Fouquieriaceae (Ericales) is a small family of eleven species of trees and shrubs endemic to mostly dry environments of Mexico and southwestern United States (Henrickson 1972, Kubitzki 2004). A recent study undertaken by Soto-Trejo et al. (2022) suggested that the family was originated in dry habitats in central-southern Mexico during the Late Miocene. Fouquieriaceae present a series of anatomical adaptations to live in seasonal arid areas, such as presence of a water-storage tissue by means of expansion rays and axial parenchyma bands (Carlquist 2000). The flowers are usually pentamerous, actinomorphic and bisexual with petals fused into a tube; the androecium consists of approximately 10-23 stamens, and the gynoecium is syncarpous with three fused carpels (Henrickson 1972, Waser 1979, Schönenberger & Grenhagen 2005, Schönenberger 2009).
The first embryological studies in Fouquieriaceae were conducted by Johansen (1936), Mauritzon (1936), Khan (1943), Amlie (1965) and Govil (1970) who provided characters associated mainly with the development of ovules and seeds. Recently, Soto-Trejo et al. (2021) described in detail the development of the female gametophyte and the ovule in Fouquieria fasciculata (Roem. & Schult.) Nash, showing the differentiation of an integumentary tapetum, a Polygonum-type embryo sac and the formation of a lateral haustorial arm in the female gametophyte. The androecium is organized in two-level with all the stamens arranged in a single series (Henrickson 1972, Schönenberger & Grenhagen 2005). In a comprehensive study of the pollen morphology of the family, Henrickson (1967) showed tricolporate pollen grains and analyzed the exine sculpturing, showing that size variation correlates directly with flower size. However, features related to the anther development, microsporogenesis and microgametogenesis have not been described in detail or are incomplete for Fouquieriaceae (Davis 1966, Govil 1970, Johri et al. 1992).
The systematic importance of embryological characters, such as the tapetum type and the presence of orbicules (or Ubisch bodies), has been particularly studied in angiosperms (Huysmans et al. 1998, Vinckier et al. 2000, Vinckier & Smets 2002a, b, Janssens et al. 2005, Verstraete et al. 2014, Song et al. 2017, Moon 2018, Ruggiero & Bedini 2020, Shamrov et al. 2021). The tapetum is innermost layer of microsporangium wall and its cells often have dense cytoplasm with one or several nuclei (El-Ghazaly 1999, Shamrov et al. 2021). The tapetal cells provides protection and essential nutrients (e.g., polysaccharides, enzymes, hormones, lipids) to pollen grain development and supplies precursors for pollen exine formation (El-Ghazaly 1999, Pacini 2010, Shamrov et al. 2021). Two main tapetum types are recognized: a) secretory type, where a layer of cells remains intact around the anther locule, and b) plasmodial type, where a multinucleate tapetal plasmodium is formed in the anther locule by fusion of tapetal protoplasts (Pacini et al. 1985, Pacini 1997, Furness & Rudall 1998, 2001, Shamrov et al. 2021). In angiosperms, a secretory tapetum has been associate with orbicules, although they are also formed from a plasmodial tapetum (Huysmans et al. 1998, El-Ghazaly 1999, Galati et al. 2007, Verstraete et al. 2014). The orbicules are sporopolenin particles that are found in the walls of the tapetal cells, which have been widely mentioned and described in species of the ANA grade, magnoliids, and monocots, varying in abundance, size, shape, and ornamentation (Vinckier & Smets 2002a, b, Verstraete et al. 2014, Ruggiero & Bedini 2020).
As mentioned previously, features related to the development of male structures have not been described in detail for Fouquieriaceae. Therefore, we study the development of the anther wall, microsporogenesis, microgametogenesis, and pollen grain morphology of Fouquieria fasciculata, to understand the embryology of the family. This species is a deciduous shrub restricted to the dry environments of the states of Hidalgo and Querétaro in central Mexico, and is characterized by a large fat swollen stem (Henrickson 1972, Zamudio 1995).
Materials and methods
Specimens of Fouquieria fasciculata were collected in the Río Estórax Canyon near the El Plátano, Querétaro, Mexico (21° 01’ 22” N; 99° 30’ 60” W) at the altitude of 910 m asl. Vouchers were deposited at the Herbarium IEB, Centro Regional del Bajío, Instituto de Ecología, A.C.
Floral buds and flowers in pre-anthesis, anthesis and post-anthesis were fixed in situ in FAA (10 % formaldehyde, 5 % glacial acetic acid, 50 % ethanol 96 %, and 35 % distilled water) for 24 hours and stored in 70 % ethanol (Johansen 1940). After that, samples were dehydrated in an ethanol series (70, 85, 96 and 100 %) and embedded in Paraplast wax following Johansen (1940) and sectioned using an American Optical 810 rotary microtome. Further, samples were also embedded in LR-W resin (London resin white) following Ruzin (1999). Sections (~2 μm) were cut using an ultramicrotome RMC-MT990, stained with 0.05 % Toluidine Blue (O'Brien et al. 1964) and mounted in Entellan (Merck). The images were obtained using an Olympus Provis AX70 optical microscope.
Scanning electron microscopy was used to describe the morphology and development of anthers and mature pollen grain. Samples were dehydrated in a graded ethanol series and critical-point dried using a CPD 030 critical-point dryer (Bal-Tec AG, Liechtenstein). Later, the samples were mounted on aluminum stubs using carbon double-sided tape and coated with gold by means of a Denton Vacuum Desk II sputter coater (Denton Vacuum, Moorestown, NJ, USA) (Bozzola & Russell 1992). Then, samples were analyzed and photographed on a JEOL JSM5310-LV scanning electron microscope at 15 kV.
Results
Androecium. The androecium of Fouquieria fasciculata consist of 10 stamens (Figure 1) organized in a single series. In early stages, the stamen primordia become enlarged starting to differentiate into anther and filament (Figure 2A, B). Then, stamens are arranged at two levels due to differences in filament length (Figure 2C, D). In the flower at anthesis, the anthers are of equal sizes but are arranged at different levels due to unequal length of filaments. The filaments present simple and unicellular trichomes on the basal portion above at the level the ovary (Figure 2E, F). Anthers are oblong-lanceolate, cordate with two lobes at the base and cuspidate at the apex (Figure 2C, D, G). The mature anthers are bilobed, dorsifixed with introrse dehiscence along two lines (Figure 2G).
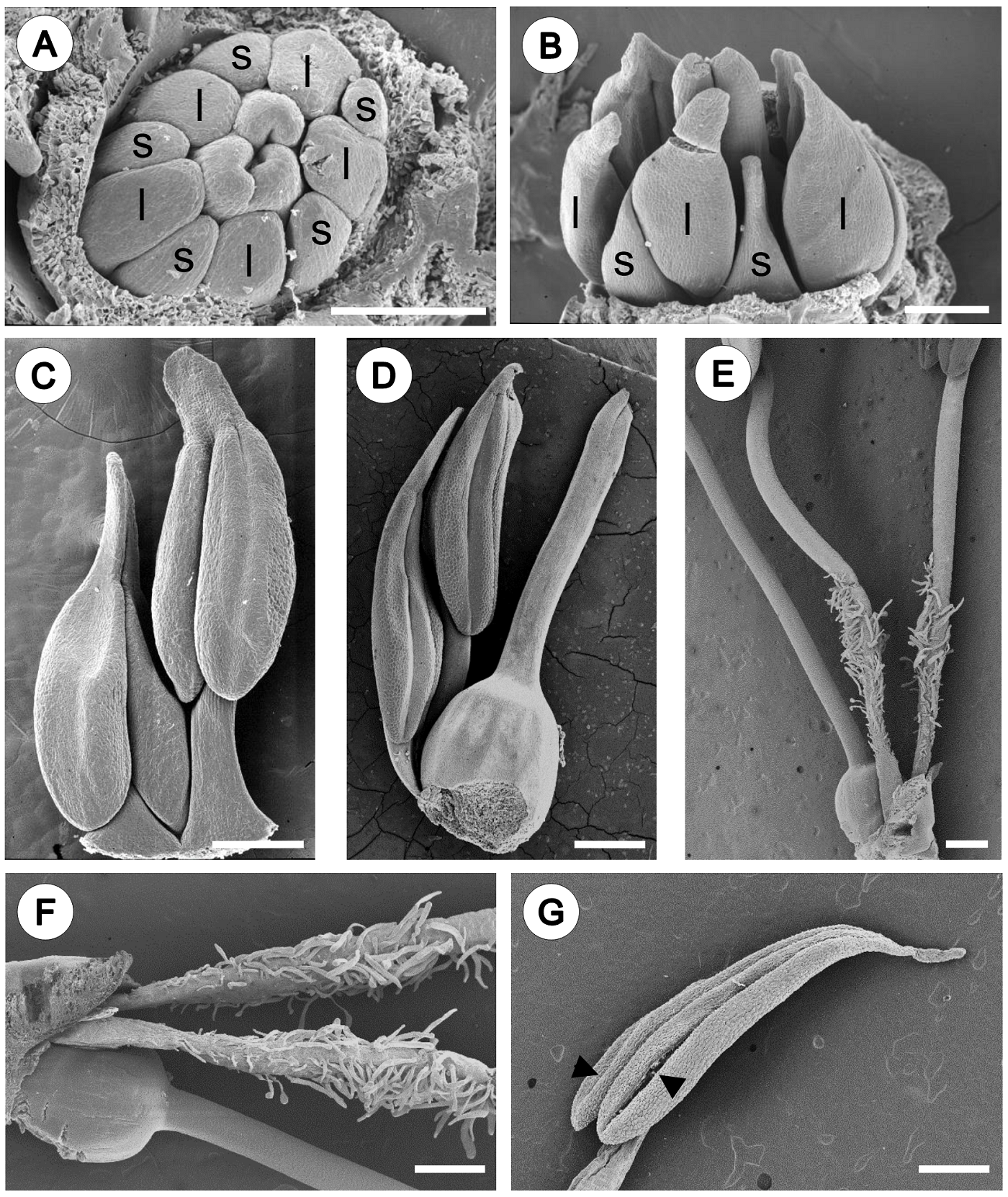
Figure 2 Androecium morphology of Fouquieria fasciculata. A-B. Young floral bud showing the stamens starting to differentiate into anther with five shorter filaments (s) and five longer filaments (l). C-D. Pre-anthesis flower, stamens are arranged at two levels due to differences in filament length. E. Flower at anthesis showing filaments with simple and unicellular trichomes on the basal portion. F. Close up of the trichomes. G. Mature anther showing two lines of dehiscence (black arrow heads). Scale bars = 200 μm (A-D) and 500 μm (E-G).
Development of the anther wall. The anther primordia develop as small hemispherical bumps surrounded by protodermis in transection (Figure 3A). Each anther primordium consisted of meristematic cells with a voluminous nucleus, the subepidermal layer increased by the number of divisions giving rise to the four anther lobes or sporangia (Figure 3B). The subepidermal cells of sporangia are larger (Figure 3B), and become differentiated into archesporial tissue, which characterize by isodiametric cells with dense cytoplasm and conspicuous nuclei (Figure 3C). Then, the archesporial cells are divided periclinally, originating a primary parietal layer and the sporogenous tissue. Periclinal divisions in the primary parietal layer generate two secondary parietal layers: external and internal (Figure 3D). The external secondary parietal layer was divided periclinally, giving rise to the endothecium and a middle layer, while the inner secondary parietal layer develops into the tapetum. Therefore, the anther wall consists of a single-layered epidermis covered by a thick cuticle, an endothecium with fibrous thickenings, one middle layer, and 1-2 nucleate secretory tapetum (Figure 3E, Table 1). According to our observations, the development of the anther wall corresponds to the Dicotyledonous type. The mature anther is bithecal and tetrasporangiate, the four lobes are separated by connective tissue (Figure 3F).
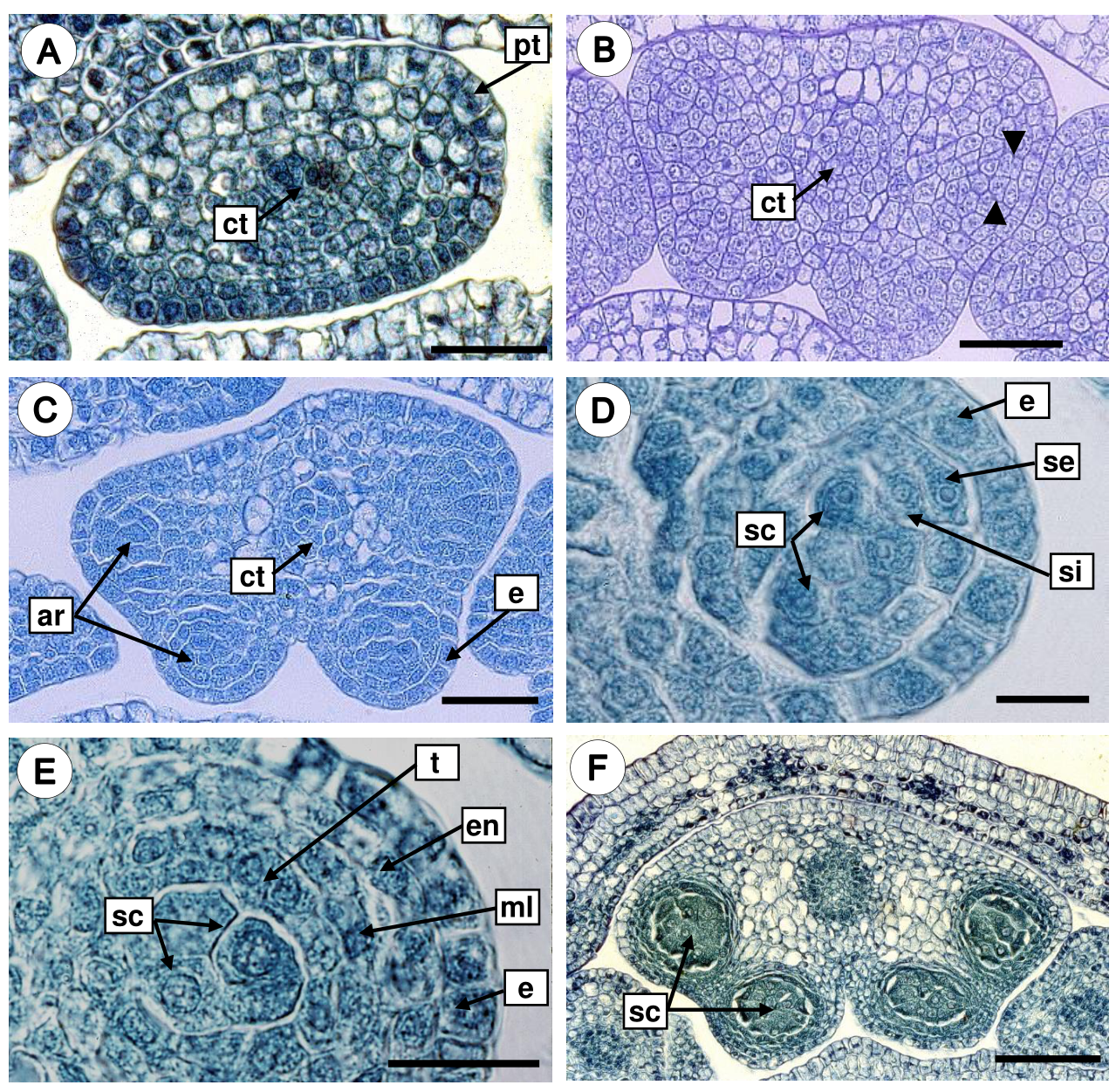
Figure 3 Development of the anther wall in Fouquieria fasciculata. A. Anther primordium surrounded by protodermis. B. Periclinal divisions in the subepidermal layer (black arrow heads) gave rise to the four anther lobes. C. Tetrasporangiate anther with arquesporial cells. D. Anther wall with external and internal secondary parietal layers and sporogenous tissue. E. Anther young wall with the four layers. F. Bilobate and tetrasporangiate anther with sporogenous cells. ar, arquesporial cells; ct, connective tissue; e, epidermis; en, endothecium; ml, middle layer; pt, protodermis; sc, sporogenous cells; se, secondary external parietal layer; si, secondary internal parietal layer; t, tapetum. Scale bars = 30 μm (A-C), 10 μm (D-E), 100 μm (F).
Table 1 Embryological characters of Fouquieria fasciculata.
| Characters | State |
|---|---|
| Number of sporangia per anther | Four |
| Type of anther wall development | Dicotyledonous |
| Epidermis | Persistent |
| Endothecium | Develops U-shaped fibrous thickenings |
| Middle layer | Not persistent |
| Tapetum | Secretory |
| Number of nuclei in a tapetal cell | One or two |
| Orbicules | Present |
| Orbicules morphology | Doughnut-shape, ruminate |
| Type of microspore tetrads | Tetrahedral |
| Number of cells in a mature pollen | Two |
Microsporogenesis and microgametogenesis. The sporogenous tissue consists of cells with conspicuous nuclei and dense cytoplasm (Figure 4A). Then, mitotic divisions give rise to microspore mother cells, which are large and spherical with a thickened callose wall (Figure 4B). The microspore mother cells undergo meiotic divisions and give rise to microspore tetrads with tetrahedral arrangement surrounded by a thick and uniform wall of callose (Figure 4C), and the tapetal cells are rectangular with thin walls, uni- or bi-nucleate (Figure 4D). Later, the callose walls of the tetrads are dissolved and the microspores are free in the anther locule, and the microspore cytoplasm presents a large central vacuole and the nucleus is displaced to a parietal position (Figure 4E). During this stage, tapetal cells increase considerably their volume and present extremely dense cytoplasm with prominent nuclei (Figure 4E). Furthermore, scanning electron micrographs of the radial and inner tangential surfaces of tapetal cells showed orbicules that vary in shape and size (Figure 4F). Two orbicules types were observed: a) doughnut-shape orbicules, where a single central perforation is present (Figure 4G), and b) aggregated orbicules, that are irregular bodies with grooves and wavy margin giving ruminate appearance (Figure 4H). The orbicules surface is granular and resemble the exine of the pollen grain.
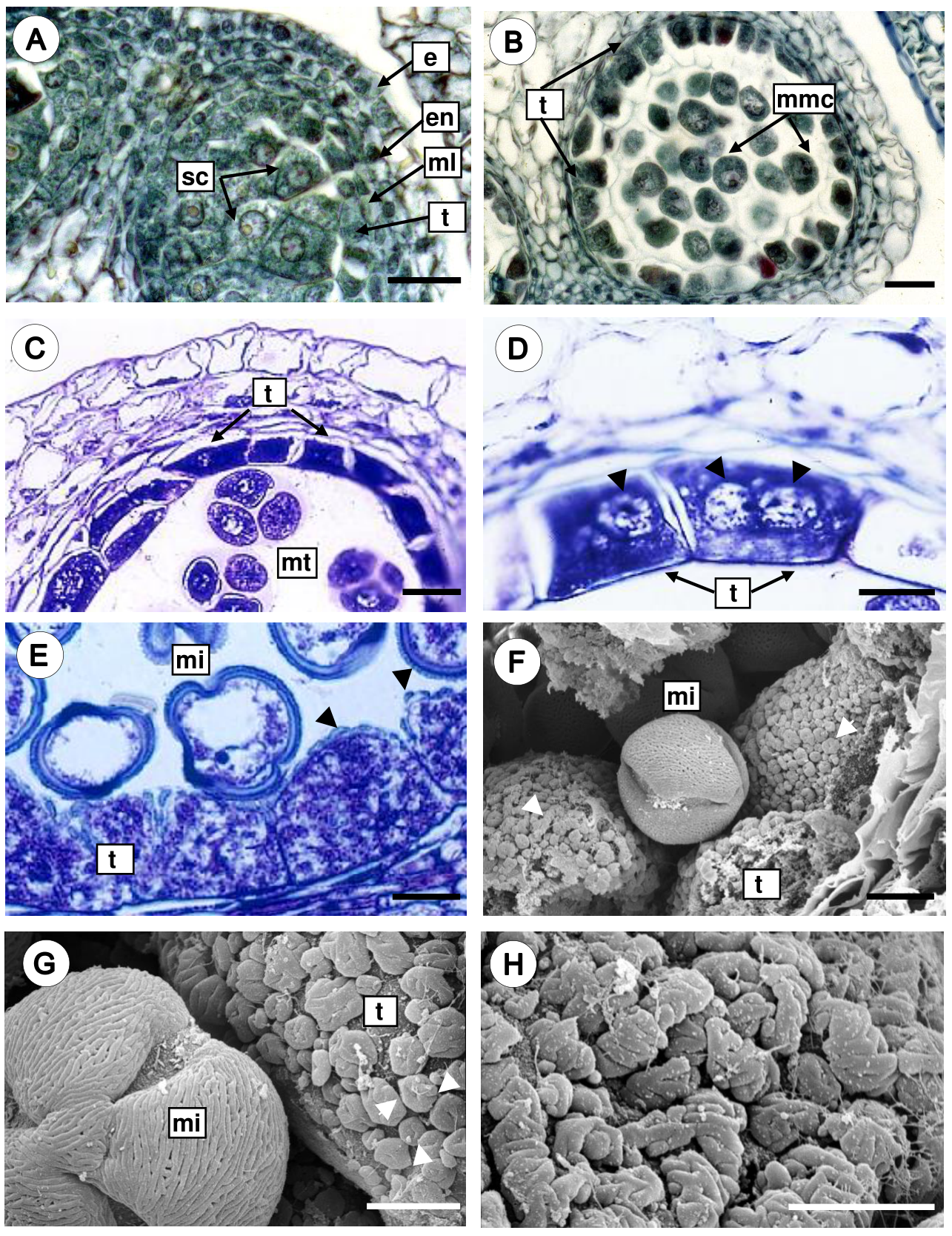
Figure 4 Microsporogenesis and microgametogenesis in Fouquieria fasciculata. A. Anther with sporogenous cells. B. Anther with microspore mother cells surrounded by the callose wall. C. Microspore tetrahedral tetrads still enclosed in callose wall. D. Tapetal cells uni- or bi-nucleate (black arrow heads) with dense cytoplasm. E-F. Free microspores with a conspicuous nucleus and a large central vacuole, and orbicules are observed on the tapetal cell walls (white and black arrow heads). G. Doughnut-shape orbicules (white arrow heads). H. Close up of the aggregate orbicules of ruminate appearance. e, epidermis; en, endothecium; mi, microspore; ml, middle layer; mmc, microspore mother cell; mt, microspore tetrahedral tetrads; sc, sporogenous cells; t, tapetum. Scale bars = 25 μm (A-D), 10 μm (E-F), 5 μm (G-H).
In mature anthers, the endothecium develops thickenings on the radial and inner tangential wall, and the cells of the middle layer and tapetum show signs of degradation. Notably, the tapetal cell persists for a long time and disintegrates just before anther dehiscence. During anther dehiscence, the parenchymal septum that separates both locules of an anther theca breaks down, and consequently a single locule is formed (Figure 5A). The endothecium cells are rectangular and developed U-shaped fibrous thickenings on their radial and inner tangential walls (Figure 5B). Finally, microspores undergo a mitotic division, forming the generative cell and the vegetative cell; thus the pollen grains are shed in the two-celled stage (Figure 5C).
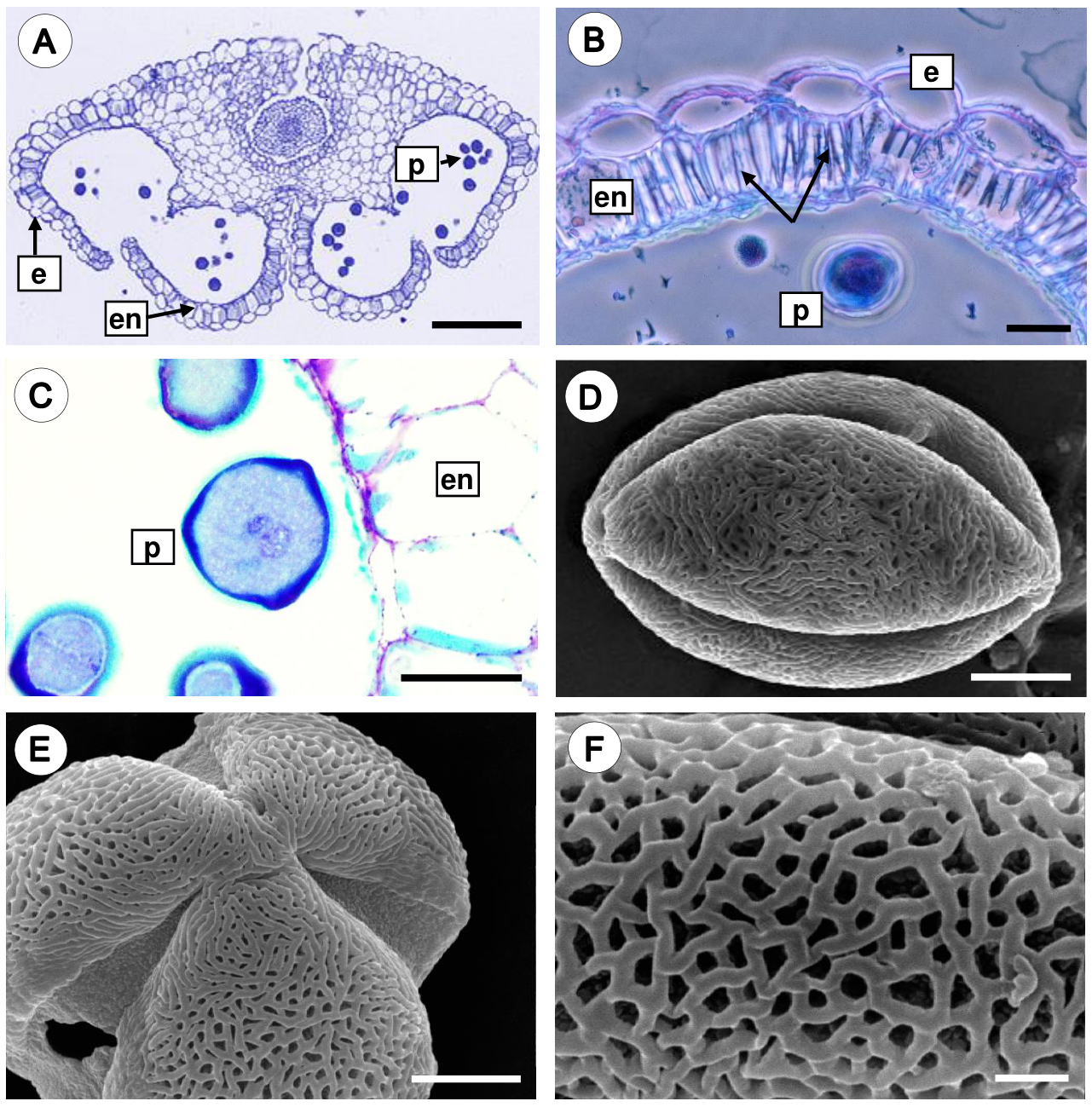
Figure 5 Mature anther and pollen grain of Fouquieria fasciculata. A. Dehiscent anther showing epidermal cells, endothecium with wall thickenings, and pollen grains. B. Detail of the endothecium cells with U-shaped fibrous thickenings (black arrows). C. The mature anther with two-celled pollen grain. D. Equatorial view of a 3-colpate pollen grain. E. Polar view of pollen grain. F. Close up of reticulate-heterobrochate exine. e, epidermis; en, endothecium; p, pollen grains. Scale bars = 200 μm (A), 20 μm (B-C), 5 μm (D-E), 1 μm (F).
Morphology of the pollen grain. The mature pollen grain is prolate, tricolporate with reticulate-heterobrochate exine (Figure 5D-F).
Discussion
In Fouquieria fasciculata, the androecium consists of ten stamens organized in a single series. During early stages, stamens are arranged at two levels due to differences in filament length. In flowers at anthesis and post-anthesis, the anthers are of equal sizes but are arranged at different levels. Our results are consistent with reported in F. columnaris (Kellogg) Kellogg ex Curran and F. splendens Engelm. by Schönenberger & Grenhagen (2005), who described in detail the androecium development.
The stamens present filaments with trichomes on basal portion above at the ovary level covering the nectary surface. According to Henrickson (1972), these trichomes reduce evaporation and present the nectar to flower visitors. Most of Fouquieriaceae species possess flowers with tubular red corollas pollinated by hummingbirds (Waser 1979, Schultheis & Baldwin 1999), thus the nectar could be obtained by these birds. But, F. fasciculata present white flowers and the pollinators are not known, thus more research is needed to determine the type of pollinators in this specie. Anther morphology is relatively uniform throughout the family Fouquieriaceae (Henrickson 1972). Anthers are oblong-lanceolate, cordate with two lobes at the base and cuspidate apex. Further, the mature anthers are tetrasporangiate, dorsifixed with introrse dehiscence along two lines, features that coincide with what has been observed by Henrickson (1972) and Johri et al. (1992).
Anther wall development is of Dicotyledonous type formed by the epidermis, fibrous endothecium, one middle layer, and a secretory tapetum with uni-binucleate cells as described by Davis (1966) for Fouquieriaceae. During the anther wall development, the tapetum presents the most important changes. Our results showed that during free microspore stage, the tapetal cells increase considerately their physiological-biochemical activity for developing microspores, pollen grains and orbicules; thus, the tapetum persist for a long time during of pollen grains formation and disintegrates just before anther dehiscence. Tapetum plays a main role in the development of the pollen grains, and some of its main functions are the production and release of callase enzyme, the formation of exine precursors, and the production and release of orbicules (Pacini et al.1985, Pacini & Franchi 1993, Pacini 1997, Huysmans et al. 1998, Bhojwani & Bhatnagar 1999, Lei & Liu 2020, Shamrov et al. 2021). In angiosperms, the distribution of tapetum types have evolved several times through the phylogeny. Most of basal angiosperms present a secretory tapetum, whereas plasmodial tapetum is relatively consistent in monocotyledons (Furness & Rudall 2001, Shamrov et al. 2021); and in eudicots the secretory tapetum is predominant (Furness 2008). Based on previous studies in Ericales, embryological characters related the development of male structures vary among the families and might be informative for understanding evolution in the order (Janssens et al. 2005, Schönenberger & Grenhagen 2005, Schönenberger 2009). Most families in Ericales have secretory tapetum with binucleate cells including family Fouquieriaceae (Johri et al. 1992).
Although Fouquieriaceae is known to have a secretory tapetum (Henrickson 1972, Johri et al.1992), orbicules have never been examined in this family. Recent studies have been focused orbicules morphological diversity in different plant species (e.g.,Song et al. 2017, Moon 2018, Galati et al. 2019, Ruggiero & Bedini 2020). In Ericales, Pelliciera (Pellicieraceae) presents aggregated orbicules with irregular shape and psilate surface (Janssens et al. 2005), while in Marcgraviaceae orbicules are mostly round or elliptic with smooth surface (Lens et al. 2005). Fouquieria fasciculata present two orbicules types: doughnut-shape orbicules and ruminate orbicules, both were abundantly distributed on the radial and inner tangential wall of tapetal cells. According to Galati et al. (2010), two or more units of orbicules could be grouped forming aggregates as in F. fasciculata. Thus, we suggested that doughnut-shape orbicules could be early stages of the ruminate orbicules; but further studies including the morphology and ultrastructure of the orbicules in Fouquieriaceae are needed to understanding their origin and development. Furthermore, the orbicules surface is granular and resemble the exine of the pollen grain, which could indicate a similar-patterned biosynthesis of sporopollenin (Ruggiero & Bedini 2020).
Studies on the structure and development of orbicules have suggested different functions, such as: a) lysis and degeneration of tapetal cells (Rowley & Erdtman 1967, Herich & Lux 1985), b) retain on their surface sporopollenin for the normal development of the microspores (El-Ghazaly & Jensen 1986, El-Ghazaly & Nilsson 1991), c) act as vectors of allergens and factors for why pollen causes allergic reactions (Vinckier & Smets 2001, Vinckier et al. 2005), d) associated to the pollen dispersion and the pollination mode (Heslop-Harrison 1968, Keijzer 1984, Pacini 1997, Galati et al. 2010, Verstraete et al. 2014). According to Galati et al. (2010), orbicules with an irregular surface and/or perforations would be common among of species pollinated by hummingbirds or long-tongued insects. Our observations in F. fasciculata showed that some orbicules have a single perforation giving them a doughnut-like appearance, which suggest that flowers could be primarily pollinated by either hummingbirds or long-tongued insects. As mentioned previously, most of Fouquieriaceae species with tubular red flowers are pollinated by hummingbirds, but the pollinators are not known for F. fasciculata, whose flowers are white. Further studies are needed to confirm the presence or absence of orbicules in other species of the family, and to understand the possible correlation between their morphology and pollinators.
Orbicules have phylogenetic and taxonomic value in several families of angiosperms, such as, Apocynaceae, Gentianaceae, Loganiaceae, Oxalidaceae, Rosaceae, and Rubiaceae (Huysmans et al. 1997, Vinckier et al. 2000, Vinckier & Smets 2002a, b, 2003, Rosenfeldt & Galati 2008, Verstraete et al. 2014, Song et al. 2017, Romero et al. 2017, Moon 2018, Ruggiero & Bedini 2020). This study showed the presence of orbicules in Fouquieriaceae for the first time, other families of order Ericales, such as Marcgraviaceae and Pellicieraceae presented consistently orbicules, while are completely absent in Balsaminaceae and Tetrameristaceae (Janssens et al. 2005). The presence of orbicules generally represents a plesiomorphic trait for angiosperms since orbicules are common in the basal clades (Verstraete et al. 2014, Ruggiero & Bedini 2020). Phylogenetic studies indicate that Fouquieriaceae is an ancient lineage that diverged from Polemoniaceae at approximately 75.5 Ma during the late Cretaceous (Schultheis & Baldwin 1999, Anderberg et al. 2002, De Nova et al. 2018); but orbicules have not been described in Polemoniaceae. More investigations are needed to clarify the systematic significance of orbicules in family Fouquieriaceae and its phylogenetic relationships in order Ericales.
Regarding the pollen morphology in Fouquieriaceae, Henrickson (1967) carried out a comprehensive study in the family and showed that the pollen grains are prolate, tricolporate, and the exine is reticulate, which is corroborated here for F. fasciculata, further the exine of this species is heterobrochate. According to Henrickson (1972), pollen grains are shed as single-celled monads, and our observations showed that are released in two-celled stage.
The present study carrying out detailed research of development of androecium in F. fasciculata and provide information on tapetum, pollen development and orbicules features that might be informative for understanding embryology and evolution in the family Fouquieriaceae. Further expanded studies about morphological and ultrastructural orbicules diversity in the Fouquieriaceae species are needed to understanding their correlation with the type of pollinators, as well as their taxonomic value at family level.











 nueva página del texto (beta)
nueva página del texto (beta)




