Any taxon showing a limited geographical distribution is considered an endemic (Anderson 1994). Endemics may be accounted for as the response to combinations of ecological features (for example, climate or topography) that restrict their geographic distribution or because of historical events (vicariance process, speciation, or isolation due to range restriction) (Crisp et al. 2001). Either way, endemics are mostly used for searching biogeographic patterns, especially endemism areas (areas sharing at least two endemic species, Morrone 1994) or to define regional provincialism (phytochorias or floristic regions).
The total percentage of vascular plant species endemic to Mexico (restricted to its political territory) reaches about 49.8 %, placing the country second in the number of endemic species in the Americas, only surpassed by Brazil (Villaseñor 2016, Ulloa-Ulloa et al. 2017). Except for Tlaxcala, all other Mexican states record at least one or more endemic species restricted to their political frontiers (Villaseñor 2016). Furthermore, knowledge of endemism in a region (natural or political) is important since several viewpoints, for example, to determine natural biogeographic regions or to offsetting conservation priorities (e.g., Villaseñor & Elias 1995, Lima et al. 2020, Villaseñor et al. 2020).
Biogeographers (e.g., Major 1988) point out that analyses of endemism should be based on floristic provinces or natural regions instead of political boundaries (e.g., Villaseñor 1990, Riemann-González 2001, Vanderplank et al. 2014, Villarreal-Quintanilla et al. 2017, Salinas-Rodríguez et al. 2017, Aragón-Parada et al. 2021, Estrada-Márquez et al. 2021, Villaseñor et al. 2021, Villaseñor & Ortiz 2022). Although the use of political boundaries is far from the ideal goal, their use is a more common practice to explain endemism patterns, especially when the results are linked to potential conservation strategies (see examples in Hobohm 2014). In Mexico, political decisions are usually made at the state rather than at the federal level when natural protected areas are decreed. By far there are more natural protected areas decreed by Mexican state and municipal governments than the federal ones. Accordingly, several Mexican states and municipalities have documented their endemic plant species; examples include the Culiacán Municipio (Vega-Aviña et al. 2000), the Comarca Lagunera (González-Zamora & Pérez-Morales 2019), or the states of Coahuila (Villarreal-Quintanilla & Encina-Domínguez 2005), Guanajuato (Zamudio & Galván-Villanueva 2011), Hidalgo (Villaseñor et al. 2022), Nuevo León (Alanís-Flores et al. 2011), Querétaro (Rzedowski et al. 2012), San Luis Potosí (De-Nova et al. 2018), Sinaloa (Pío-León et al. 2023), or Veracruz (Castillo-Campos et al. 2005).
While the study of plant endemism using political boundaries has been a common practice, there remain many Mexican states without their endemic species assessment. For this reason, the main goal of this study is to assess the number and taxonomic distribution of the vascular plant species endemic or nearly endemic to the state of Puebla which lacks a formal analysis of its plant endemism. A second goal is to evaluate their geographic distribution within the state and to determine areas of concentration of such endemics and their phylogenetic diversity, identifying areas of endemism potentially valuable to be considered in future conservation strategies in the state and to refine the biogeographic regionalization of the state.
Materials and methods
Study area. The state of Puebla is in east-central Mexico including a surface of 34,306 km2 and adjoins the states of Hidalgo, Veracruz, Oaxaca, Guerrero, Morelos, State of Mexico, and Tlaxcala. Its surface is equivalent to Belgium or the state of Maryland in the United States. It is bound by the coordinates 17° 52’ and 20° 50’ N and 96° 43’ and 99° 04’ W (Figure 1). The state includes important mountain peaks of Mexico: the Citlaltépetl (5,610 m asl), the Popocatépetl (5,500 m asl), and the Iztaccihuatl (5,220 m asl); these volcanic zones have the lowest recorded temperatures and can reach 0 °C or below (CONABIO 2011, Rodríguez-Acosta et al. 2014). It includes seven of the 19 biogeographic provinces in the country (CONABIO 2011), and six of the phytogeographic provinces (Rzedowski 1978). According to the classification of the major biomes of Mexico (Villaseñor & Ortiz 2014), 42.3 % of the extension of Puebla corresponds to temperate forest, 24.6 % to seasonally dry tropical forest, 18.4 % to xeric scrub, 7.7 % to humid tropical forest, and 7 % to humid mountain forest.
Figure 1 The state of Puebla (gray area) divided into 1° latitude and longitude grid cells. The numbering follows a nomenclature used at the national level (see Cruz-Cárdenas et al. 2013) and the numbered grid cells were considered as the distributional limits of the nearly endemic species.
It is a state with contrasting climates due to its position and geographical extension, in which temperate climates predominate, followed by warm, semi-warm, semi-dry, dry, semi-cold, and cold climates, respectively. By its latitudinal position, it is in an area where the trade winds and high temperatures predominate (> 29 °C); however, the altitudinal gradient provided by its topography causes the ascent, cooling, and condensation of the humidity conveyed by the winds, producing an orographic shadow effect. Precipitation conditions are mainly determined by tropical cyclones in summer and cold fronts in winter. Inland where the altitude is less than 1,000 m, the average annual temperature can be higher than 24 °C, but at altitudes that exceed 4,000 m, the average annual temperature ranges from 2 to 4 °C as average annual temperature (CONABIO 2011).
The northern and central parts of the state have good levels of humidity, ranging from 750 to 4,250 mm of average annual rainfall. For the south of the state, rainfall is lower, with an average ranging between 350 to 650 mm of annual rainfall. Various factors have been fundamental for soil formation in this state (e.g., climate, relief), which results in an edaphic mosaic of 21 soil units, with Leptosols, Regosols, Phaeozems, and Andosols being the most representative soil types in its territory, since together they occupy 69.1 % of the surface (CONABIO 2011).
Sources of information. Two main sources of information were used in this study. The first is a literature review dealing with plant endemism in Mexico, especially those focusing on the central part of the country where the state of Puebla is located. The second comes from two Mexican most important internet-accessed databases, the SNIB from the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO: www.conabio.gob.mx) and IBData from the Instituto de Biología, Universidad Nacional Autónoma de México (UNAM) (www.ibdata.ib.unam.mx). Each species data point of Puebla endemics was thus derived from herbarium specimens documenting their geographical occurrences. The georeferenced records from both databases were combined into a new database (using Microsoft Access) containing the records of the species considered in this study as the Puebla endemics. The final database included 4,303 records for 356 endemic or nearly endemic species. Records for species not included in the two consulted databases were obtained from the consultation of additional sources, such as Tropicos (www.tropicos.org) or directly from the protologues where the species were published. Inconsistencies in the data analyzed were corrected; for example, the use of different names (synonyms) attributed to the same species. Nomenclature follows the accepted names used by Villaseñor (2016).
Endemism patterns. A species was considered endemic to Puebla if the known records restrict its distribution inside the political boundaries of the state. In addition, a species was considered nearly endemic if its distribution goes not far from these frontiers without surpassing the administrative limits of its neighboring states. Incidence estimates of species distribution and chorological analyses were done by dividing the state of Puebla by 1° × 1° latitude and longitude grid cells (approximately 11,368 km2 surface). Nearly endemics were selected when their distribution does not overpass one or sometimes two grid cells far from the Puebla state’s political boundaries (see Figure 1).
The use of such a coarse scale (1° latitude and longitude) allowed better control data and made the results comparable with other studies using the same scale. Its size efficacy has been demonstrated and tested in several biodiversity and biogeographic studies, either floristic or faunistic (Morrone & Escalante 2022, Hurlbert & Jetz 2007, Kreft & Jetz 2010, Cruz-Cárdenas et al. 2013, Munguía-Lino et al. 2016). In addition, finer scales, at least with Mexican plant data, generally result in under-sampled grids. Once detected the Puebla major endemic centers at such grid cell size, further analyses using finer scales can be applied to the data to recover smaller centers of endemism.
The total number of endemic species by grid (endemic richness) was first summarized. Then the grid´s weighted endemism (WE) was calculated (by weighting each species by the inverse of its range), as well as the corrected weighted endemism (CWE, dividing the grid´s weighted endemism by the total endemic richness), which measures the proportion of endemics by grid related to the total endemic richness (Crisp et al. 2001). Maps indicating these richness and endemism values were performed.
To determine the biome occurrence of species, each georeferenced point was overlapped against a shapefile with the main biomes of Mexico (Villaseñor & Ortiz 2014). In this way, the biome type where the point intersected was classified. The incidence of endemic species by biome was thus calculated for each grid cell.
Spatial phylogenetic analysis. For phylogenetic inferences, a species-level phylogenetic tree was created using the “phylo.maker” function implemented in R package version 4.1.3 (R Core Team 2022) V.PhyloMaker2 (Jin & Qian 2022). This package uses the largest dated phylogeny for plants, GBOTB.extended.tre (Smith & Brown 2018) with names standardized according to The Plant List (www.theplantlist.org, now superseded by World Flora Online, www.worldfloraonline.org). Scenario 3 was chosen because it has been shown that a community phylogenetics study based on a phylogeny produced in this way is equivalent to a species-level based phylogeny (Qian & Jin 2021), allowing this approach to be reliably used to reconstruct plant phylogenies and measuring phylogenetic diversity (Shrestha et al. 2021, Qian et al. 2022).
The set of species records and phylogeny described above were analyzed in Biodiverse version 3.1 (Laffan et al. 2010). Record coordinates were converted to a presence within a grid of 1° latitude and longitude grid cells. The methods and metrics described by Mishler et al. (2014) and Thornhill et al. (2016) were used to calculate species richness (SR), weighted endemism (WE), phylogenetic diversity (PD), phylogenetic endemism (PE), relative phylogenetic diversity (RPD), and relative phylogenetic endemism (RPE) for each grid cell. Compared to taxonomic indices of diversity and endemism, phylogenetic indices provide additional information about the evolutionary relationships between species occurring within a community, helping to distinguish between close and distant species.
A figure of 999 spatial randomizations was performed on the data set, as described by Mishler et al. (2014), and compared with the observed results to estimate the significance of PD, RPD, and RPE, applied in the categorical analysis of neo and paleo-endemism (CANAPE). The randomization algorithm used repeatedly shuffles the identities of the taxa found in each grid cell of a set of occurrences, keeping the number of taxa per cell and the number of cells per taxon constant. This null model assumes that the occurrences of a taxon show no spatial autocorrelation or correlation with the occurrence of other taxa, thus including the expected correlation between species richness and endemism, and the corresponding phylogeny-based measures. Our goal was to detect areas that have values markedly higher or lower than expected, given the number of species present (Mishler et al. 2020).
CANAPE is a two-step process that estimates the contribution of PE by branches that are longer or shorter than expected, for grid cells that first show up as significantly high in PE (Mishler et al. 2014). The test uses a ratio (the RPE) obtained by comparing the PE measured on the original tree as the numerator with the phylogenetic endemism measured on a comparative tree where all branches are of equal length as the denominator. This proportion was estimated for each randomization and compared with the observed RPE proportion (Thornhill et al. 2016). The first step of CANAPE determines whether cells are centers of high phylogenetic endemism by applying a one-tailed test to PE with the observed tree and the comparative tree. Then, for cells that pass these tests, the second step applies a two-tailed test for RPE to identify cells that are significantly dominated by taxa from restricted ranges and short branches (centers of neo-endemism), taxa with restricted ranges and long branches (centers of paleo-endemism), or both (centers of mixed endemism). Areas of mixed endemism can be further subdivided and called centers of super-endemism if both the numerator and denominator of relative phylogenetic endemism are highly significant (Mishler et al. 2014).
Results
The grid cell division of the state of Puebla and its neighboring territory comprises 16 sampled cells and 4,303 occurrence records for 356 endemic or nearly endemic vascular plant species. Among the species, there are 60 strictly Puebla endemics and 296 shared with neighboring states. Table 1 shows the taxonomic dispersal of the endemic species by major taxonomic groups. Except for basal Angiosperms, lacking any endemism, the other major groups have species that evolved mostly inside Puebla’s political territory.
Table 1 Number of endemic or nearly endemic vascular plant species recorded in Puebla arranged by major taxonomic groups. In parenthesis the number of Puebla strict endemics. Flowering plants according to the APG IV schedule (APG IV 2016).
| Taxonomic group | Families | Genera | Species |
|---|---|---|---|
| Ferns and Monilophytes | 2 | 2 | 3 |
| Gymnosperms | 1 | 2 | 2 |
| Basal Angiosperms (including Austrobaileyales and Nymphaeales) | 0 | 0 | 0 |
| Magnoliids (including Chloranthales) | 2 | 3 | 3 |
| Monocots | 10 | 24 | 50 (10) |
| Eudicots (including Ceratophyllales) | 3 | 5 | 5 (1) |
| Rosids (including Saxifragales) | 24 | 68 | 128 (23) |
| Asterids (including Caryophyllales and Santalales) | 26 | 90 | 166 (25) |
| 68 | 194 | 356 (60) |
The flora of Puebla includes 5,415 vascular plant species, distributed among 248 families, and 1,607 genera. The 356 endemic or nearly endemic species distribute in 68 families, and 194 genera. Table 1 indicates the distribution of the species along the hierarchical groups. More specifically, the strict Puebla endemics (60 species) concentrate on 28 families, and 46 genera. Among the genera with the greatest number of strict Puebla endemic species are Salvia (six species), Acourtia, Bidens, Davilanthus, Hechtia, Perymenium, Polygala, Sedum, and Tillandsia, each one with two species; the other genera with strict endemics record a single species (See Table S1, Supplementary material).
Species richness by grid cell. The species mean range of endemic or nearly endemics is 2.3 grid cells, with a Median of two and a Mode of one. Ocotea disjuncta Lorea-Hern. and Oreopanax flaccidus Marchal (both recorded in four states and seven grid cells) are the widest-distributed endemic species; on the contrary, 95 species are recorded only in a single grid cell each. The percentage of rare endemics, those distributed in just one to three grid cells reaches the figure of 289 (82.6 % of the total).
Table 2 indicates the total endemic or nearly endemic species recorded by grid cells. Likewise, Figure 2 shows the geographic distribution of the scores of the three estimated indices (Table 2). Grid cells 211 and 227 located at the southeastern edge of the state record the largest number of endemics (endemics alpha diversity). Likewise, both grid cells record the highest Weighted endemism scores (WE), suggesting a significant correlation between these two scores. In fact, once estimated the correlation values among all the grid cells, the two scores show a significant correlation (R = 0.985).
Table 2 Species richness (Total species, SR), Weighted endemism (WE), and Corrected weighted endemism (CWE) values per grid cells of the endemic and nearly endemic species of Puebla (see Figure 1).
| Grid | SR | WE | CWE |
|---|---|---|---|
| 164 | 2 | 0.9 | 0.3 |
| 177 | 9 | 2 | 0.22 |
| 178 | 30 | 13.28 | 0.37 |
| 179 | 20 | 12.39 | 0.52 |
| 193 | 2 | 0.37 | 0.19 |
| 194 | 47 | 18.94 | 0.37 |
| 195 | 75 | 41.81 | 0.45 |
| 196 | 27 | 14.57 | 0.36 |
| 209 | 4 | 6.33 | 0.58 |
| 210 | 82 | 44.12 | 0.45 |
| 211 | 279 | 176.36 | 0.52 |
| 212 | 17 | 8.7 | 0.33 |
| 225 | 12 | 5.44 | 0.34 |
| 226 | 18 | 9.41 | 0.32 |
| 227 | 159 | 68.34 | 0.38 |
| 228 | 115 | 40.1 | 0.32 |
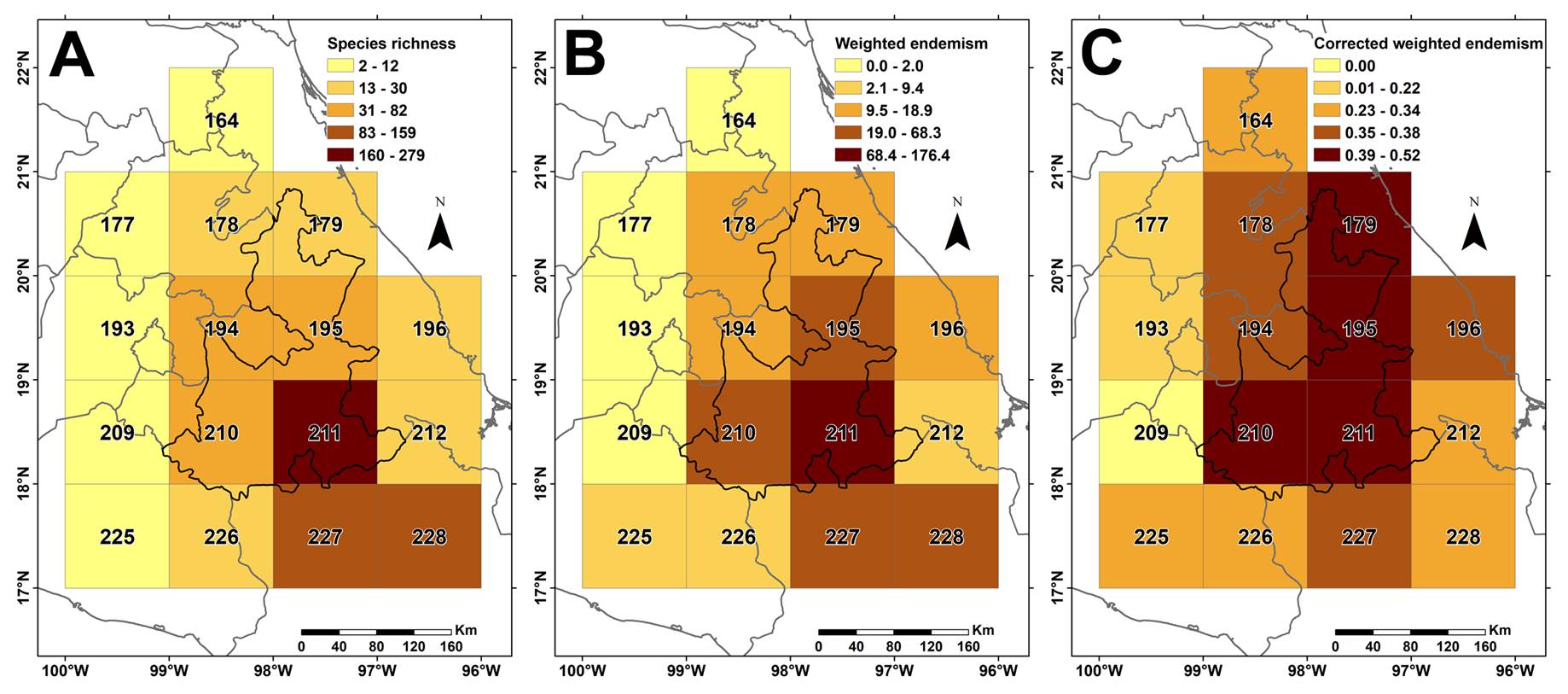
Figure 2 A. Total species richness recorded in each 1° latitude and longitude grid cell (SR). B. Weighted endemism (WE). C. Corrected weighted endemism (CWE).
A different situation is observed when the third score (Corrected weighted endemism, CWE) is evaluated. The CWE values per grid cell are more balanced than the other scores (Table 2), and the importance of grid cells changes substantially, now highlighting grid cells 209, 179, and 211 as the more important ones.
The distribution of endemic species by major clades among the grid cells is indicated in Table 3. The same trends as with total species (Table 2) are observed when endemism is dissected among major clades. The larger number of species is observed in grids 211 and 227. In the former two grid cells, Monocots, Rosids, and Asterids are better represented. However, it is interesting to point out special cases. For example, Fern’s endemics are better represented in grid cell 228 (without Puebla’s territory), indicating they correspond to near endemics shared especially with the northern part of the state of Oaxaca. On the other hand, the Eudicots, although a group not too rich in endemic species, (5 species in total), represent the sister group of the two other more recently derived lineages (Asterids and Rosids) with contrasting chorological patterns: one species is, for example, representative of the portion in Puebla of the Sierra Madre Oriental (Berberis trifolia (Schltdl. & Cham.) Schult. & Schult. f., growing in humid mountain and temperate forests), whereas two additional species mostly restrict their distribution to the seasonally dry tropical forests or the xerophytic scrubs of southern Puebla (e.g., Buxus mexicana Brandegee and Clematis malacocoma W.T. Wang).
Table 3 Number of endemic or nearly endemic species in each of the grid cells used to subdivide the state of Puebla.
| 164 | 177 | 178 | 179 | 193 | 194 | 195 | 196 | 209 | 210 | 211 | 212 | 225 | 226 | 227 | 228 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferns and Monilophytes | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 4 |
| Gymnosperms | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Magnoliids | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Monocots | 0 | 2 | 4 | 3 | 0 | 4 | 11 | 3 | 1 | 9 | 38 | 2 | 2 | 3 | 23 | 15 |
| Eudicots | 0 | 1 | 2 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 |
| Rosids | 0 | 2 | 7 | 6 | 0 | 18 | 24 | 10 | 1 | 31 | 97 | 5 | 4 | 3 | 49 | 47 |
| Asterids | 1 | 3 | 13 | 10 | 2 | 21 | 37 | 11 | 2 | 41 | 138 | 9 | 6 | 12 | 84 | 49 |
Species richness by biome. Biomes do not distribute uniformly within the state; there are grid cells comprising the five major biomes (for example, 211 or 228), whereas others only record a single biome in its territory (grid cells 164, 177, or 193). Table 4 indicates the number of species recorded by grid cell according to the biome scored based on the collecting geographical coordinates. Most likely the higher species richness of several grid cells is the response of a greater environmental heterogeneity, resulting in the occurrence of more biomes.
Table 4 Number of endemic or nearly endemic species by biome in each of the cells used to subdivide the state of Puebla. Empty cells indicate the absence of the biome. BHM = Humid mountain forest, BTEM = Temperate forest, BTHU = Humid tropical forest, BTSE = Seasonally dry tropical forest, MXE = Xerophytic scrubland.
| 164 | 177 | 178 | 179 | 193 | 194 | 195 | 196 | 209 | 210 | 211 | 212 | 225 | 226 | 227 | 228 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BHM | 3 | 22 | 13 | 10 | 32 | 24 | 28 | 11 | 1 | 2 | 17 | |||||
| BTEM | 19 | 9 | 2 | 29 | 54 | 21 | 17 | 89 | 9 | 12 | 59 | 35 | ||||
| BTHU | 6 | 5 | 8 | 8 | 4 | 3 | ||||||||||
| BTSE | 3 | 51 | 54 | 14 | 15 | 43 | 29 | |||||||||
| MXE | 8 | 7 | 23 | 36 | 6 | 1 | 51 | 218 | 7 | 13 | 145 | 88 |
The occurrence of endemics in most grid cells is related to heterogeneity triggered by the existence of different biomes; the larger number of biomes the larger number of endemic species (e.g., grid cells 211 and 228). Likewise, the response of the plant lineages to such heterogeneity is linked to their occurrence among the distinct biomes (Table 5). There are major taxonomic groups mostly restricted to three or fewer biomes (Eudicots, Ferns, Gymnosperms, Magnoliids); in contrast, the more diversified lineages (Asterids, Monocots, Rosids) record endemic species in all the five major biomes. Additionally, each biome records species that apparently evolved and restrict their geographic distribution in it (Table 5).
Table 5 Number of endemic or nearly endemic species of Puebla among the five major biomes in the state. In parenthesis, the number of species with geographic distribution restricted to the biome.
| BHM | BTEM | BTHU | BTSE | MXE | |
|---|---|---|---|---|---|
| Ferns and Monilophytes | 3 (1) | 2 | 1 | ||
| Gymnosperms | 1 | 1 | |||
| Magnoliids | 1 | 1 | |||
| Monocots | 5 (2) | 22 (5) | 3 | 7 (4) | 32 (7) |
| Eudicots | 1 | 1 | 4 | ||
| Rosids | 8 (3) | 26 (8) | 3 (1) | 23 (13) | 74 (9) |
| Asterids | 17 (4) | 58 (12) | 2 | 33 (10) | 110 (17) |
| Total species | 36 (10) | 101 (25) | 7 (1) | 63 (27) | 220 (33) |
Spatial phylogenetic analysis. Only 94 species out of 356 considered in this study are included in the phylogeny used (Jin & Qian 2022) and this set was used in the analysis. PD shows a pattern like the distribution of species richness, with grid cell 211 having the highest proportion of distantly related taxa, and from here the neighboring squares decrease in value as they move away from it (Figure 3). The RPD values greater than 1 indicate an overrepresentation of lineages with long branch lengths (early diversified); they are observed in grid cells in the transition between the Gulf of Mexico Coast and the Sierra Madre Oriental provinces, decreasing southwards. In contrast, PE patterns (same as WE), show that cells with higher values also contain a high presence of lineages with a restricted range. Finally, RPE shows as relevant the grid cells in the vicinity of the Gulf of Mexico Coast and the Sierra Madre Oriental (same as RPD), with an overrepresentation of lineages with restricted ranges and long branch lengths; the values of this index decrease as the cells move away from this zone.
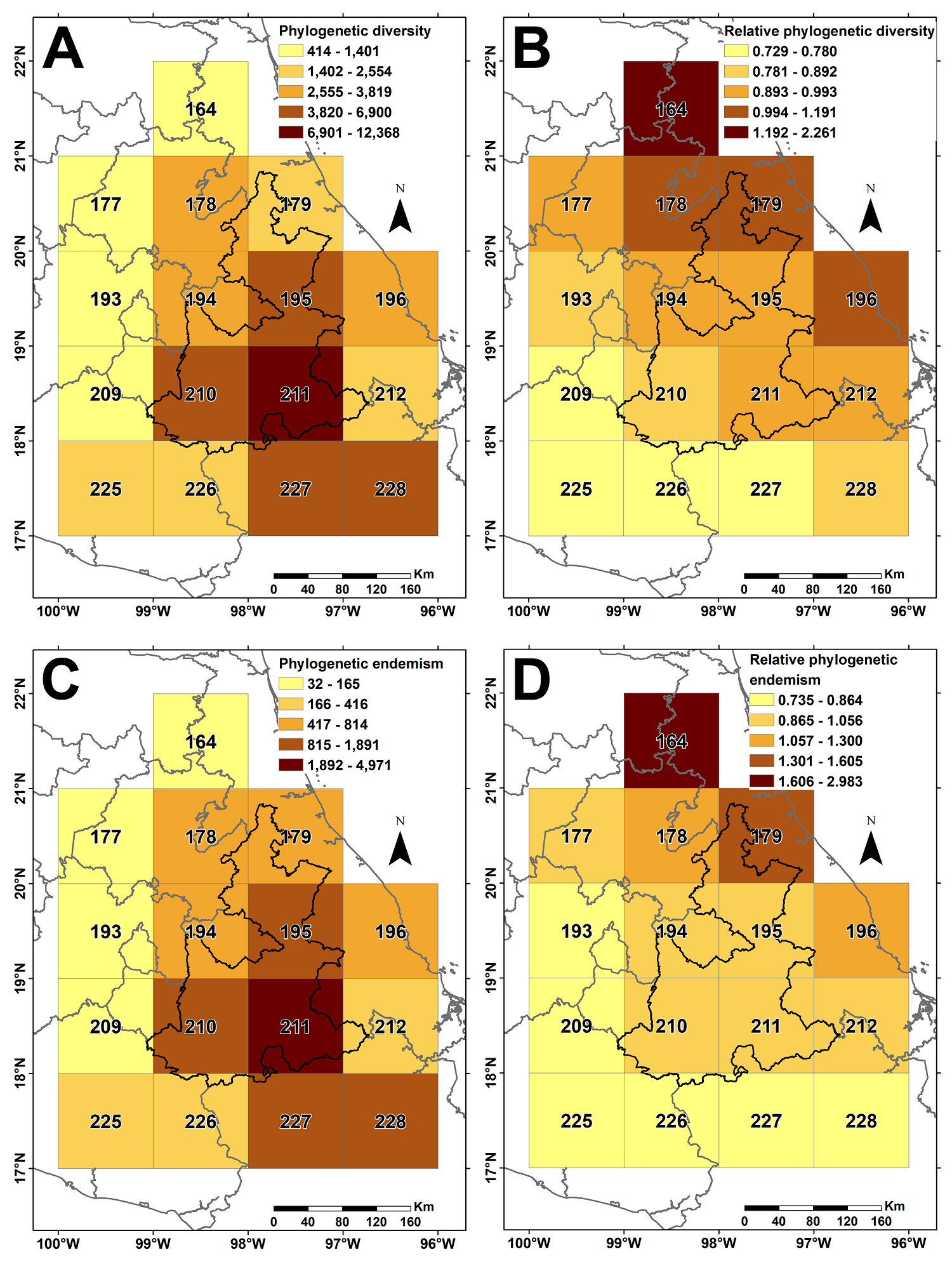
Figure 3 A. Phylogenetic diversity (PD) recorded in each 1° latitude and longitude grid cell. B. Relative phylogenetic diversity (RPD). C. Phylogenetic endemism (PE). D. Relative phylogenetic endemism (RPE).
The CANAPE analysis identifies cells 164, 178, 179, and 196 as centers of paleo-endemism, located on the limits of the Gulf of Mexico Coast and the Sierra Madre Oriental provinces (Figure 4). Likewise, it recovers as centers of mixed endemism cells 210, 211, and 225, located in the adjoining Balsas Depression, the Tehuacán-Cuicatlán Valley, and the Sierra Madre del Sur (Figure 4). The paleo-endemism center includes nine species restricted to its territory whereas the mixed endemism center includes 86 species (see Table S1, Supplementary material).
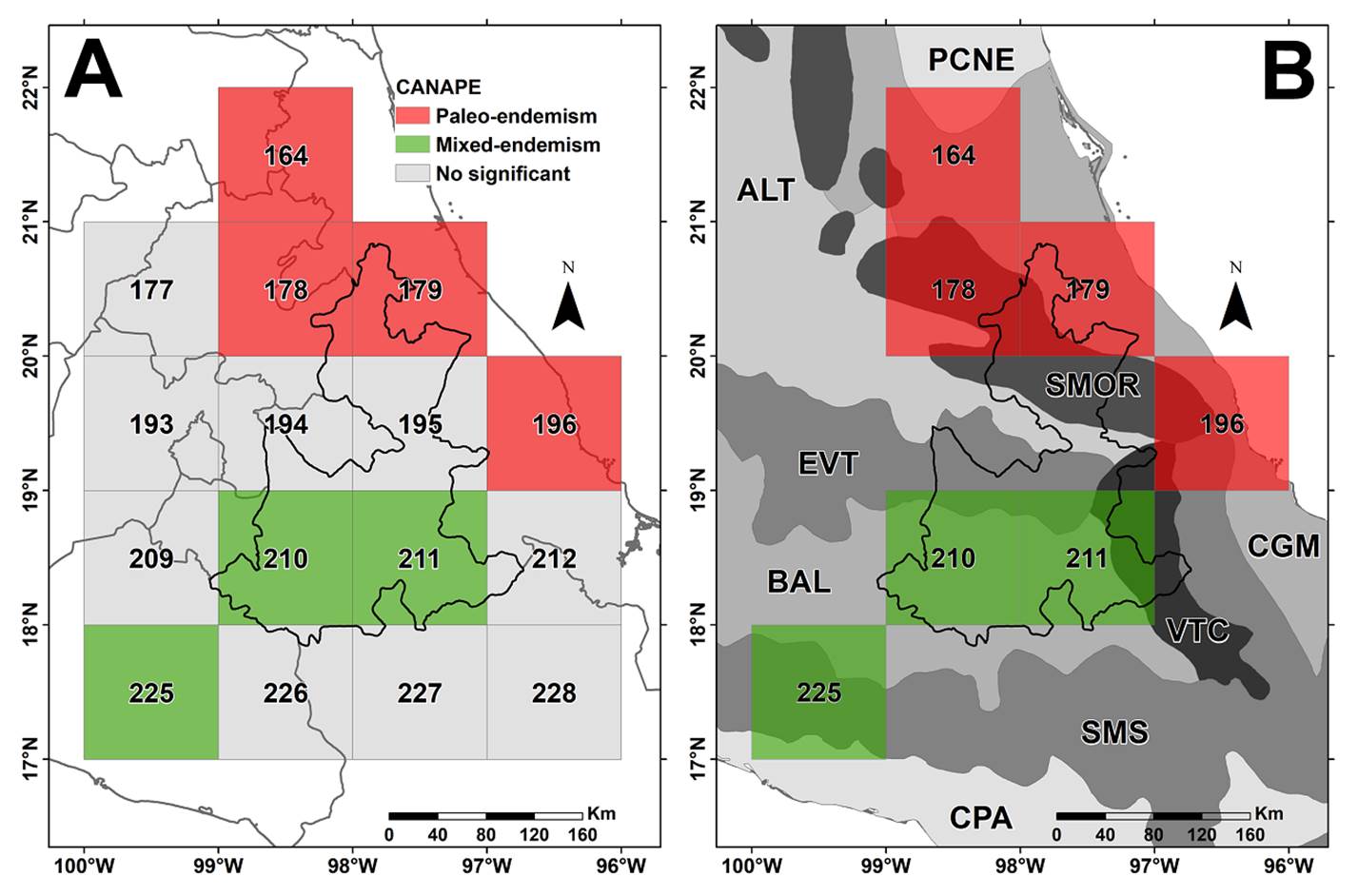
Figure 4 A. Centers of paleo- and mixed endemism in the state of Puebla; grid cells in gray color indicate no significant phylogenetic endemism. B. Centers of endemism according to the floristic provinces (Rzedowski 1978) included in the grid cells. The floristic provinces (Rzedowski 1978) are indicated to show the placement of the grids identified. Acronyms: ALT = Altiplanicie, BAL = Depresión del Balsas, CGM: Costa del Golfo de México, CPA = Costa Pacífica, EVT = Eje Volcánico Transversal, PCNE = Planicie Costera del Noreste, SMOR = Sierra Madre Oriental, SMS = Sierra Madre del Sur, VTC = Valle de Tehuacán-Cuicatlán.
In percentage values, 47.4 % of the endemic species characterizing the center of paleo-endemism distribute in humid mountain forest, 31.6 % in temperate forest, and 15.8 % in tropical humid forests; no species is found either in seasonally dry tropical forest or xerophytic scrub. In contrast, in the mixed center of endemism, 43.1 % of its characteristic endemic species distribute in xerophytic scrubs and 24.4 % in seasonally dry tropical forests; the percentage of species occurrence in the other biomes is negligible. Table 6 (see also the Table S1, Supplementary material) includes the endemic species characterizing both paleo- and mixed centers of endemism across the major taxonomic groups.
Table 6 Number of endemic or nearly endemic species of Puebla characterizing the two centers of endemism in the state of Puebla (Figure 4). Only the main taxonomic groups including species are included. Above diagonal data of paleo-endemism center/below diagonal center of mixed endemism.
| Families | Genera | Species | |
|---|---|---|---|
| Gymnosperms | 1/1 | 1/1 | 1/1 |
| Magnoliids | 2/3 | 2/3 | 2/3 |
| Monocots | 4/8 | 4/13 | 4/20 |
| Rosids | 2/18 | 4/36 | 5/45 |
| Asterids | 6/16 | 7/33 | 7/54 |
The center of paleo-endemism corresponds roughly with the Relative phylogenetic endemism (RPE) area (Figure 3D), whereas the center of mixed endemism with the Phylogenetic diversity (PD) and Phylogenetic endemism (PE). The most relevant floristic provinces involved in these two centers of endemism are the Costa del Golfo de México (Mexican Gulf Coast, CGM) and the Sierra Madre Oriental (SMOR) in the center of paleo-endemism, and the Depresión del Balsas (Balsas Depression, BAL) and the Valle de Tehuacán-Cuicatlán (Tehuacán-Cuicatlán Valley, VTC) in the center of mixed endemism.
Discussion
The number of 60 strict endemic species in the state of Puebla appears low. There are neighboring states with a larger number of strict endemics, such as Guerrero, Oaxaca, or Veracruz (Castillo-Campos et al. 2005, Villaseñor 2016). However, when near endemics are also evaluated, this number rises considerably, highlighting the important role of the state as a center of endemism. There are few studies in Mexico approaching endemism levels as here proposed. Examples are those for Coahuila (Villarreal-Quintanilla & Encina-Domínguez 2005), recording 350 taxa (species plus subspecific taxa), Nuevo León (Alanís-Flores et al. 2011), recording 191 species, San Luis Potosí (De-Nova et al. 2018) citing 336 taxa, or Sinaloa (Pío-León et at. 2023) recording 209 endemics. These case studies highlight the importance of considering not only the strict endemics but also those distributed closely in neighboring political entities. In this way, the role of endemics in Puebla is featured, placing Puebla among the state’s richest in endemic species.
The highest richness values and WE concentrate in the southern edge of the state, the CWE identifies additional grid cells (194 and 209) in its northern and western parts (Table 2). As discussed by Crisp et al. (2001), CWE is uncorrelated with species richness, allowing to identify endemism centers undetected by the other two indices (Total richness and WE, Table 2), such as grid cells 179 or 209 (0.52 and 0.58 CWE scores respectively). In addition, CWE is poorly correlated with Total species richness (R = 0.396) as WE is.
Table 2 illustrates how the use of three different indices shows contrasting values. Total species richness endemic or nearly endemic to Puebla concentrates especially in its state’s southern part, a trend followed by WE. This latter index puts emphasis on grid cells that, although not necessarily rich in total species, include high proportions of species geographically restricted (Crisp et al. 2001). Examples include those grid cells located in the north half of the state (178, 179, 194, 195), which do not contain as many species as those in the southern half but record higher WE values, indicating important numbers of species with restricted distribution.
Endemism richness patterns. The endemic and near Puebla endemics concentrate mostly in its southern half (Figure 2). Alpha diversity (total number of species by grid cell) is strongly correlated with the WE score, showing the same distributional patterns. This correspondence between the two scores highlights the narrow distribution of most species; the higher the WE score the narrow the species distribution. A scoreless correlation with the total species richness is the CWE, indicating the occurrence in the northern tip of the state an important region that outstands by either its high proportion of total species or endemic ones.
In northern Puebla, grid cell 179 is notable; although it is not rich in endemic or nearly endemic species (Table 2), it has a highly Corrected weighted endemism (CWE) index (Figure 3) and is part of the northern paleo-endemism center. This grid cell includes nine endemic species restricted to its territory (Acourtia gracilis L. Cabrera, Commelina bravoa Matuda, Croton rosarianus Mart.-Gord. & Cruz-Durán, Iresine papantlana Loes., Jacquinia morenoana Cast.-Campos & Medina Abreo, Philodendron subincisum Schott, and P. oliverianum Trel.). In southern Puebla, in contrast, grid cell 211 is important, including the richest number of endemics although showing a moderate CWE index. It constitutes the core of the mixed center of endemism (Figure 4), including 85 species restricted to its territory (see Table S1, Supplementary material); examples of such species are Carex tehuacana Reznicek & S. González, Davilanthus davilae (Panero & Villaseñor) E.E. Schill. & Panero, Habranthus medinae L.O. Alvarado & García-Mend., Hechtia aquamarina I. Ramírez & C.F. Jiménez, Jatropha riojae Miranda, Menodora tehuacana B.L. Turner, Perymenium tehuacanum Villaseñor & Panero, Physaria sinuosa (Rollins) O'Kane & Al-Shehbaz, Randia pueblensis Borh. & E. Martínez, Salvia gavilanensis Martínez-Ambriz, Fragoso & Mart. Gord., and Sedum hernandezii J. Meyrán.
Phylogenetic diversity. The coexistence in the state of Puebla of endemic species belonging to 68 distinct families and 194 genera implies many evolutionary events that account for its phylogenetic diversity dispersal. Most of its territory includes places where at least one endemic species occurs (Table 3), suggesting the state has played an important role in the Mexican plant diversity, where intermingle clades of longer history mixed with younger taxonomic groups. This becomes evident in the two centers of endemism identified by the CANAPE analysis (Table 6).
Using a finer grid-cells scale (0.5 decimal degrees), Sosa et al. (2018) placed Puebla as part of a large center of mixed endemism; roughly similar results were obtained by Mishler et al. (2020) analyzing all of North America. In contrast, in this study, using a coarser scale (1 degree) two centers of endemism were recovered, one paleo-endemic center of endemism in northern Puebla (grid cells 164, 178, 179, and 196) and a mixed center of endemism in its southern part (grid cells 210, 211, and 225). Sosa et al. (2018) support the argument that dry forests played important roles either as cradles or museums of diversity, however, it is evident that other biomes played also equivalent roles, as this study revealed. Among the nine species characterizing the center of paleo-endemism (in northern Puebla), five of them distribute in humid mountain forest (BHM), four in temperate forests (BTEM), and along the humid tropical forest (BTHU) there are a couple of species shared with the two previous biomes (Table 6); notably, none of the nine species occur in tropical seasonally dry forest (BTSE) or xeric scrubs (MXE) (Table S1, Supplementary material).
Considering the 86 species depicting the center of mixed endemism, the pattern is reversed, since 79 of the species are found mainly in xeric scrub (MXE) and seasonally dry tropical forest (BTSE), while only five species distribute in temperate forest (BTEM), two in humid mountain forest (BHM), and one species is shared between BHM and humid tropical forest (BTHU).
Among representatives of the basal (long) branches of the phylogeny in this mixed endemism center, mostly diversified in the xerophytic environments are Dioon caputoi De Luca, Sabato & Vázq. Torres (Zamiaceae, Gymnosperm), Buxus mexicana Brandegee (Buxaceae, Eudicot), or Aristolochia pueblana J.F. Ortega & R.V. Ortega (Aristolochiaceae, Magnoliid).
This center of mixed endemism shows the same pattern of high species richness in dry forests reported by Sosa et al. (2018), but the center of paleo-endemism, although with fewer distinctive species, shows the relevance of temperate and humid biomes in the evolution and diversification of the state's flora. The results of this study emphasize not only the role dry forests played in the evolution of the endemic Mexican flora but at least in Puebla other biomes also contributed to this explosive diversification in situ.
Conclusions
The state of Puebla includes in its territory areas that meet the criteria to be considered centers of species endemism (Haffer 1981). The species recorded exclusively inside several grid cells of the network used, constitute sets of endemic species with restricted and quite congruent ranges, especially related to the biomes occurring there. Geologic history and past climate studies will help to support the conclusion that they constitute furthermore centers of origin. Undoubtedly, the grid cells’ endemism needs to be nested in larger areas, such as floristic or biogeographic regions, because they constitute subsets of larger natural regions with common biological history. Examples are grid cells 211 and 227, the two with the richest alpha diversity mostly concentrated in the xerophytic shrublands characteristics of the Tehuacán-Cuicatlán Valley floristic province (Rzedowski 1978).
Although the use of political borders in the assessment of plant endemisms can be widely criticized, the results here exposed indicate they can afford important information to most people not knowledgeable of Mexican provincialism. In addition, they provide evidence for multiple uses in the future, such as evaluating the role that the state or federal natural protected areas play in a state’s conservation program. Strategies toward a better understanding of plant endemism, as proposed here, may provide a better comprehension of the chorology of Mexican endemism, and justify the diversity and/or evolutionary role of natural protected areas in the conservation of Mexico’s biodiversity.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.3299











 nueva página del texto (beta)
nueva página del texto (beta)



