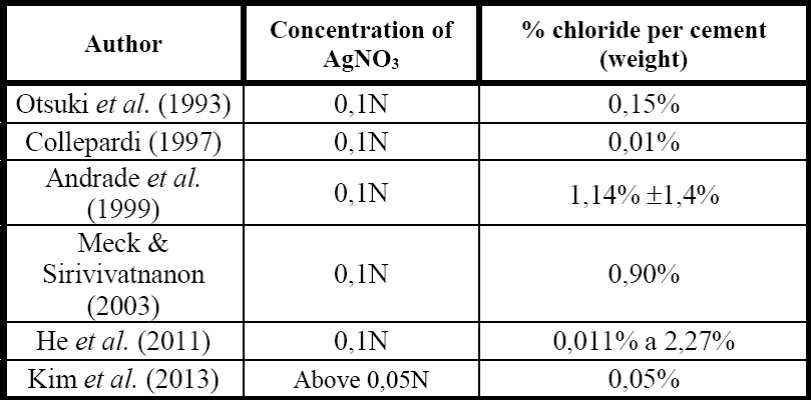
Recently, Kim et al. (2013) reassessed the variables that can influence the technique. The study intended to identify if the is a change in color by altering the pH of the environment, the concentration of AgNO3 and the chloride content. Additionally, if the rated water/cement ratio influence towards the concentration of chlorides in the color-changing border (border-color change), and if the colorimetric method could be applied on site to real structures. The evaluated items and their details are shown in Table 1.
Initially the tests were made at pH = 12 and change in chloride concentrations and silver nitrate solutions were made, as shown in Figure 5. According to Kim et al. (2013), the color change was more clearly observed for concentrations of AgNO3 over 0,03N. As the silver nitrate concentration increases, color change was observed change. In low concentrations, this change was not clearly displayed and can generate errors (especially in concentrations of 0.03 and 0,04N). Therefore, to measure the depth of penetration of chloride ions, the authors recommend using silver nitrate concentration to 0.05N.

Figura 5 Determination of optimal concentration of the silver nitrate and the minimum chloride content (KIM et al., 2013).
Following the study by Kim et al. (2013), four test specimen were submerged in seawater for 3 months, were subjected to spray test of silver nitrate to different concentrations of AgNO3, as shown in Figure 6.
By analyzing the values held by different authors, it can be said that the concentration of AgNO3 solution most suitable for the colorimetric method is 0.1N. However, there is still no consensus on the chloride content which leads to color change. Even the most current research (HE et al., 2011 and KIM et al., 2013) have not yet reached values close to each other, as shown in Table 2
To evaluate the influence of pH on the coloration, Kim et al. (2013) tested, as Figure 7 shows, several silver nitrate and chloride concentrations at different pH values.
The results evidenced when the pH is below 10, the extent of penetration of chlorides turns to become impractical (Figure 8). Therefore, when the structure is exposed to the attack by chlorides and CO2, the depth of carbonation should be measured before the depth of chlorides. When the carbonation depth exceeds the penetration of chlorides, it is impossible, according to Kim et al. (2013) determining a second variable (penetration free chlorides) by spraying silver nitrate.

Figure 8 a) Staining to pH = 10 b) Staining to pH = 11 C) Staining to pH = 12 d) staining to pH = 13 (KIM et al., 2013)
The ratio water/cement did not influence the concentration of chlorides in the color-change boundary (Kim et al., 2013), ie, it does not affect the turning point to the colorimetric method.
The colorimetric method using silver nitrate spray can be used at least as a first step to quantify the penetration into the concrete of chlorides (Bouny et al., 2007). In the case of structures exposed to marine environments and CO2, the use of silver nitrate spray method becomes complicated, it is necessary to associate the method with other tests (JUCÁ, 2004; FRANCE, 2011; MOTA 2011). Kim et al. (2013) applied the colorimetric method to reinforced concrete structures exposed to chlorides and highways exposed to deicing salts to confirm the applicability of the test. As carbonation was deeper than the penetration of chlorides, it was impossible to apply the colorimetric method, since the spray is silver nitrate, the color change region would indicate the presence of carbonation and not just contamination chlorides.
5. OTHER METHODS RENDERING COLORIMETRIC SILVER NITRATE
Since 1970, three colorimetric methods based on AgNO3 (AgNO3 + fluorescein, AgNO3 + AgNO3 and K2CrO4) have been proposed to measure the depth of penetration of chloride ions in the concrete field and in the laboratory. Both methods are as follows (He et al, 2011.):
AgNO3 + fluorescein: in the 70s, Collepardi et al. (1970; 1972) developed a colorimetric method to determine the free chloride contained in concrete, in which, firstly, a fluorescein solution (1g / L in a solution of 70% ethyl alcohol in water) was sprayed on a concrete section with chloride penetration. Then it was applied to 0.1 mol / L silver nitrate solution. Immediately after spraying silver nitrate, there was the formation of Ag2O and AgCl. Fluorescein a weak organic acid, which dissociates in solution in a yellowish green ion. This method was defined as the Italian Standard 79-25 (1978).
AgNO3 + K2CrO4: In this method, first, a solution of 0.1 mol / L AgNO3 pH = 3-5 is sprayed onto a concrete section. After one hour of natural drying, applies the K2CrO4 solution (5 wt%). Since potassium chromate solution is sprayed, the zone contaminated by chlorides turns yellow due to the formation of AgCl blank, and then applying the K2CrO4 solution yellowish leaves.
Comparison of three methods: Colorimetric method which uses only silver nitrate is the simplest of them all; using potassium chromate and fluorescein requires higher reaction time to achieve better coloring effect. The use of AgNO3 results in a clear change in most cases is therefore the most rendered method. In Figure 9 it can be seen that the color change limit between the zone containing chloride and the zone free of chlorides is visible in the three cases. Among the three, the use of fluorescein and AgNO3 + AgNO3 method are very similar. However, the method fluorescein + AgNO3 does not evidence a very clear boundary.
4. CONCLUSIONS
The purpose of this article was to produce an overview of the state of the art on the use of the colorimetric method by spraying silver nitrate. In this context we attempted to assess the applicability of the method and following are some observations made with the study:
The type of cement influences the results of the colorimetric method by combining chlorides. As the concrete passes from 28 to 56 days, for example, it combines more chlorides, reducing the free chlorides, which are responsible for the color change;
It is recommended that the concentration of 0.1N AgNO3, as this allows a clear color change, but studies with 0.1N above concentrations were found and could be developed to verify if there was improvement in contrast;
There is still no consensus on the chloride content which leads to discoloration, because the studies found out the subject to be very contradictory with very disparate findings together;
When the pH of the concrete is less than 10, or the carbonation is deeper than the chloride penetration, the colorimetric method cannot be used all alone. One should employ a realkalisation technique, but this kind of practice to enable the use of the method is not merely an idea as an effective procedure which is not quite defined into the technical environment.
In cases where the attack is exclusively chlorides, the colorimetric method is a qualitative technique efficient, practical and low cost.
Referencias
Bouny, B. V. et al (2007), AgNO3 spray tests: advantages, weaknesses, and various applications to quantify chloride ingress into concrete. Part 1: Non-steady-state diffusion tests and exposure to natural conditions Materials and Structures, p. 759-781. [ Links ]
Cavalcanti, A. N.; Cavalcanti, G. A. D. (2010), Inspeção técnica do píer de atracação de Tambaú Concreto e construção, v. 57, p. 45-55. [ Links ]
França, C. B. (2011), Avaliação de cloretos livres em concretos pelo método de aspersão de solução de nitrato de prata Dissertação de Mestrado. Universidade Católica de Pernambuco, Recife, Brasil. [ Links ]
Helene, P. (1993), Contribuição ao estudo da corrosão em armaduras de concreto armado 231 p. Tese (Livre docência) - Escola Politécnica, Universidade de São Paulo, São Paulo, Brasil. [ Links ]
He, F. et al (2012), AgNO3-based colorimetric methods for measurement of chloride penetration in concrete Construction and Building Materials, v. 26, n. 1, p. 1-8. [ Links ]
He, F. et al. (2011), Calculation of chloride concentration at color change boundary of AgNO3Construction and Building Materials, v. 41, n. 11, p. 1095-1103, 2011. [ Links ]
Jucá, T. R. P. (2002), Avaliação de cloretos livres em concretos e argamassas de cimento Portland pelo método de aspersão de solução de nitrato de prata Dissertação (mestrado), Universidade Federal de Goiás. Goiânia, Brasil. [ Links ]
Kim, M. et al2013 Application of the colorimetric method to chloride diffusion evaluation in concrete structures Construction and Building Materials. v. 41, p. 239-245. [ Links ]
Marcondes, G. N. (2012), Adição de nanotubos de carbono em concretos de cimento Portland - absorção, permeabilidade, penetração de cloretos e propriedades mecânicas Dissertação (mestrado). Universidade Federal do Paraná, Curitiba, Brasil. [ Links ]
Marriaga, J. L.; Claisse, P. (2003), Influencia de la adición de escoria de alto horno em la penetración de lós cloruros en el concreto Ingenieria e investigatión, v.31, p. 38-47, 2011. [ Links ]
Meck, E.; Sirivivatnanon V. Field indicator of chloride penetration depth. Cement and Concrete Research, v.33, p.1113-1117. [ Links ]
Medeiros, M. H. F. (2008), Contribuição ao estudo da durabilidade de concretos com proteção de superficial frente à ação de íons cloreto Tese (doutorado). Escola Politécnica da Universidade de São Paulo, São Paulo, Brasil. [ Links ]
Medeiros, M. H. F.; Hoppe Filho, J.; Helene, P. (2009), Influence of the slice position on chloride migration tests for concrete in marine conditions Marine Structures, v. 22, p. 128-141. [ Links ]
Mota, A. C. M. (2011), Avaliação da presença de cloretos livres em argamassas através do método colorimétrico de aspersão da solução de nitrato de prata Dissertação (mestrado). Escola Politécnica da Universidade de Pernambuco, Recife, Brasil. [ Links ]
Otsuki, N.; Nagataki, S; Nakashita, K. (1992), Evaluation of AgNO3 solution spray method for measurement of chloride penetration into hardener cementitious matrix materials ACI Materials Journal. v. 89, n. 6, p. 587-592. [ Links ]
Pereira, L. F. C.; Cincotto, M. A. (2001), Determinação de cloretos em concreto de cimentos Portland: influência do tipo de cimento Boletim Técnico da Escola Politécnica da Universidade de São Paulo, São Paulo, Brasil. [ Links ]
Silva, F. G. (2006), Estudo de concretos de alto desempenho frente à ação de cloretosTese de Doutorado, Universidade de São Paulo, São Carlos, Brasil. [ Links ]
Trindade, G. H. (2011), Durabilidade do concreto com cinza de casca de arroz natural sem moagem: mitigação da reação álcali-sílica e penetração de cloretos Dissertação (mestrado) Universidade Federal de Santa Maria, Santa Maria, Brasil. [ Links ]
Yuan, Q.; Shi, C.; Schutter, G.; Audenaert, K.; Deng, D. (2008), Effect of hydroxyl ions on chloride penetration depth measurement using the colorimetric methodCement and concrete research, v. 38, n. 10, p. 1177-1180. [ Links ]
Received: January 07, 2015; Accepted: May 27, 2015











 text in
text in