In Bolivia, there are 65 species of Bromeliaceae in the genus Puya Molina, which shows a wide geographical distribution from the tropical lowlands to the páramo vegetation of the Andes (500-4,500 m asl; Smith & Downs 1974, Jabaily & Sytsma 2013, Krömer et al. 2014). Fifty-six are endemic to the country, including Puya ctenorhyncha L.B. Sm. (Krömer et al. 1999, 2014). Puya plants are terrestrial or saxicolous (rarely epiphytic), have a shrubby habit (Benzing 2000) and their spiny leaves usually form big rosettes, which develop large inflorescences with many showy flowers (Varadarajan & Brown 1988, Benzing 2000). These flowers are very attractive to hummingbirds (Trochilidae) and represent an important food resource especially at high elevations (Kessler & Krömer 2000, Krömer et al. 2006, Kessler et al. 2020).
Puya flowers are generally long, tubular, and wide, which allows for many hummingbird species to access the nectar (Smith 1969, Krömer et al. 2006, Gonzalez & Loiselle 2016). Many researchers report that hummingbirds are the main floral visitors and pollinators of several Puya species (García-Meneses & Ramsay 2012, Hornung-Leoni et al. 2013, Restrepo-Chica & Bonilla-Gómez 2017, Gonzalez et al. 2019, Kessler et al. 2020, Velásquez-Noriega et al. 2020); however, a few species of bats and moths also consume their nectar and might act as pollinators (Hornung-Leoni & Sosa 2005, Krömer et al. 2006, Aguilar-Rodríguez et al. 2019). Passerine birds were observed using the plant’s inflorescences as well, mainly as perches, but they also chew the corollas, often destroying the flowers (Rees & Roe 1980, Salinas et al. 2007, Hornung-Leoni et al. 2013, Velásquez-Noriega et al. 2020).
Phenological patterns and the abundance of resources during different phenological periods (e.g., the emergence of flowers and the amount of nectar available in a flower; Stiles 1978, Gonzalez & Loiselle 2016) shape when pollinators, granivores, herbivores, and seed dispersers exploit particular plants (Fenner 1998). Bromeliads usually have a unimodal annual flowering period (Benzing 2000, Machado & Semir 2006, Pool-Chalé et al. 2018) and Puya species show a well-delimited flowering period. However, not all plants flower each year, so the number of individuals with flowers varies annually (Janeba 2017, Restrepo-Chica & Bonilla-Gómez 2017, Velásquez-Noriega et al. 2020, Franco-Saldarriaga & Bonilla-Gómez 2021). From the visitors' perspective, nectar resources may not follow a direct relationship with plant abundance, making multiyear phenological studies more adequate.
The composition and concentration of sugars in nectar are tightly associated with particular flower visitors, therefore, they are considered as being part of the floral syndrome (Freeman et al. 1984, Scogin & Freeman 1984, Baker & Baker 1990). Hummingbirds are the primary pollinators of bromeliad species that have conspicuous inflorescences usually with red bracts and contrasting violet, orange, or yellow coloured tubular flowers, which produce sucrose rich nectar (Baker & Baker 1990, Kessler & Krömer 2000, Krömer et al. 2006, 2008, Ornelas et al. 2007, Kessler et al. 2020). The morphological characteristics of Puya flowers thus indicate that their nectar must be a crucial resource for hummingbirds.
The aim of this study was to describe the reproductive phenology of P. ctenorhyncha, its nectar production, and sugar composition, as well as to determine the assemblage of its floral visitors and their activity pattern in the montane cloud forest of Bolivia. Considering a previous study on the closely related species P. atra L.B. Sm., which also grows in this region (Velásquez-Noriega et al. 2020), we hypothesise that hummingbirds are the most important visitors for P. ctenorhyncha because of similar morphological and floral characteristics between both species.
Materials and methods
Study area. The study was conducted in the Nor Yungas province, La Paz Department, Bolivia, along the so-called “Death Road” (Camino de la Muerte) that connects the cities of La Paz and Coroico. Our study population grows along a transect of the road between the villages of Chuspipata to Sacramento, between 2,500-3,000 m asl in the humid montane cloud forest (16° 16' 8.16" S, 67° 47' 7.80" W - 16° 17' 7.88" S, 67° 49' 3.30" W; Figure 1). The Yungas cloud forest is characterised by high humidity that results from the orographic shock of humidity-laden winds hitting the mountains. The Yungas get torrential rains and frequent drizzles throughout the year (Ribera-Arismendi 1995). The study area shows a mean annual temperature of 10.1 ºC, average relative humidity of 97.5 %, and high precipitation of 3,000 mm (Bach et al. 2003). The plant community is dominated by Cyatheaceae (tree ferns), Piperaceae, Rubiaceae, Ericaceae, Orchidaceae, and Melastomataceae, and there is a high dominance of climbers, vascular epiphytes, and mosses.
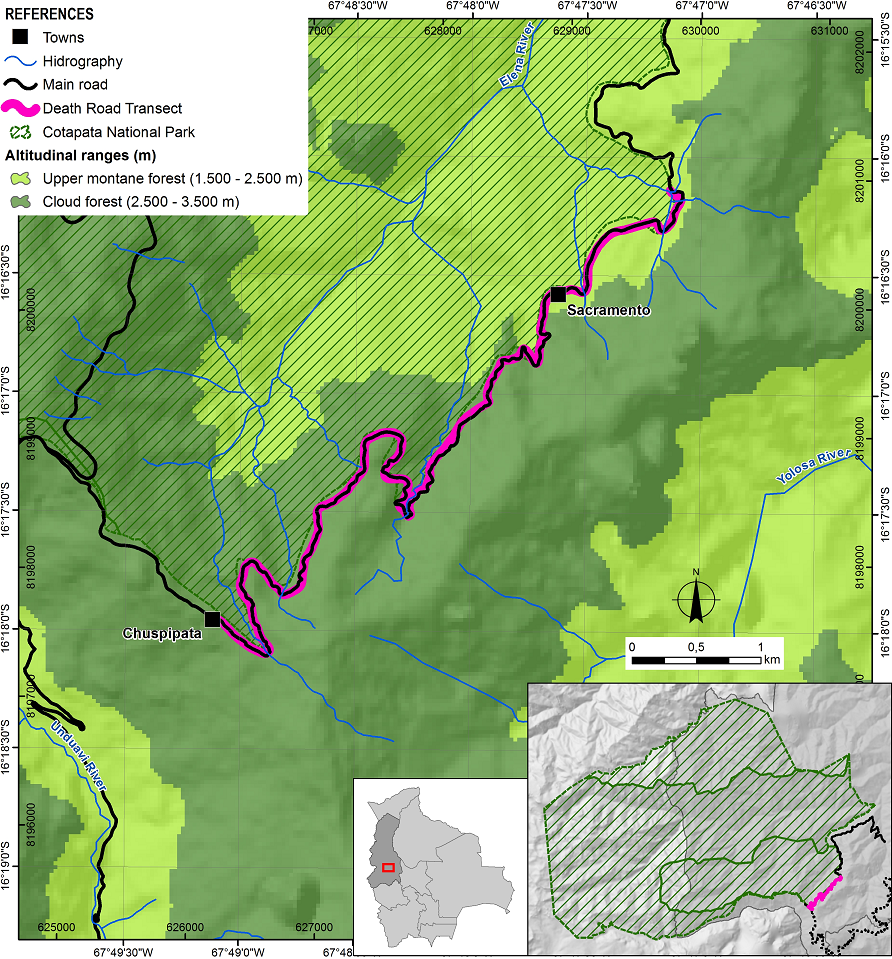
Figure 1 Location of the study area along the “Death Road” from Chuspipata to Sacramento in the Yungas montane cloud forest in La Paz Department, Bolivia.
Study species. Puya ctenorhyncha is endemic to Bolivia, where it has a restricted distribution between 2,500-4,050 m asl in the Department of La Paz (Krömer et al. 1999, 2014), and has been categorised as Near Threatened by the IUCN in the Red List of Threatened Species (Mercado Ustariz et al. 2020). It is a shrubby plant with a basal rosette of 1-1.2 m in diametre and 1.2 to 2 m in height (Smith 1969, Smith & Downs 1974). It grows on rocky slopes either in isolation or in dense patches (Figure 2). Inflorescences are about 1 m long, pendulous, densely white pubescent, and somewhat woolly (Krömer 2000). The sepals are free, green, triangular-subulate, 26-30 mm long, 6-7 mm wide in the base; petals are free, oblanceolate, about 41-46 mm long, 10-20 mm wide with a light greenish-yellow colour; stamens are all equal in length, shorter than the petals; filaments whitish, filiform, 27-32 mm long; anthers yellow, linear, 7-10 mm long; style linear, 37-40 mm long; stigma divided into 3, ca. 3 mm long, recurved, green-yellowish. It is locally known as “Bear Flower” (Flor de Oso) since its inflorescences and rosettes serve as food for the Andean bear (Tremarctos ornatus; Figure 2). A voucher of the species was deposited at the National Herbarium of Bolivia in La Paz (P. Velásquez 2, LPB).

Figure 2 A. Flowering plants of Puya ctenorhyncha growing on slopes along the “Death Road” trail, B. Group of plants of P. ctenorhyncha in different phenological stages, C. Peduncle of inflorescence eaten by an Andean bear, D. Rest of a rosette after being visited by the Andean bear.
Assessment of floral biology and reproductive phenology. We visited the study transect each month from January 2018 to December 2019 to record the reproductive phenology of the plants, following the methods described in Velásquez-Noriega et al. (2020). We observed and recorded the phenophase of plants using binoculars and a digital camera during each visit, monitoring about 216 flowering plant individuals distributed unevenly on rocks and steep slopes along the study transect. We did not individually mark each plant as the rocky terrain made it difficult to reach them all.
We described the phenophases in the following categories: (1) Bud set, from the first appearance of the young inflorescence within the rosette until it reaches its maximum height of about 100 cm with peduncle, bracts, and buds of the immature flower separated; (2) Flowers, when the green-yellow corollas appear in the inflorescence; (3) Implanted flowers, when all corollas become twisted and the peduncle and the petals acquire a brown-yellow colour because they begin to wilt; (4) Fruits, when the fruits are immature, green and non-dehiscent; and (5) Open fruits, when the capsules are dehiscent and release seeds, and the whole plant turns dark brown (Figure 3). A plant phenology diagram was elaborated based on all these data (Pereira & Quirino 2008, Rodrigues Marques & De Lemos Filho 2008).

Figure 3 Phenological stages of Puya ctenorhyncha A. Bud set, B. and C. Flowers, D. Implanted flowers, E. Fruits, F. Open fruits with seeds. Photographs: B. G. Archondo, E. and F. E. Cuba.
Determination of nectar characteristics. To prevent floral visitors from accessing the floral nectar, four inflorescences were completely covered with tulle bags. We could not cover more inflorescences with bags, because the access to most of the plants was difficult as they were growing on steep rocky slopes. We harvested the total floral nectar volume about one hour after the beginning of the anthesis, between 7:00-7:30 h, using 80 μL micro capillaries (Scogin & Freeman 1984). We could only measure each flower once because flowers were removed from the inflorescence to obtain the nectar. A total of 12 individual flowers were measured to estimate the mean floral nectar volume produced and to determine its sugar composition (García & Hoc 1998). The floral nectar sugar concentration was measured from eight flowers using a hand refractometre (Cole-Parmer RSA-BR90S, range: 0-42 %, United Kingdom) directly in the field, and the total amount of sugar was calculated following Corbet (2003). Additionally, we transported nectar samples in a container with silica gel to the laboratory for nuclear magnetic resonance spectroscopy (Hölscher et al. 2008, Wenzler et al. 2008). Staff at the “Laboratorio de Biorgánica” of the Instituto de Investigaciones Químicas at Universidad Mayor de San Andrés, La Paz performed the analyses.
Observation of floral visitors. We assessed both, diurnal and nocturnal flower visitors. We observed 29 flowering individuals in six areas between 8:00 and 19:00 h over 31 days, for a total of 341 hours in 2019 (Canela & Sazima 2005). Given the low availability of flowering P. ctenorhyncha individuals, we chose an observation area if at least one individual was in the flower phenophase. Observation areas were at least 100 m apart from each other (Gonzalez & Loiselle 2016).
We divided the observation time into 11 one-hour intervals to analyse the activity patterns of floral visitors throughout the day, measuring the number of visits per hour per species (Woods & Ramsay 2001). This interval assignment allowed us to compare the daily activity patterns between floral visitors and P. ctenorhyncha. For hummingbirds, we considered a “visit” event when an individual sipped nectar from a plant by inserting its bill into a flower within a one-hour interval (Vázquez et al. 2005). For invertebrates, we recorded a “visit” event when the animal clearly touched the reproductive structures of the plant. We calculated the frequency of visits for each animal species using the number of visits in each hour interval.
Nocturnal visits of P. ctenorhyncha were evaluated by placing three camera traps (Denver WCT-8010, Denmark) near flowering plants, located, due to logistical and safety reasons, in slightly different observation areas from those where diurnal observations were taken, for five consecutive nights. The cameras were scheduled to take one picture every five seconds over a 12-hour period each night (18:00-6:00 h). Additionally, direct observations were made during the night between 19:00-23:00 h, for a total of 20 hours in five consecutive days (Aguilar-Rodríguez et al. 2014).
Results
Assessment of floral biology and reproductive phenology. Puya ctenorhyncha has an acropetal flowering. During the two years of observation, the 193 monitored inflorescences had an average of 15 ± 7 open flowers per day (CV: 49.94 %; range: 1-33 flowers). In the first year the maximum number of flowering individuals (69) was recorded in July, while in the second year a maximum of 14 flowering individuals were recorded in June. The flowers always opened early in the morning (~ 6:00 h) and remained open between 72 to 96 hours.
The complete phenological cycle of P. ctenorhyncha lasted about 12 months (Figure 4). The first stage (development of buds) started in March 2018 with a peak in May. Fully open flowers were observed between April and August 2018 showing a unimodal pattern, and the implanted flowers were present mainly in September and October 2018. Fruits developed from November 2018 to February 2019 and open fruits started to release their seeds in March 2019, although seed dispersal lasted until December 2019. Interestingly, seed dispersal was also present from January to April 2018, as a result of flowers from 2017. Although the highest peak of buds was in May 2018, another small peak was observed in April 2019, likely as a new phenological cycle started and lasted until December 2020. Similarly, lower flowering and implanted peaks were observed in 2019 from May to June and from July to October, respectively (Figure 4).

Figure 4 Reproductive phenology stages of Puya ctenoryncha along the “Death Road” in the Yungas montane cloud forest of La Paz, Bolivia, during 2018 and 2019. Every point represents the percentage of sampled individuals per month belonging to each respective stage (bud set, flower, implanted, fruit, and open fruit).
Determination of nectar characteristics. The total nectar volume per flower averaged 31.26 ± 19.08 µL (SD 17.43) and 468.9 µL per inflorescence per day (n = 12 flowers from four individuals) with an average sugar concentration of 8.56 ± 4.5 °Bx ranging from 2-16 %. Nectar composition included 2 % β-fructofuranose, 2 % β-fructopyranose, 3 % α-glucopyranose, and 7 % β-glucopyranose, traces of sucrose, and 86 % of water.
Observation of floral visitors. During our diurnal observations, we recorded visits to 29 of the 193 flowering individuals of P. ctenorhyncha, by two hummingbird species (both sexes; Figure 5): the Collared Inca (Coeligena torquata Boissonneau 1840) with 77 % of visits and the Violet throated Starfrontlet (Coeligena violifer Gould 1846) with 23 % of visits. Only one male of Amethyst throated Sunangel (Heliangelus amethysticollis d'Orbignye and Lafresnaye 1838) visited the plant once and sipped the flowers twice. Additionally, some unidentified flies (Diptera) were observed in the flowers, but none of them touched either the anthers or stigma. During the night no flower visits by bats, moths, or other nocturnal insects have been detected by camera traps and direct observations. The only recorded visitor we observed in the camera trap recordings was C. torquata at 18:45 h.

Figure 5 A. Female of Coeligena violifer in hovering flight visiting a flower of Puya ctenorhyncha, B. Male of C. violifer perching in the observation area, C. Male of C. torquata perching in the observation area, D. Male of C. torquata visiting the plant. Photographs: A. B. Téllez-Baños, B. E. Cuba.
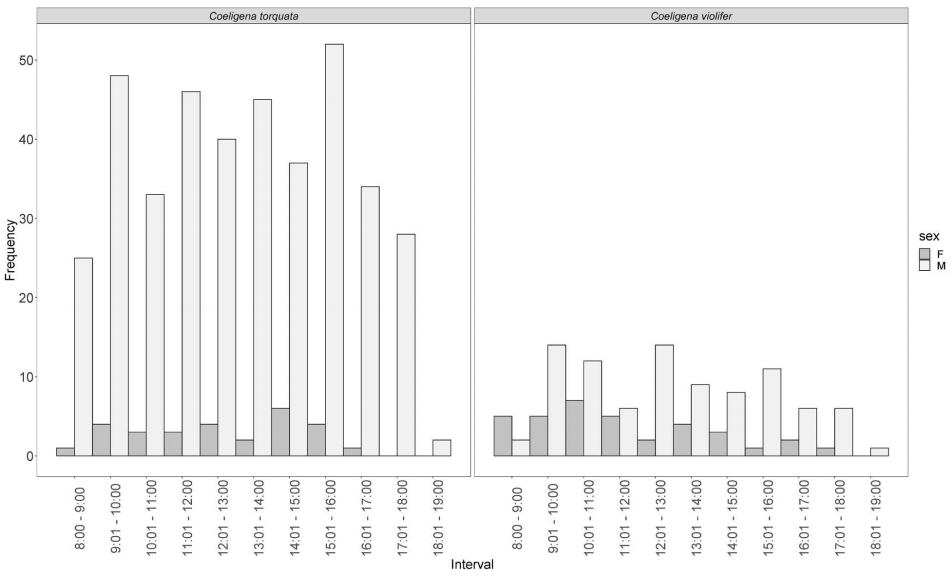
The following analysis of the activity patterns of floral visitors therefore only includes the two species of Coeligena. Males of C. torquata had the highest visiting frequency (2,157 visits) with a peak of activity between 15:01-16:00 h (Figure 6). Females of C. torquata visited flowers 145 times, with a peak of activity between 13:01-14:00 h. Males of C. violifer (483 visits) just realised the 22 % of visits of C. torquata males, and their peak of activity was between 9:01-10:00 h and 12:01-13:00 h; while females of C. violifer visited flowers as frequently as female C. torquata (147 visits in total), with a peak between 9:01-10:00 h (Figure 6).
Discussion
Assessment of floral biology and reproductive phenology. The flowering phenology of P. ctenorhyncha may be classified as annual and unimodal, coincident with other species of Puya (Benzing 2000, Restrepo-Chica & Bonilla-Gómez 2017, Velásquez-Noriega et al. 2020). At the population scale, however, the complete phenological cycle, from buds until seed dispersal, lasts up to 24 months. Puya ctenorhyncha shows a similar pattern as P. atra (Velásquez-Noriega et al. 2020), which is usually found at higher elevations (3,000-3,500 m asl) along the “Death Road” (Krömer 2000), although both species occur sympatrically in a few sites in the Yungas montane cloud forest. The flowers of P. ctenorhyncha are available in the dry season when most other plant species in the Yungas ecoregion are not flowering (Serrudo-Gonzáles et al. 2012), which indicates that they represent an important food resource, especially for hummingbirds (Krömer et al. 2006). Our results and other preliminary observations suggest that the populations of flowering individuals of several Bolivian Puya species are cyclical; when there were many individuals in the flowering stage, the flowering period was longer and followed by a year with few flowers and a short period of blooming.
Phenological data suggest that P. ctenorhyncha shows a "cornucopia" strategy, where a large number of flowers is produced daily over a time span of several weeks (Kessler et al. 2020). In contrast, most Bromeliaceae, specifically epiphytic species, show a "steady state" flowering strategy, with plants producing relatively few flowers per day over extended periods of several weeks or months (Kessler et al. 2020). The acropetal and asynchronous flowering has been previously identified as an advantageous strategy to attract flower visitors and pollinators due to the increase of flower longevity and foraging resources (Knight et al. 2005). The same mechanism might occur in P. ctenorhyncha where flowers are available for about four months and thus offer stable resources for their pollinators. Our study population had flowers available for a long period, and even individual flowers remained open for about 72 hours; however, the only observed visitors were diurnal.
Determination of nectar characteristics. The mean volume of nectar produced by P. ctenorhyncha per inflorescence per day is higher than in P. chilensis Molina (358.27 µL; Hornung-Leoni et al. 2013), but similar to P. atra (432.4 µL; Velásquez-Noriega et al. 2020). Lower values per day have been reported for other Puya species, for example, P. alpestris Gay. (120.88 µL), P. coerulea Miers (62.78 µL), P. raimondii Harms (21.7 - 49.5 µL), P. venusta Phil. (23.65 µL), and P. hamata (4.8-18.3 µL; Woods & Ramsay 2001, Hornung-Leoni et al. 2013). The concentration of dissolved sugar in P. ctenorhyncha (8.56 ± 4.5 °Bx) was lower than reported for P. alpestris (12.16 ± 0.51 °Bx) and P. chilensis (12.56 ± 1.63 °Bx), while other species such as P. hamata (18.3 ± 8.2 °Bx), P. raimondii (20.1 ± 0.60 °Bx), P. venusta (22.93 ± 2.93 °Bx), and P. coerulea (22.78 ± 0.35 °Bx) showed even higher values (Woods & Ramsay 2001, Hornung-Leoni et al. 2013). Thus, the sugar concentration of P. ctenorhyncha is not consistent with other trochilophilous Puya species, but is more similar to those of chiropterophilous bromeliads (e.g., Pitcairnia, Vriesea, Werauhia; Baker & Baker 1990, Krömer et al. 2008).
The water content in the nectar of our study species was higher (86 %) than in P. atra (62 %; Velásquez-Noriega et al. 2020). Thus, the nectar is relatively diluted, which matches with the preferences reported for other angiosperm species with the hummingbird pollination syndrome (Baker & Baker 1990). Hexose solutions have higher osmolarity, and therefore lower evaporation rates than sucrose solutions, however, these former sugars also tend to require more water for their formation (Abrahamczyk et al. 2017). Besides, it is possible that the plant at this elevation with high atmospheric humidity absorbs water from the environment. There is no data on water content available for other Puya species, although hummingbirds depend on the nectar reward for both water and energy, albeit they complement their diet with arthropods (Wolf et al. 1976, Calder 1979).
The nectar of P. ctenorhyncha is mainly composed of fructose and glucose (i.e., hexose-rich) and had only a small proportion of sucrose, which is similar to P. atra (Velásquez-Noriega et al. 2020) and other Puya and bromeliad species pollinated by bats or passerine birds (Scogin & Freeman 1984, Baker & Baker 1990, Baker et al. 1998, Krömer et al. 2008). In P. alpestris the presence of these three sugars has been reported as well, although in different proportions and mostly classified as sucrose-rich, while this plant is mainly visited by passerine birds (Hornung-Leoni et al. 2013). In contrast, P. venusta, P. coerulea, and P. chilensis were also shown to be hexose-rich and are visited by passerine birds as well (Hornung-Leoni et al. 2013), consistent with the pollination syndrome for passerines. However, we did not record any visits by bats or passerines, which might suggest that sugar composition may not always be associated with a certain type of flower visitors, as observed in other plant groups, such as Alooideae, Papilionoideae, Proteaceae, and Ericaceae (van Wyk 1993, Barnes et al. 1995, Nicolson & Fleming 2003). Abrahamczyk et al. (2017) reported that pollinators are sensitive to the proportion of sugars constituted by sucrose, while hexoses do not provide evidence of a specific pollinator syndrome.
Hummingbirds usually prefer sucrose-rich nectar (Freeman et al. 1984, Baker & Baker 1990, Galetto & Bernardello 1992, Nicolson & Fleming 2003, Krömer et al. 2008) even though this sugar has been reported only in a small proportion for P. ctenorhyncha. Physiologically they can also digest fructose and glucose as alternative energy sources as they feed on a mixture of sugars (Chen & Welch 2014). Thus, it remains possible that a putative phylogenetic constraint on nectar features is, like other characters, more or less relaxed in different taxonomic groups (Schmidt-Lebuhn et al. 2007). Nonetheless, more studies of other plant families and Bromeliaceae genera are needed to get firm conclusions.
Observation of floral visitors. Although some of the flower characteristics of P. ctenorhyncha are consistent with the floral syndrome of hummingbirds as its main visitors (e.g., tubular, scentless flowers, exerted stamens, anthesis in the morning, abundant nectar production), there are no red bracts with contrasting corollas. Nevertheless, hummingbirds also visit Puya species with green or yellow flowers and unshowy brown bracts or woolly inflorescences, such as P. trianae L.B. Sm. in Colombia (Restrepo-Chica & Bonilla-Gómez 2017), P. raimondii in Peru (Salinas et al. 2007), and P. atra near our study area (Velásquez-Noriega et al. 2020). Hummingbirds can discriminate a wide range of colours, including not spectral ones (Altshuler 2003, Stoddard et al. 2020), which would allow them to recognise the untypical flower colours of Puya species even when they have been trained to associate red colours with nectar reward (Maruyama et al. 2013). It is likely that the visits of the hummingbirds are more related to the big size of the inflorescence and their numerous flowers than their colour, as their size may make them attractive relative to other plants with small flowers in the Yungas montane cloud forest.
Hummingbirds have been reported as the main visitors and putative pollinators for most Puya species, regardless of bill morphology or body size (Woods et al. 1998, Salinas et al. 2007, García-Meneses & Ramsay 2012, Hornung-Leoni et al. 2013, Restrepo-Chica & Bonilla-Gómez 2017, Aquino et al. 2018, Gonzalez et al. 2019), while passerine birds or bats might act as secondary visitors. Only two long-billed hummingbirds frequently visited the P. cternorhyncha flowers, even though five other hummingbird species with shorter bills live in the study area: Heliangelus amethysticollis (which visited the plant only once), Adelomyia melanogenys Fraser 1849, Aglaiocercus kingie Lesson 1832, Metallura tyrianthina Lodiges 1832, and Chaetocercus mulsant Bourgier 1842 (Velásquez-Noriega et al. 2023). Thus, P. cternorhyncha flowers may be accessible to short billed species.
The main floral visitor of P. ctenorhyncha is C. torquata, which seems to monopolise an abundant and stable resource ("cornucopia"), as we observed that one individual could stay at one observation area all day. In our study, C. torquata showed a territorial and aggressive behaviour compared to C. violifer, which acted more as a trapliner. A traplining strategy involves hummingbirds feeding on renewable food resources from isolated plant patches along reused routes (Feinsinger & Colwell 1978). It appears that body size determines interspecies dominance among hummingbirds (Bribiesca et al. 2019) and given that C. torquata is the largest hummingbird in our study, it could monopolise feeding sites by chasing, attacking, and remaining vigilance against the approach of other individuals. In areas of the Yungas where C. torquata is absent, C. violifer is the most aggressive hummingbird (Serrudo-Gonzáles et al. 2012) and can adopt the cornucopia pattern (Kessler et al. 2020). Regarding nocturnal floral visitors, none have been reported, although the characteristics of the flowers and its nectar, such as the greenish-yellow petals, the nectar composition, and the concentration of sugar, would appear to be adaptations to attract nocturnal visitors. However, only 7 % of all bromeliads in Bolivia are known to be pollinated by bats (Kessler & Krömer 2000), suggesting that there might be many more undetected cases (Aguilar-Rodríguez et al. 2019).
Our study showed that Puya ctenorhyncha is visited almost exclusively by two species of hummingbirds (Coeligena torquata and C. violifer). Its flowering period is annual, its nectar sugar is composed mainly of fructose, glucose, and water, with only a small proportion of sucrose. The main floral visitor and putative pollinator of P. ctenorhyncha is C. torquata, which guarded flowers from other hummingbirds. Regarding the activity of visitors, the males of both species ate at flowers more frequently than females. Future studies should look at these plant-bird interactions in more detail to determine if the plant´s reproductive success depends on these hummingbirds as pollinators and if the plant´s floral phenology pattern leads to changes in foraging patterns of hummingbirds. This information would be key to develop adequate conservation and management strategies for the protection of Puya ctenorhyncha, a species under critical threat due to its restricted distribution in an endangered habitat.











 nova página do texto(beta)
nova página do texto(beta)