Bryophytes reproduce by spores and asexually by specialized structures (e.g., gemmae, tubers, bulbils) or clonal growth (a division of a genetic individual into new clones) (During 1992, Frey & Kürschner 2011). Both propagules’ production, dispersal, and establishment contribute to population dynamics at large and local scales (Herben 1994, Söderström 1994, Stieha et al. 2014). In addition, each mode of reproduction has intrinsic features which allow them to play different ecological roles. Spores can be dispersed long-distance by wind or water currents and colonize new habitats; in contrast, asexual propagules facilitate adaptive habitat exploitation in a localized area (During 1979, Kimmerer 1991a, Newton & Mishler 1994, Löbel & Rydin 2009, Brzyski et al. 2014, Patiño & Vanderpoorten 2018). Production of asexual propagules seems more frequent in dioicous than monoicous mosses (Longton 1992, Laenen et al. 2016). Thus, for example, in Mexico, 21 % of the known dioicous mosses reproduce both sexually and asexually, compared to 5 % of monoicous taxa (Peña-Retes & Delgadillo-Moya 2018). The moss family Calymperaceae is an excellent representative of a dioicous tropical plant group with both modes of reproduction producing specialized asexual foliar gemmae and sporophytes (spores) (Reese 1993, 1994).
The production of specialized asexual propagules, such as gemmae, is crucial in bryophyte population dynamics because it contributes to the occupation of nearby areas with similar habitats. Gemma production may be influenced by environmental variables, like rainfall, and colony features such as colony size, number of shoots able to produce gemmae, i.e., gemmiferous shoots and the place of gemma formation on the leaf (Laaka-Lindberg 1999, Algar-Hedderson et al. 2013). Reports on tropical Calymperaceous mosses indicate that gemma output responds to seasonal patterns where more gemmae are produced during constant rainfall (Odu & Owotomo 1982, Egunyomi & Olarinmoye 1983, Cerqueira et al. 2016).
Shoot density, or the number of shoots per area, could be a good estimator of the gemma production. For temperate bryophytes, shoot density and gemma production could be negatively correlated (Kimmerer 1991b, Algar-Hedderson et al. 2013), or they do not seem to have a significant effect (Laaka-Lindberg 1999). In contrast, the number of gemmiferous shoots a species can produce is variable and positively correlated with gemma output. For example, the percentage of gemmiferous shoots in Calymperaceae varies from 86 % of shoots per unit area for Syrrhopodon simmondsii Steere, to 25 % for Calymperes palisotii Schwaegr and 0 % for S. annotinus Reese & Griffin (Egunyomi & Olarinmoye 1983, Pereira et al. 2016). Nonetheless, the effect of shoot density on gemma output in tropical mosses is known only from indirect data, and it is uncertain whether this variable affects gemma production directly.
The gemma development could also be controlled by the occurrence of sexual structures (antheridia and archegonia) and sporophytes, suggesting a trade-off between sexual versus asexual reproduction modes. In some species, shoots developing gametangia and sporophytes produce fewer or no asexual propagules than sterile shoots (Kimmerer 1994, Laaka-Lindberg 2001, Manyanga et al. 2011, Algar-Hedderson et al. 2013, dos Santos et al. 2022). In Camlyperaceous species of lowland Amazonia, the number of archegonia and perichaetia (male sexual branch) per shoot was lower when gemmae co-occurred (Pereira et al. 2016), suggesting differential resource allocation for modes of reproduction. However, this pattern seems to be species-specific and is not a standard feature for all Calymperaceae.
This research aims to identify whether rainfall, shoot density, number of gemmiferous shoots, and sexual structures determine gemmae output per shoot and per cm2 in two tropical mosses with different reproductive strategies. Two epiphytic Calymperaceous mosses were studied, Calymperes afzelii Sw., mainly asexual, and Syrrhopodon incompletus Schwägr., with sexual-asexual reproduction. Specifically, we aim to i) investigate how two tropical epiphytic mosses with contrasting reproductive strategies differed in gemma output; ii) assess the pattern of gemma production through an annual interval; and iii) analyse the correlation of rainfall, shoot density, number of gemmiferous shoots, and sexual structures with the number of gemmae per shoot and cm2.
Materials and methods
Study area and selected species. The Agua Blanca State Park is located in the Macuspana municipality of Tabasco state, southeastern Mexico (17° 35'-17° 38’ N, 92° 25'-92° 29’ W), covering an area of 20.25 km2 with elevations between 100 and 200 m. The vegetation is tropical rainforest and the mean annual temperature ranges from 23 to 26 °C with a mean annual rainfall between 2,100 to 3,200 mm (Zarco-Espinosa et al. 2010).
Calymperes afzelii and Syrrhopodon incompletus are epiphytic mosses that occur sympatrically in the study area. Both species are dioicous (unisexual) and produce gemmae adaxially on leaf apices (Reese 1993, 1994) that become detached through the rupture of an abscission cell at the base of gemma or stalk (tmema). Normally, the cell rupture is triggered by raindrop impact and mechanical effect on leaves that eject the gemma (Egunyomi & Olarinmoye 1983, Reese 1997). Gemma size has been related to dispersal ability because it affects the length of time gemmae stay in air currents (During 1992, Reese 2001) (Figure 1). The two selected species belong to Calymperaceae. Calymperes afzelii is a pantropical moss with dimorphic leaves differentiated into gemmiferous (producing gemmae) and sterile ones (Reese 1993, 1994). Like other species of the genus, C. afzelii produces gemmae frequently, but sporophytes seem rare (Reese 1997, 2007). In contrast, S. incompletus shows a disjunct distribution between the Americas and Africa, produces foliar clavate gemmae with a papillose surface on non-dimorphic leaves, and sporophytes are developed recurrently (Reese 2001, Ellis & Pressel 2020). Two varieties of this species are recognized in Mexico, S. incompletus var. incompletus Schwägr. and var. berteroanus (Brid.) Reese (Reese 1994), with the former occurring at the study site.
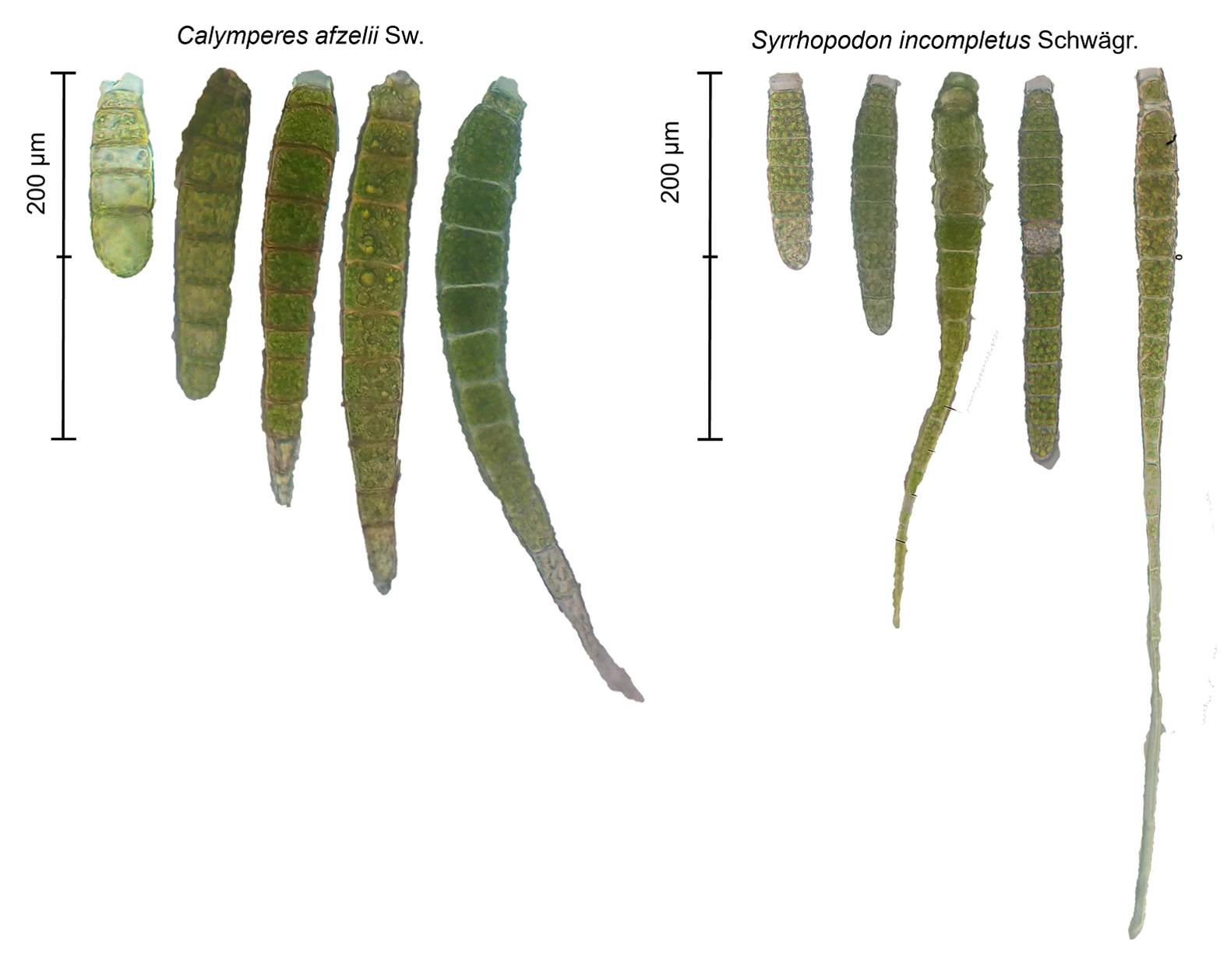
Figure 1 Gemmae of Calymperes afzelii Sw. and Syrrhopodon incompletus Schwägr. Mean gemma size was 156 µm (± 32) for C. afzelii and 130 µm (± 35) for S. incompletus based on 259 observations. Gemma size differs between species (U = 49 920, P = < 2.2e-16), but there is also variation within species. Gemmae with appendage tips are common in S. incompletus
Sampling design. An exploratory visit was conducted to identify trees where the species occurred. Three 10 × 20 m plots separated by at least 100 m were established (Figure 2). Within each plot, seven selected trees were marked for chronological sampling. Characteristics of the sampled trees were measured for habitat comparison purposes (Table 1): area covered by the moss species, tree species identity, diameter at breast height (DBH; 1.30 m), moss position relative to sunlight, bark type and its water holding capacity (WHC), and bark pH. The area covered by mosses on each tree was measured by marking the perimeter on 40 × 200 cm plastic bands (up to 1.7-2 m high) and analysing it on image editing software. Bark WHC was analyzed following Levia & Herwitz (2005) and Levia & Wubbena (2006) but using bark weight without volume and expressed as a percentage (i.e., the difference between saturated and dry weights divided by dry weight). Bark pH was calculated using a standard pH-electrode, following Kricke (2002).

Figure 2 Geographic location of the study area in Mexico (upper left) and Tabasco state (upper right). The spatial representation of the sampling plots (P) and trees (T) within the Agua Blanca State Park is displayed at the bottom of the figure. Trees with the occurrence of Calymperes afzelii Sw. are represented in blue, and those of Syrrhopodon incompletus Schwägr. in red. Each tree was coded according to plot and within plot number (e.g., P2-T3).
Table 1 Characteristics of sampled trees where Calymperes afzelii Sw. and Syrrhopodon incompletus Schwägr. were found. Tree code is based on plot number (see Figure 2 for reference). Moss species occurred only on trees where the cover area is indicated. The diameter at breast height (DBH), moss position on each tree, tree bark type, bark water holding capacity (WHC) and pH are also indicated. The moss species grew on different trees.
| Plot 1 | Plot 2 | Plot 3 | |||||
|---|---|---|---|---|---|---|---|
| Tree code: | P1T1 | P1T2 | P2T1 | P2T2 | P2T3 | P3T1 | P3T2 |
| Calymperes afzelii | 2,937.19 cm² | 112.63 cm² | 278.35 cm² | 194.42 cm² | |||
| Syrrhopodon incompletus | 3,455.66 cm² | 1,661.08 cm² | 869.96 cm² | ||||
| Tree species | Spondias mombin | Spondias mombin | - | Spondias mombin | Guazuma ulmifolia | Dialum guianense | Dialum guianense |
| DBH (cm) | 172 | 93 | 138 | 141 | 152 | 180 | 160 |
| Position | 230SW | 307NW | 60NE | 220SW | 120SE | 275W | 255W |
| Bark type | Furrowed | Furrowed | Porous | Furrowed | Furrowed | Smooth | Smooth |
| WHC (%) | 78 | 70 | 133 | 111 | 233 | 175 | 167 |
| pH | 7.5 | 7.7 | 6 | 7.3 | 6.9 | 6 | 6.5 |
Moss samples were collected using grids with 1 cm2 mesh. Three grids for each moss species were sampled every two months from September 2015 to November 2016. Samples were collected from randomly selected trees with repetition to avoid sampling bias. A 1 cm2 square was randomly selected from each grid (8 visits × 3 squares). All shoots were taken from each square and put in individual microtubes with water (Figure 3). Sample size was chosen to avoid the excessive thinning of both moss populations. Voucher specimens were deposited at MEXU herbarium following the first author collection numbers (Escolástico-Ortiz 335, 336, 337).
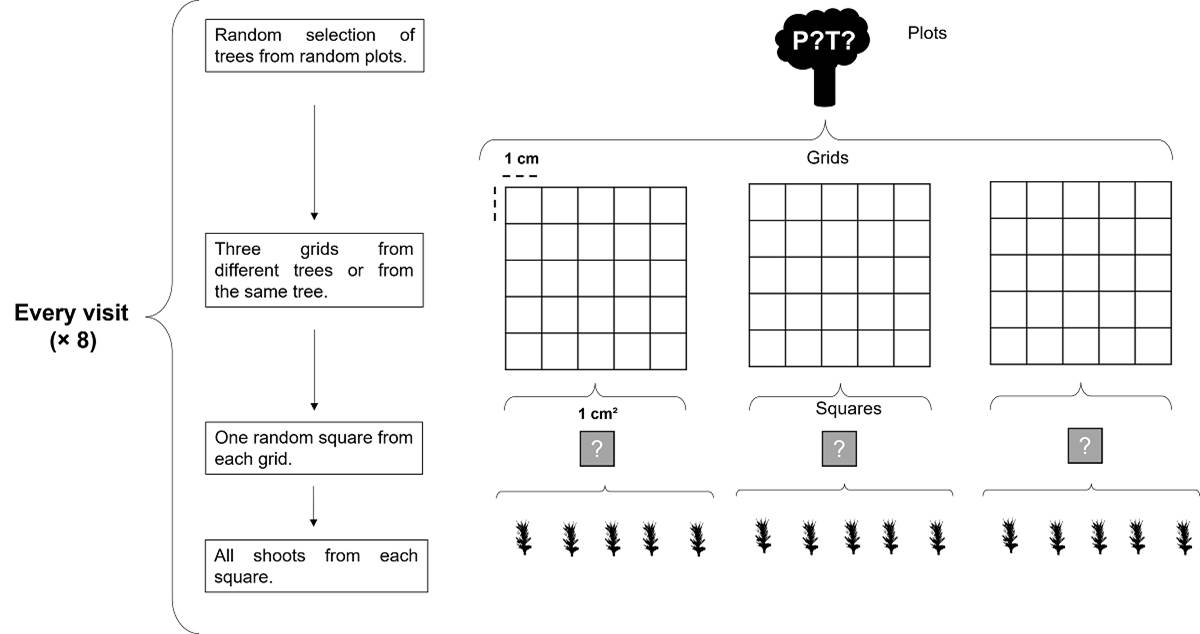
Figure 3 Sampling design based on a random selection of trees where Calymperes afzelii Sw. and Syrrhopodon incompletus Schwägr. occurred. In each of the eight visits from September 2015 to November 2016, three grids were placed on the selected trees, and three random squares of 1 cm2 were sampled for each moss species. From each square, all shoots were collected in microtubes.
Gemma production, gemmiferous shoots, sexual structures, and rainfall. Gemma production per shoot was measured from five randomly selected shoots per square (1 cm2). Based on a previous report concerning gemma viability, broken gemma or fragments with less than five cells were not considered for the analyses (Egunyomi & Olarinmoye 1983). Similarly, gemmae from the same square were summed up to estimate mean production per area (Figure 3). Estimates were made for every visit during the study period (8 visits × 3 squares × 5 shoots). The number of shoots and gemmiferous shoots per square was recorded as shoot density. Only gemmiferous shoots with at least ten gemmae were considered for the analyses. The occurrence of fertile shoots with archegonia, antheridia or sporophytes were also recorded for each square. The presence of sexual structures on shoots was registered for shoot scale analyses and the percentage of sexual shoots per square for the area scale (1 cm2). Data on sexual structures and sporophytes were not recorded for September and November 2015.
Daily mean precipitation was acquired from the automatic meteorological station Dos Patrias located 40 km west of the study site. We calculated the mean of the daily mean precipitation (mm) for 60 days prior to each visit and used those values in the statistical analyses.
Statistical analyses. The following colony features were statistically compared between moss species: mean gemma production per shoot and per area (1 cm2), percentage of gemmiferous shoots and shoots carrying sexual structures per area. Mann-Whitney tests were used to compare the variables due to the non-Gaussian distributed data. In addition, the mean number of gemmae per shoot and per visit and mean daily rainfall per month were used to examine temporal patterns of gemma production through the study period.
Regression models were employed to examine the effect of rainfall, shoot density and occurrence of sexual structures on gemmae production at shoot and cm2 scales. We examined the gemma production per shoot as a function of rainfall, shoot density and the occurrence of sexual structures for both species. First, the number of gemmae per shoot ( response variable) was assessed to identify the distribution type resulting in non-Gaussian distributed data. Thus, the data were fitted against theoretical general distributions. The negative binomial distribution was selected because it models count data well and, compared to the Poisson distribution (variance equal to the mean), it can account for the variability of the data. Due to the repeated counts of gemmae per square, we selected generalized linear mixed models (GLMM), which help model non-Gaussian distributed data with nested design or repeated measures because they can deal with autocorrelation and estimate the effects of random and fixed variables. The packages lme4 v. 1.1.29 (Bates et al. 2015) and lmerTest v. 3.1.3 (Kuznetsova et al. 2017) were used to construct the model performing maximum likelihood with Laplace approximation. The models were fitted using the number of gemmae per shoot as the response variable, the rainfall values, shoot density per square and the presence of sexual structure as fixed effects and the squares as random effects. A negative binomial error distribution was used with a log link function.
The gemma production per area (cm2) was investigated using the rainfall, shoot density per square, and percentage of gemmiferous and sexual shoots per square as the predictor variables. The area scale data also fitted better the negative binomial distribution, but in this case, generalized linear models (GLM) were applied (i.e. no random effects). The models were fitted using the number of gemmae per shoot as response and rainfall values, shoot density per square, percentage of gemmiferous and sexual shoots per square as the predictors. A negative binomial error distribution was used with a log link function with the MASS v. 7.3.55 package (Venables & Ripley 2002).
In all cases, the models were validated by evaluating the residual distribution and the assumption of normal distribution. In addition, for GLMMs, the normal distribution of the random effects was also assessed. All statistical analyses were conducted in R v. 4.1.3 (R Core Team 2022).
Results
Calymperes afzelii and Syrrhopodon incompletus, grew on different trees (Table 1). C. afzelii was found only on Spondias mombin L. trees with furrowed bark and S. incompletus on Dialum guianense Steud. with smooth bark and higher WHC. These observations suggest differential preferences of mosses for host trees.
Overall, the mainly asexual species C. afzelii has a higher gemma production per cm2 and gemmiferous shoots, while the sexual-asexual S. incompletus, covers a more extensive area on trees and has a higher shoot density (cm2) (Table 2). However, shoots bearing sexual structures (%) did not significantly differ between moss species and no sporophytes were recorded for C. afzelii during the study period. The mean production of gemmae in C. afzelii indicates a relatively constant development of gemmae. For S. incompletus, there was a high variation in the mean number of gemmae through time.
Table 2 Comparison of moss cover area, gemma production and colony features in Calymperes afzelii Sw. and Syrrhopodon incompletus Schwägr. Means for each variable are presented, followed by their standard errors and sample size (n) for each species. Finally, results from Mann-Whitney U test are shown with their respective p-values (P < 0.05 *).
| Variable | Calymperes afzelii | Syrrhopodon incompletus | n | Mann-Whitney U | p-value |
|---|---|---|---|---|---|
| Moss cover on trees (cm2) | 3,521 | 5,985 | |||
| Gemmae per cm2 | 146 ± 24 | 132 ± 197 | 24 | 431 | 0.003* |
| Shoot density per cm2 | 9 ± 2 | 11 ± 3 | 24 | 155 | 0.006* |
| Gemmiferous shoots (%)/cm2 | 70 ± 34 | 36 ± 38 | 18 | 239 | 0.012* |
| Shoots bearing sexual structures (%)/cm2 | 32 ± 28 | 51 ± 28 | 18 | 112 | 0.115 |
The gemma production per shoot for both species showed a seasonal pattern related to rainfall. Both moss species developed more gemmae per shoot during rainy months (September, November, and January) and fewer in the drier months (March, May, and June) (Figure 4). Rainfall was higher in September 2016, but November 2015 exhibited the most significant number of gemmae per shoot in both species.
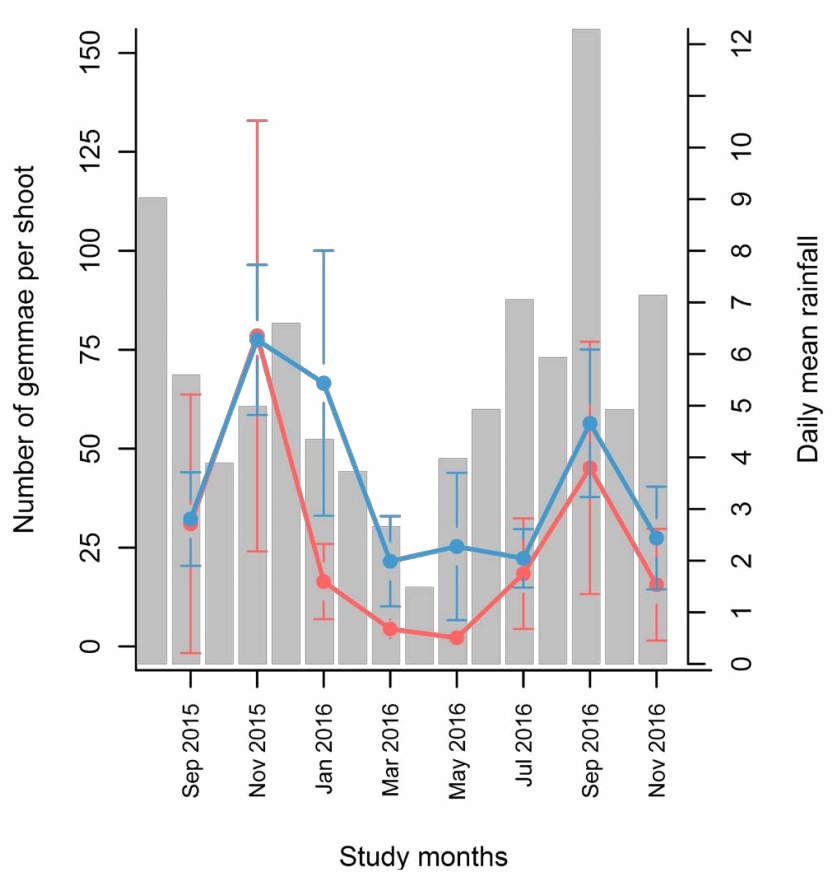
Figure 4 Gemmae production per shoot of Calymperes afzelii Sw. (in blue) and Syrrhopodon incompletus Schwägr. (in red) in every visit from September 2015 to November 2016. Mean number of gemmae per shoot and 95 % confidence intervals are presented for each visit in the two species. Sample size was n = 15. Daily mean precipitation (mm) for 60 days prior to each visit is shown as gray bars.
The GLMM estimates of the gemma production at shoot level confirmed the positive effect of rainfall on the number of gemma per shoot (Table 3). In addition, sexual structures, such as antheridia and archegonia, seem positively related to gemma output per shoot in C. afzelii. The rest of the studied variables do not significantly affect gemma production for both species; however, sexual structures are negatively related to the production of gemmae in S. incompletus.
Table 3 Summary of GLMMs (Negative binomial) of the number of gemmae per shoot as a function of rainfall, shoot density and presence of sexual structures. Coefficients (β), standard errors (SE) and p-values are given for each variable per moss species (P < 0.01 *; P < 0.001**). The variation among squares (random effect) is presented as SE. The amount of rainfall is positively associated with the number of gemmae produced per shoot. The occurrence of a sexual structure on a shoot for C. afzelii has a statistically marginal effect on gemmae production. The sample size is n=87 per species.
| Calymperes afzelii | Syrrhopodon incompletus | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | Estimate | SE | z-value | p-value | Estimate | SE | z-value | p-value |
| (Intercept) | 0.572 | 1.020 | 0.56 | 0.5750 | -0.244 | 1.203 | -0.20 | 0.8392 |
| SD Squares | 0.792 | 1.052 | ||||||
| Rainfall | 0.219 | 0.090 | 2.42 | 0.0155 * | 0.388 | 0.122 | 3.17 | 0.0015 ** |
| Shoot density | 0.157 | 0.102 | 1.53 | 0.1261 | 0.050 | 0.079 | 0.63 | 0.5264 |
| Sexual structure | 0.441 | 0.177 | 2.48 | 0.0131 * | -0.046 | 0.220 | -0.21 | 0.8344 |
The GLM results indicated that gemma production per cm2 mainly responds to the intrinsic ability of shoots to develop gemmae (i.e., number of gemmiferous shoots per cm2) and rainfall in both species (Table 4). All other studied variables did not significantly affect the production of gemmae per cm2 at the study site.
Table 4 Summary of GLMs (Negative binomial) of the number of gemmae per cm2 as a function of rainfall, shoot density, and percentage of gemmiferous and sexual shoots. Coefficients (β), standard errors (SE) and p-values (P < 0.05*; P < 0.001**) are given for each moss species. The rainfall and the percentage of gemmiferous shoots per area positively affect the gemma output per cm2. The sample size is n =18 per species.
| Calymperes afzelii | Syrrhopodon incompletus | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | Estimate | SE | z-value | P-value | Estimate | SE | z-value | p-value |
| (Intercept) | 2.827 | 0.497 | 5.68 | 1.32e-08 ** | 1.406 | 0.950 | 1.47 | 0.1391 |
| Rainfall | 0.094 | 0.042 | 2.21 | 0.0272 * | 0.253 | 0.102 | 2.48 | 0.0130 * |
| Shoot density | -0.033 | 0.051 | -0.65 | 0.5135 | 0.105 | 0.065 | 1.61 | 0.1065 |
| Gemmiferous shoots (%) | 2.698 | 0.328 | 8.22 | 2e-16 ** | 2.522 | 0.646 | 3.90 | 9.5e-05 ** |
| Sexual shoots (%) | -0.146 | 0.404 | -0.36 | 0.7183 | -1.233 | 0.724 | -1.70 | 0.0885 |
Discussion
Asexual reproduction by specialized gemmae in the epiphytic mosses C. afzelii and S. incompletus is influenced by rainfall and the species’ intrinsic ability to produce gemmiferous shoots. These results are among the first quantitative estimates of moss gemma output in a tropical environment.
Moss species differed in tree preferences and may indicate a significant effect on moss colonization. For instance, C. afzelii grows on trees with bark that maintains a humid microhabitat where propagules are more likely to germinate (Barkman 1958). Similarly, S. incompletus occurs on trees with high WHC. Both species inhabited trees with relatively neutral pH (6-7.7), which are conditions reported to favour gemma germination (Löbel & Rydin 2010). Even though these observations are noteworthy, a different sampling approach is needed to accurately evaluate the host tree preferences of moss species.
For C. afzelii, gemmae seemed to be the only means of persistence at Agua Blanca State Park; in contrast, S. incompletus relies on both gemmae and sporophytes to reproduce (Table 2). Based on the total moss cover and the estimated gemma output per cm2 (Table 2), C. afzelii and S. incompletus populations at the study site can produce a mean of 514,066 ± 84,504 and 790,020 ± 1,179,045 gemmae every two months. Gemma output per unit area was positively correlated with moss species’ percentage of gemmiferous shoots. The recorded percentage of gemmiferous shoots for C. afzelii and S. incompletus are similar to those reported for other congeneric species, such as C. mitrafugax Florsch. (60 %) and S. fimbriatus Mitt (28 %) in an Amazonian forest in Brazil (Pereira et al. 2016).
The absence of sporophytes of C. afzelii in Agua Blanca Park seems to be a common feature for North American populations (Reese 2007). Regression models also indicate that the presence of sexual structures is related to higher gemmae production in C. afzelii. However, this positive relationship is unclear to interpret, and it may reflect the recurrent gemmae production of this species regardless of the shoot condition. For S. incompletus, the negative correlation between the number of gemmae and presence of sexual structure, although not significant, has also been reported in other Calymperaceae, with gemmae being less numerous in shoots producing archegonia (Pereira et al. 2016). This pattern is more evident in the tropical moss Fissidens flaccidus Mitt., where shoots with a high reproductive allocation, in terms of sexual structure weight, produce fewer gemmae (dos Santos et al. 2022). In addition, the authors also found sex differences for gemma output, with males producing fewer gemmae than females. Sex-specific traits are well known in other mosses (e.g., McLetchie & Puterbaugh 2000, Brzyski et al. 2014, Slate et al. 2017, dos Santos et al. 2022) and could help understand tropical mosses’ reproductive ecology better.
Gemma production through an annual period depended on rainfall for both species, as shown in the temporal production plot (Figure 4) and the regression models (Tables 3-4). This pattern has also been reported for C. afzelii in Western Africa (Odu & Owotomo 1982) and other Calymperaceous species (Egunyomi & Olarinmoye 1983, Reese 1984, Cerqueira et al. 2016). In addition, we hypothesize that some of the registered gemma output may reflect events of dispersal due to rainfall rather than low gemma production, such as the case of January 2016 for S. incompletus (Figure 4). Once gemmae are formed, they can be ejected by the mechanical action of rainfall (Egunyomi & Olarinmoye 1983, Pohjamo et al. 2006). Interestingly, gemmae resist dry environmental conditions before germinating, which can confer an advantage during months with low precipitation (Coe et al. 2021).
The number of gemmiferous shoots is a good predictor of gemma output per unit area in both species, as demonstrated by the regression models, and it has been exemplified in the liverwort Lophozia ventricosa (Dicks.) (Algar-Hedderson et al. 2013). Additionally, high shoot density may facilitate water retention in the colony and maintain suitable humid conditions for gemma development and persistence before dispersal.
Our results exemplify how rainfall and colony features influence the production of asexual propagules in two tropical mosses with different reproductive strategies. For both moss species, sex ratios, sex-specific traits and phenology data are desired to complement the information on asexual reproduction and its ecological implications. Overall, propagule production (sexual or asexual) is the first stage of dispersal and identifying its controlling variables will serve to understand the population dynamics of epiphytic mosses in tropical environments.











 nueva página del texto (beta)
nueva página del texto (beta)



