The rivers and vegetation that grows along their banks create an ecologically important system (Nilsson & Svedmark 2002). The rivers are continuous systems that redistribute water, nutrients, sediments, and energy throughout the landscape from the upper basin to the middle and lower zones (Nilsson & Svedmark 2002). Along the riverbanks, terrestrial and aquatic communities interact depending on the altitudinal zonation in which they are found (Garrido et al. 2010). The rivers and their associated vegetation are considered highly complex natural systems whose spatial and temporal dynamics determine the ecosystems’ functioning (Cotler 2010, Semmens & Ancona 2019).
The riverbanks are transitional zones between terrestrial, aquatic, and underground systems, where rich and heterogeneous plant communities known as riparian vegetation are formed (Postel & Richter 2003, Parker et al. 2016, Cabette et al. 2017, Maseyk et al. 2017). Therefore, riparian vegetation communities are considered high biodiversity concentration sites that also work as biological corridors (Bennett et al. 2014).
The altitudinal zonation of the basins affects different characteristics, such as water quality, organic matter accumulation, ecosystem processes, and plant communities’ composition (Chen et al. 2017). The riparian vegetation is formed by generalist and specialist species. The generalist species are found throughout the altitudinal rank because of their capacity to tolerate different elevations, temperatures, and flood patterns (Nilsson & Svedmark 2002). Besides elevation, the characteristics of the vegetation are also affected by hydrological and geomorphic properties like the flood regime, soil types, sediment deposition, and slope, which influence the soil’s humidity and nutrient content, among others (Bendix & Hupp 2000).
Rivers and riparian vegetation offer relevant environmental benefits like water supply, sediment retention, and absorption of pollutants from human activities such as agrochemicals and household wastewater (Anbumozhi et al. 2005, Flores-Díaz et al. 2018). In addition, riparian vegetation helps control floods, keeps the tributary rivers’ structure, and serves as a habitat for wildlife, having a high impact on the ecosystems’ functioning, soil’s fertility, and water balance (Robles-Diaz-de-Leon & Kangas 1998, Postel & Richter 2003, Cotler et al. 2010, Bennett et al. 2014).
Riparian vegetation zones are rich in resources for developing productive activities, which results in highly disturbed ecosystems worldwide (Flores-Díaz et al. 2014). However, in agricultural landscapes riparian vegetation is frequently a remnant of the original vegetation displayed as vegetation stripes linked to the riverbanks’ topography. The vegetation composition of these remnants differs from that in adjacent areas due to a higher presence of invasive or human-introduced species in anthropic landscapes (Nilsson & Svedmark 2002).
In Mexico, 45 % of riparian corridors are degraded due to hydrologic alteration of the rivers, urbanization, extraction of riverbank materials, and disorderly use of land (Garrido et al. 2010). These disturbances occur even in areas destined for the conservation of biological diversity and ecosystem services. This work analyzes part of the Sierra Chincua Priority Terrestrial Region (Arriaga et al. 2000) that belongs to the Monarch Butterfly Biosphere Reserve (MBBR), one of the most emblematic protected areas of Mexico.
The MBBR is located in the central part of the country at the state border between Michoacán and the State of Mexico. It is characterized by a volcanic relief covered by temperate forests that serve as shelter for millions of monarch butterflies (Danaus plexippus L.) who migrate from Canada and the United States to overwinter (Brower 1999). The MBBR is part of the Trans-Mexican Volcanic Belt physiographic province, which is characterized by its biological richness and endemism of different groups of organisms such as amphibians and plants (Flores-Villela 1993, Rzedowski 1991, Delgadillo et al. 2003). The unique biodiversity of this physiographic province is composed of elements from the Holarctic and Neotropical biogeographic regions (Cornejo-Tenorio et al. 2003). The dominant vegetation types are coniferous forests, including sacred fir forests (Abies religiosa (Kunth) Schltdl. & Cham. and pine forests (Pinus spp.), associations of pine-oak forests (Pinus spp., Quercus spp.), a few remnants of mountainous cloud forest, scrublands, and grasslands (Giménez et al. 2003, Cornejo-Tenorio & Ibarra-Manríquez 2008, Carbajal-Navarro et al. 2019).
The territory of the MBBR is divided into more than 100 properties, mainly of communal lands owned by ejidos and indigenous communities (Ramírez et al. 2019b). These communities have used their territories and resources since pre-Hispanic times (Farfán et al. 2007, Ramírez et al. 2019a, Venegas-Pérez et al. 2020), making the reserve a site of high biocultural significance. Therefore, it is protected under a Biosphere Reserve category to harmonize biological conservation and socioeconomic development. It is also divided into a core zone, where human activities are restrained, and a buffer zone, where sustainable productive activities are allowed (CONANP 2001). Nevertheless, the reserve faces complex natural, socioeconomic, and political conditions that threaten the conservation objectives (Barkin 2003, Honey-Rosés 2009, Carbajal-Navarro et al. 2019, Guzmán-Aguilar et al. 2020).
The reserve’s management plan has not included the riparian vegetation as an object of conservation, nor are there any published reports about its conservation condition. Therefore, due to the lack of information about this essential element in the landscape, we present in this paper an ecological characterization of the riparian vegetation of the Senguio Microbasin in the state of Michoacán, which is one of the critical areas for the location of monarch butterfly’s overwintering colonies.
The objective of our work was to characterize the riparian vegetation of this microbasin by analyzing its composition, structure, and diversity in relation to its protection status (core zone, buffer zone, and influence area outside the reserve) and land use categories (conserved forests, disturbed forest, farmland). This information reinforces the importance of riparian systems of the reserve and could be considered for further management actions.
Materials and methods
Study area. The Senguio Microbasin is located in the municipality of Senguio, in eastern Michoacán, Mexico, has an extension of 8,610 ha, part of which is included in the core (1,740 ha) and buffer (2,274 ha) zones, its remaining area being outside the reserve. It ranges in elevation from 2,100 to 3,300 meters and is covered by temperate fir forests (Abies religiosa) in elevations above 2,800 meters, mixed pine (Pinus sp.) and oak (Quercus sp.) forests at mid-elevations, and farmland and human settlements at the bottom (Figure 1). The top and mid-elevations are part of a complex volcanic mountain range, where andosols and luvisols sustain the natural vegetation, and the bottom area is a valley with flat slopes where farmland activities are developed, mainly on vertisols, and, to a lesser extent, on luvisols (INEGI 2001). The river network of the Senguio Microbasin is formed by 14 perennial streams, having a total longitudinal extent of 66.1 km. The longest stream is the Ailes river (17.3 km), followed by the Agua Caliente, Los Yugos, Paso de Arrieros, San Ramón, Senguio, and Zapatero, which range in length from 6 to 8 km. The top of the Zapatero river is one of the critical areas for the establishment of monarch butterfly overwintering colonies (Vidal & Rendón‐Salinas 2014, Saunders et al. 2019).
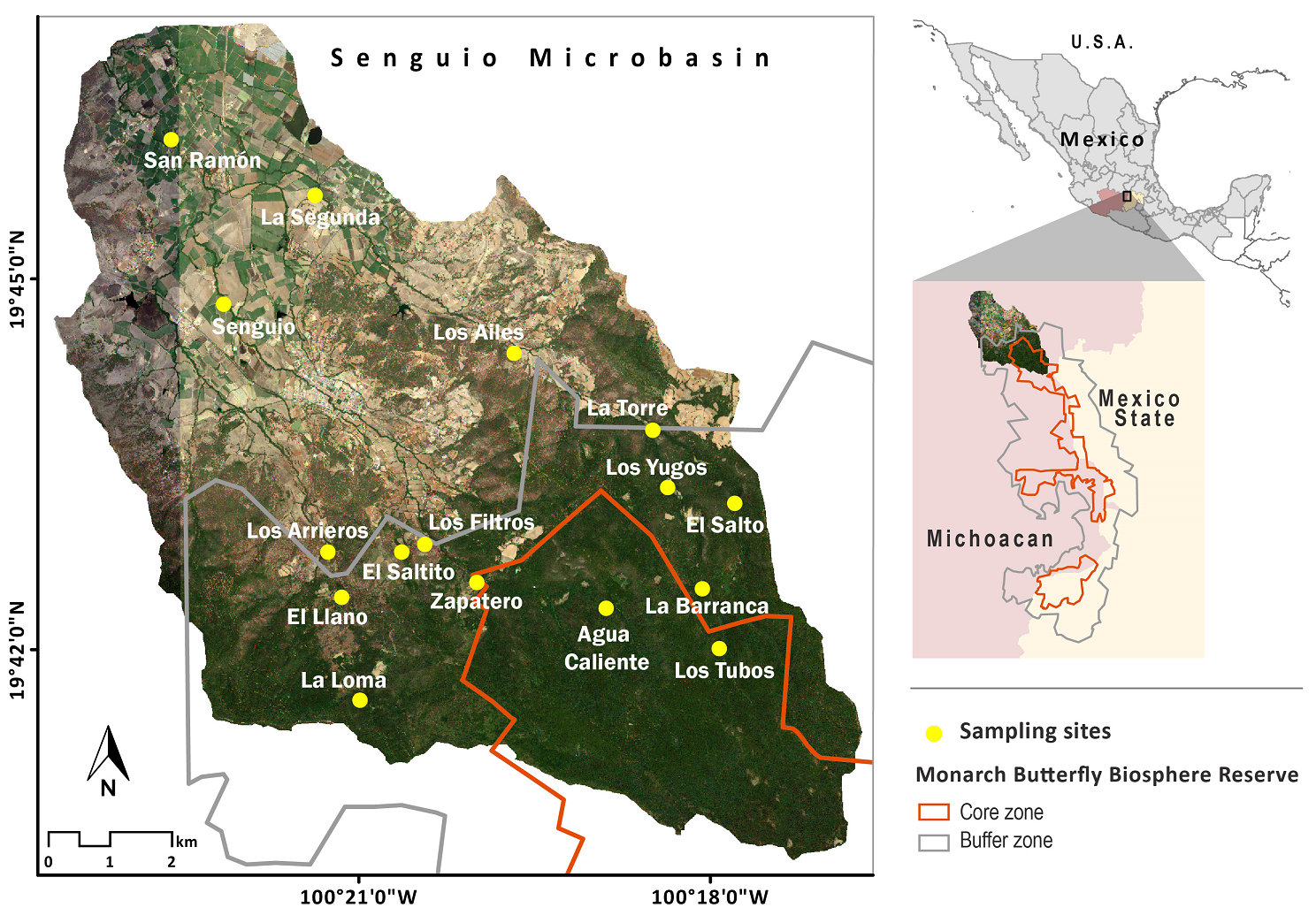
Figure 1 Location of the Senguio Microbasin within the Monarch Butterfly Biosphere Reserve, and distribution of sampling sites.
Management categories. We generated a land-use map through visual interpretation of a Spot 5 image from 2014. We merged the multispectral bands with the panchromatic image to obtain a 5 meters resolution pan-sharpened image using Qgis software. The delimitation of the microbasin was obtained from a series of GRASS Hydrology tools available in Qgis software. The polygons of the reserve’s core and buffer zones were downloaded from the CONANP (2001) web page, and the perennial rivers from INEGI (2001).
The 1,200 meters elevation range of the studied microbasin covers land use (conserved forests, disturbed forests, shrubland, farmland, human settlements) and protection status (core zone, buffer zone, influence area outside the reserve) gradients. The highest part of the microbasin corresponds to the core zone of the MBBR and is covered by conserved forests (CCZ); the middle part corresponds to the buffer zone that contains both conserved forests (CBZ) and highly disturbed forests and areas open to agriculture and pasture (DBZ). The lowest part is in the influence area outside the MBBR. It is covered by fragments of conserved forest (CIA) as well as a mix of human settlements, agriculture and grassland, croplands, and patches of disturbed forests (DIA) (Figures 2 and 3).
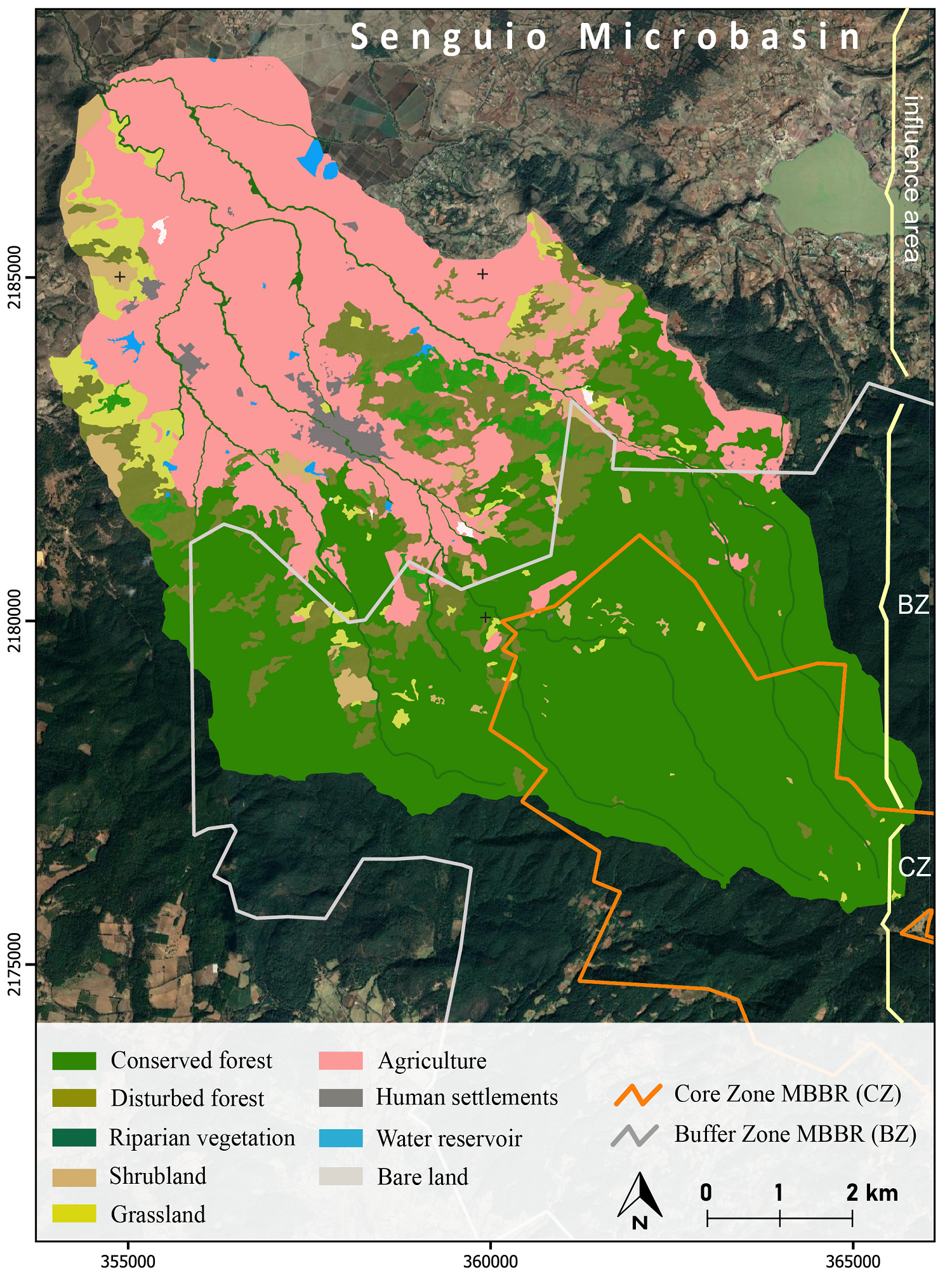
Figure 2 Land cover and land use map of the Senguio Microbasin, Monarch Butterfly Biosphere Reserve, 2014.
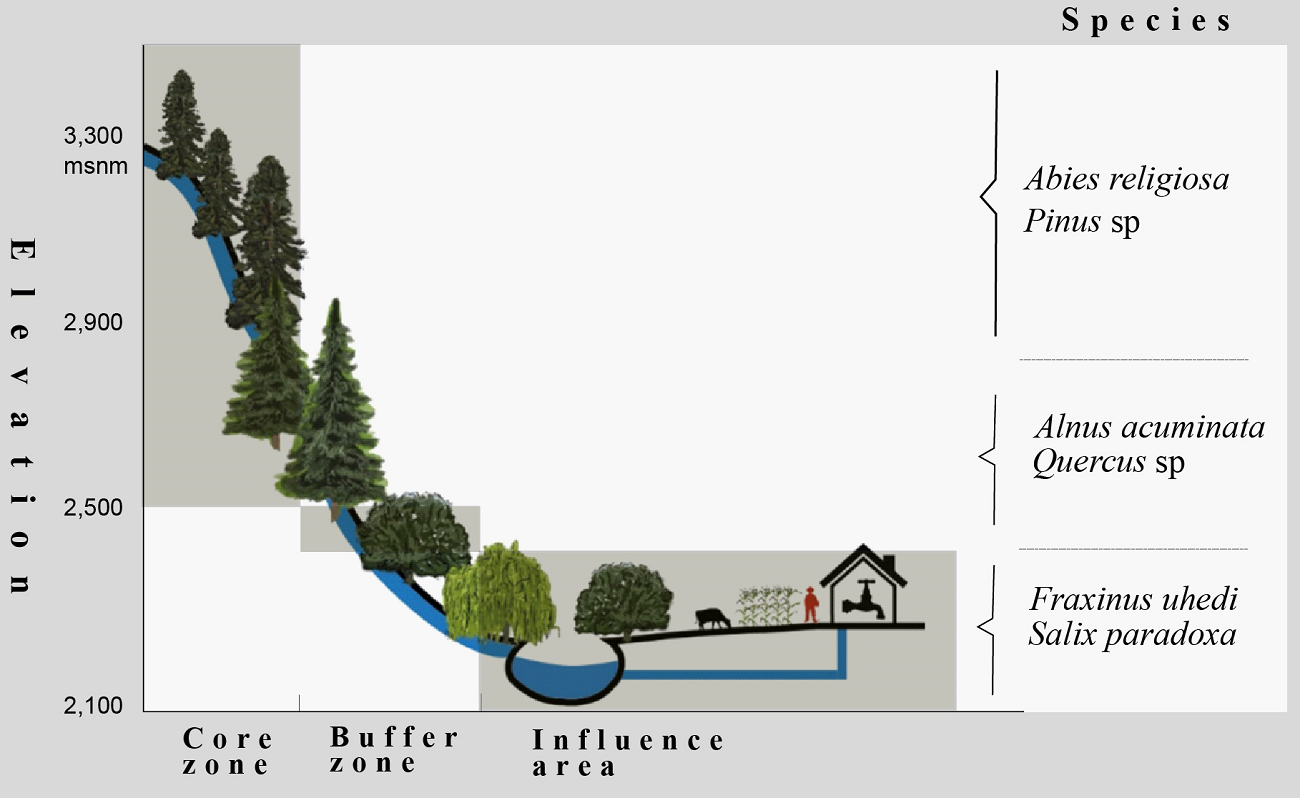
Figure 3 Profile of the riparian vegetation along the elevation and protection-management gradients. (Illustration: Vito Vangeles)
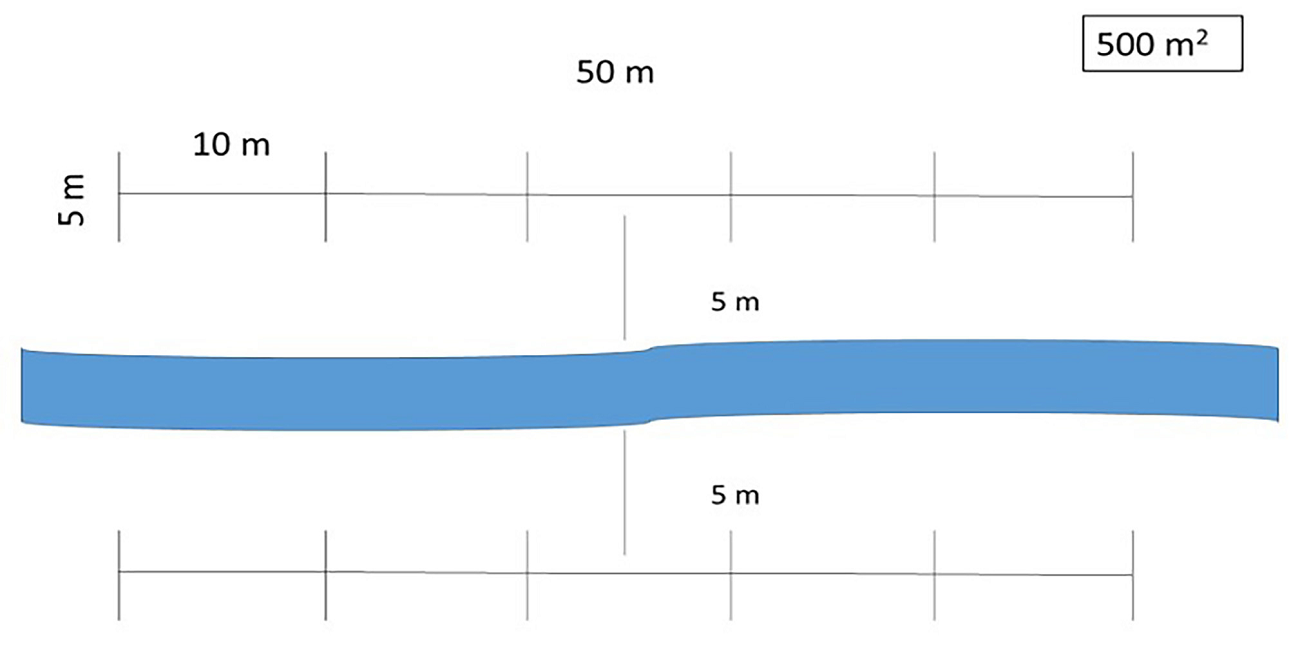
Vegetation sampling.- From February 2016 to February 2017, the sampling of the riparian vegetation was conducted, including three replicas from each of the previous five categories (Table 1). Sampling of the vegetation followed the 500 m2 quadrat approach (Valiente-Banuet et al. 2000, Vallejo et al. 2014, 2015, Zermeño-Hernández et al. 2020) adjusting sampling sites to cover both sides of the riverbed (Figure 4). All the specimens of woody species were recorded, and the height and diameter of their canopies were estimated. The diameter at breast height (DBH) was measured for woody plants with trunks over 1 cm in diameter. Most species were identified in situ or vegetative parts were collected for taxonomic identification in the laboratory to avoid specimen extraction.
Table 1 Sampling design
| Number of sites | Protection status | Land use | Abbreviation |
|---|---|---|---|
| 3 | Core zone | Conserved forest | CCZ |
| 3 | Buffer zone | Conserved forest | CBZ |
| 3 | Buffer zone | Highly disturbed forest and farmland | DBZ |
| 3 | Influence area | Conserved forest | CIA |
| 3 | Influence area | Highly disturbed forest, farmland and human settlements | DIA |
Statistical analysis. We calculated the Importance Value Index (IVI) for each species and protection and management category. IVI is a qualitative indicator, calculated by adding relative frequency, abundance, and biomass percentages, that shows species composition and identifies the most important species (Vallejo et al. 2014). The biomass was estimated using the height and crown radius values of each species of shrubs and trees, in the case of trees, considering the DBH.
The analyzed parameters were richness, composition, α and β diversity, and plant community structure. We separated shrubs data from the trees for the data analysis because they have different ecological processes (biomass, photosynthetic rate, etc.) on their distribution along with the riparian vegetation. To assess differences in community structure and species composition, we calculated α and β diversity levels metrics.
We verified the effectiveness of the sampling effort by estimating the sample coverage for each site (Chao & Jost 2012) using iNext software (Hsieh et al. 2016). This estimator is sensitive to species with one (singletons) or two (doubletons) individuals (Chao & Jost 2012). The sample coverage varied from 0.92 to 0.99 for trees and from 0.89 to 0.99 for bushes (Table 2). The sample coverage mean values for the five categories and the group of plants (trees or bushes) were higher than 0.94 (Table 2). Therefore, the sampling effort was adequate, and the results were not biased by the differences in the integrity of the sampling among the sites (Chao & Jost 2012).
Table 2 Trees and shrubs sample coverage, number of individuals, species richness (0D), common species ( 1 D), and dominant species (2D) total and mean values with standard error for the five land use categories.
| CCZ (n = 3) | CBZ (n = 3) | DBZ (n = 3) | CIA (n = 3) | DIA (n = 4) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mean | Total | Mean | Total | Mean | Total | Mean | Total | Mean | ||
| Trees | Chat | 0.99 | 0.97 ± 0.01 | 0.99 | 0.96 ± 0.2 | 0.98 | 0.96 ± 0.2 | 0.98 | 0.96 ± 0.02 | 0.99 | 0.98 ± 0.00 |
| N | 234 | 78 ± 14.18 | 191 | 63.6 ± 25.69 | 167 | 55.66 ± 8.69 | 163 | 54.33 ± 30.02 | 354 | 88.5 ± 3.75 | |
| 0D | 16 | 8 ± 1.73 | 18 | 10.3 ± 1.86 | 13 | 7.33 ± 1.33 | 15 | 7.66 ± 2.40 | 9 | 7.25 ± 0.25 | |
| 1D | 7.37 | 4.21 ± 1.49 | 10.33 | 5.95 ± 0.45 | 8.24 | 4.68 ± 1.36 | 8.81 | 5.07 ± 1.60 | 5.17 | 4.53 ± 1.7 | |
| 2D | 4.69 | 3.10 ± 0.97 | 6.38 | 4.42 ± 0.87 | 7.05 | 3.84 ± 1.24 | 4.78 | 4.01 ± 1.47 | 4.09 | 3.60 ± 0.21 | |
| Shrubs | Chat | 0.98 | 0.96 ± 0.03 | 0.99 | 0.98 ± 0.00 | 0.98 | 0.96 ± 0.01 | 0.98 | 0.98 ± 0.01 | 0.97 | 0.95 ± 0.01 |
| N | 486 | 162 ± 156.92 | 622 | 207.33 ± 61.67 | 307 | 102.33 ± 1.76 | 379 | 126.33 ± 21.40 | 396 | 99 ± 20.37 | |
| 0D | 29 | 12.33 ± 3.28 | 27 | 13.33 ± 3.17 | 19 | 9.33 ± 1.45 | 20 | 10 ± 2.08 | 29 | 10.75 ± 2.09 | |
| 1D | 9.82 | 6.46 ± 2.35 | 10.72 | 7.14 ± 1.03 | 8.46 | 5.08 ± 1.10 | 10.92 | 6.11 ± 1.13 | 9.49 | 5.27 ± 0.84 | |
| 2D | 6.61 | 4.68 ± 1.60 | 7.03 | 5.4 ± 0.45 | 5.32 | 4 ± 1.11 | 9.27 | 5.08 ± 0.92 | 6.17 | 3.99 ±0.69 | |
For α diversity, we calculated on each site Hill numbers (Chao et al. 2006) that have the same unit (the effective number of species) and are also useful to evaluate diversity patterns with a different number of individuals. Particularly, we considered the Hill numbers with an order of 0 ( 0 Dα, richness of species) that are non-sensitive to the abundance of species and provide a disproportionate weight to the exotic species; with an order of 1 ( 1 Dα, Shannon exponential entropy) that can be interpreted as the numbers of common species in the communities; and with an order of 2 ( 2 Dα, Simpson inverse concentration) which favor the dominant species and are, consequently, interpreted as the numbers of very abundant or dominant species in the community (Chao et al. 2006).
To establish differences in the species turnover among five management categories, we estimated β diversity according to Hill numbers: q Dβ = q Dγ / qDα, where qDγ is the accumulated number (regional) of species in the total number of samplings and qDα is the average number of species per category of protection. The qDβ is interpreted as the “effective number of totally different communities” (Jost 2007), and it varies from 1 (when all the sites or communities are identical) and N (when all the N sites are entirely different from each other). To explore the differences in the β diversity, we first compared the species turnover among the five management categories. Finally, the turnover rate was calculated within each category, building a matrix that has β diversity values from each pair compared to each protection category. Finally, the taxonomic metrics of α and β diversity were estimated utilizing the package “Entropart” for R (Marcon & Herault 2015).
Generalized linear models (GLM) were used to evaluate the different attributes of the community and the number of individuals ( 0 Dα, 1 Dα, 2 Dα) in the five management categories. We also tested a second model, adding the altitude as a cofactor to determine whether our response variables influenced that variable. We used a Gaussian error propagation for 1 Dα and 2 Dα and a Poisson error distribution for the number of individuals and richness of species ( 0 Dα). In the cases where the model obtained high values of over dispersion (> 2.0), a Quasi-Poisson error distribution was calculated (Crawley 2013).
Differences on community structure along management categories gradient were assessed using Non-Metric Multi-Dimensional Scaling (MDS) that considers the distance-based measures of Chao (Chao et al. 2005). This analysis reduces the dimensional space and arrange relationships among management categories according to the composition of trees or bushes and number of individuals per specie (relative abundance). Finally, we applied permutational MANOVA (function ADONIS) to determine whether vegetation land use explained a significant amount of variation in the community structure of trees and shrubs. All statistical analyses were performed using R software “Vegan: Community Ecology Package” (Oksanen et al. 2020).
Results
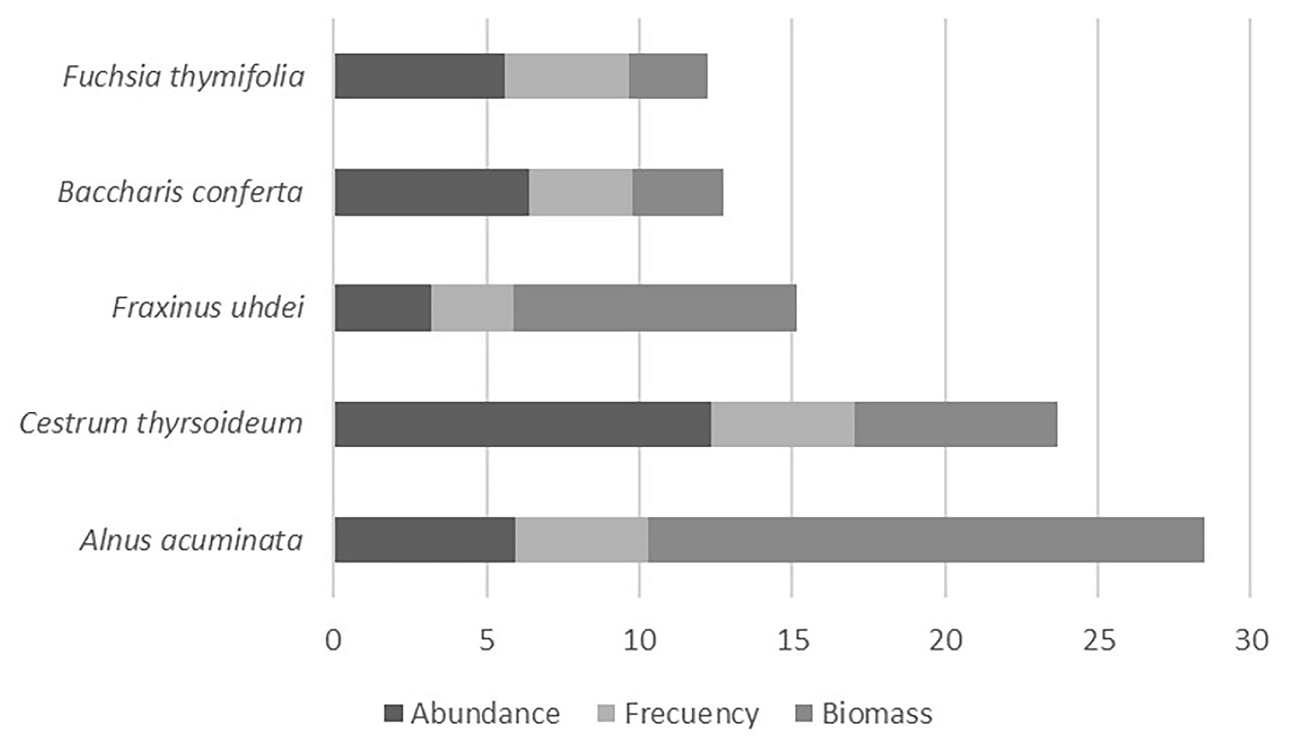
We registered 99 species of woody plants that belong to 68 genera and 38 families (Table 3). The dominant family was Asteraceae, followed by Solanaceae (Figure 5). The most abundant genera were Cestrum L. (Solanaceae), Senecio L. (Asteraceae) and Quercus L. (Fagaceae). The most important species based on their IVI were Alnus acuminata Kunth (Betulaceae) and Cestrum thyrsoideum Kunth, due to their biomass and abundance and frequency, respectively (Figure 6). The 99 % of the registered species are native being two individuals of Vinca major we recorded in the DBZ the only exotic plants (1 %).
Table 3 List of riparian vegetation species and the management unit was sampled in the Senguio micro-basin, Michoacán. Monarch Butterfly Biosphere Reserve.
| Family | Species | Management Unit |
|---|---|---|
| Amaranthaceae | Iresine diffusa Hum. & Bonpl. Ex Willd | DBZ |
| Apocynaceae | Vinca major L. | DBZ |
| Aquifoliaceae | Ilex discolor Hemsl | CCZ, CBZ |
| Araliaceae | Oreopanax echinops (Schltdl. & Cham.) Decne. & Planch. | CIA |
| Oreopanax xalapensis (Kunth) Decne. & Planch. | CCZ, CBZ | |
| Asparagaceae | Agave sp. | DIA |
| Betulaceae | Alnus acuminata Kunth | CCZ, CBZ, CIA, DBZ, DIA |
| Cactaceae | Opuntia sp. | DIA |
| Caprifoliaceae | Symphoricarpos microphyllus (Humb. & Bonpl. ex Schult.) Kunth | DIA |
| Sambucus nigra L. | CBZ | |
| Clethraceae | Clethra mexicana DC. | CCZ, CBZ, CIA |
| Cistaceae | Helianthemum glomeratum Lag. ex DC. | DIA |
| Asteraceae | Ageratina glabrata (Kunth) R.M.King & H.Rob. | CIA, DIA |
| Ageratina mairetiana (DC.) R.M.King & H.Rob. | CCZ, CBZ | |
| Ageratina pichinchensis (Kunth) R.M.King & H.Rob. | CBZ, DBZ | |
| Archibaccharis hirtella (DC.) Heering | CCZ, CBZ, CIA | |
| Baccharis conferta Kunth | CCZ, CBZ, CIA, DBZ, DIA | |
| Baccharis heterophylla Kunth | CCZ, CBZ, CIA, DBZ, DIA | |
| Coreopsis petrophiloides B.L.Rob. & Greenm. | DIA | |
| Montanoa grandiflora (DC.) Sch.Bip. ex Hemsl. | CIA, DIA | |
| Piqueria pilosa Kunth | CBZ | |
| Rumfordia floribunda DC. | DBZ | |
| Senecio albonervius Greenm. | CCZ, CIA, DBZ | |
| Senecio angulifolius DC. | CCZ, CBZ | |
| Senecio barba-johannis DC. | CBZ | |
| Senecio callosus Sch. Bip. | CIA | |
| Senecio cinerarioides Kunth | CBZ, DBZ, DIA | |
| Senecio stoechadiformis DC. | CIA, DIA | |
| Sigesbeckia jorullensis Kunth | CIA | |
| Stevia monardifolia Kunth. | DIA | |
| Stevia salicifolia Cav | DIA | |
| Verbesina grayii (Sch.Bip.) Benth. ex Hemsl. | CBZ | |
| Verbesina oncophora B.L. Rob. & Seaton | CIA, DBZ | |
| Celastraceae | Celastrus pringlei Rose | CCZ |
| Convolvulaceae | Ipomoea arborescens (Humb. & Bonpl. ex Willd.) G. Don | CIA |
| Cornaceae | Cornus disciflora Moc. & Sessé ex DC. | CBZ |
| Cornus excelsa Kunth | CBZ, CIA, DBZ, DIA | |
| Cupressaceae | Cupressus lusitanica Mill. | CCZ, CBZ, DBZ |
| Ericaceae | Arbutus xalapensis Kunth | CCZ, CBZ |
| Arctostaphylos pungens Kunth. | DIA | |
| Chimaphila umbellata (L.) Nutt. | CCZ | |
| Comarostaphylis discolor (Hook.) Diggs | CCZ, CBZ | |
| Fabaceae | Acacia angustissima (Mill.) Kuntze | DIA |
| Desmodium densiflorum Hemsl. | CBZ | |
| Eysenhardtia polystachya (Ortega) Sarg. | CIA | |
| Fagaceae | Quercus castanea Nee | CBZ |
| Quercus gentryi C.H.Mull. | CCZ, CBZ, CIA, DBZ | |
| Quercus laurina Humb. & Bonpl. | CCZ, CIA, DBZ | |
| Quercus rugosa Née | CBZ | |
| Quercus sp. | CCZ, CBZ, CIA, DBZ | |
| Garryaceae | Garrya laurifolia Benth. | CBZ |
| Geraniaceae | Geranium seemannii Peyr. | CBZ, DBZ |
| Grossulariaceae | Ribes ciliatum Humb. et Bonpl. ex Roem et Schult. | CCZ, CBZ, |
| Lamiaceae | Salvia gesneriiflora Lindl. & Paxton | CIA. DIA |
| Salvia mexicana L. | DIA | |
| Clinopodium macrostemum (Moc. & Sessé ex Benth.) Kuntze | CCZ, CBZ | |
| Oleaceae | Forestiera phillyreoides (Benth.) Torr. | DIA |
| Fraxinus uhdei (Wenz.) Lingelsh. | CBZ, DBZ, DIA | |
| Onagraceae | Fuchsia microphylla Kunth | CCZ, CBZ |
| Fuchsia obconica Breedlove | CCZ, CBZ, DIA | |
| Fuchsia thymifolia Kunth | CCZ, CBZ, CIA, DBZ, DIA | |
| Orobanchaceae | Lamourouxia xalapensis Kunth | CCZ, CBZ |
| Pentaphylacaceae | Ternstroemia lineata DC. | CCZ, CBZ, DBZ |
| Pinaceae | Abies religiosa (Kunth) Schltdl. & Cham | CCZ, CBZ |
| Pinus pseudostrobus Lindl. | CCZ, CBZ | |
| Pinus leiophylla Schiede ex Schltdl. & Cham | CBZ | |
| Polygalaceae | Monnina ciliolata DC. | CCZ, CBZ, CIA, DBZ, DIA |
| Ranunculaceae | Clematis dioica L. | CBZ |
| Rhamnaceae | Ceanothus caeruleus Lag | DBZ |
| Frangula mucronata (Schltdl.) Grubov | CBZ | |
| Rhamnus microphylla Humb. & Bonpl. ex Schult. | CIA | |
| Sageretia minutiflora (Michx.) C. Mohr | CIA, DBZ, DIA | |
| Rosaceae | Acaena elongata L. | CCZ, CBZ |
| Crataegus mexicana Moc. & Sessé ex DC. | CBZ, CIA, DBZ, DIA | |
| Prunus prionophylla Standl. | CBZ, DBZ | |
| Prunus serotina Ehrh. | CCZ, CBZ, CIA, DIA | |
| Rubus sp. | CCZ, CBZ, CIA, DBZ, DIA | |
| Rubiaceae | Bouvardia longiflora (Cav.) Kunth | DIA |
| Salicaceae | Salix paradoxa Kunth | DIA |
| Saxifragaceae | Heuchera orizabensis Hemsl | CCZ |
| Scrophulariaceae | Buddleja cordata Kunth | CCZ |
| Buddleja sessiliflora Kunth | DIA | |
| Solanaceae | Cestrum fulvescens Fernald | DIA |
| Cestrum roseum Kunth | CIA, DBZ | |
| Cestrum sp. | CIA | |
| Cestrum thyrsoideum Kunth | CCZ, CBZ, CIA, DBZ, DIA | |
| Cestrum tomentosum L.f. | DIA | |
| Cestrum nocturnumL. | CCZ | |
| Croton adspersus Benth. | CIA, DBZ, DIA | |
| Physalis coztomatl Dunal | DIA | |
| Solanum argentinum Bitter & Lillo | CCZ, CBZ, CIA, DBZ | |
| Solanum nigricans M. Martens & Galeotti | CCZ, CIA, BDZ, | |
| Styracaceae | Styrax argenteus C. Presl | CCZ, CIA, DBZ |
| Verbenaceae | Lantana hirta Graham | CIA, DIA |
| Lippia mexicana G.L.Nesom | CCZ | |
| Sp 1. | DBZ | |
| Sp 2. | CCZ | |
| Sp 3. | DBZ | |
| Sp 4. | DIA |
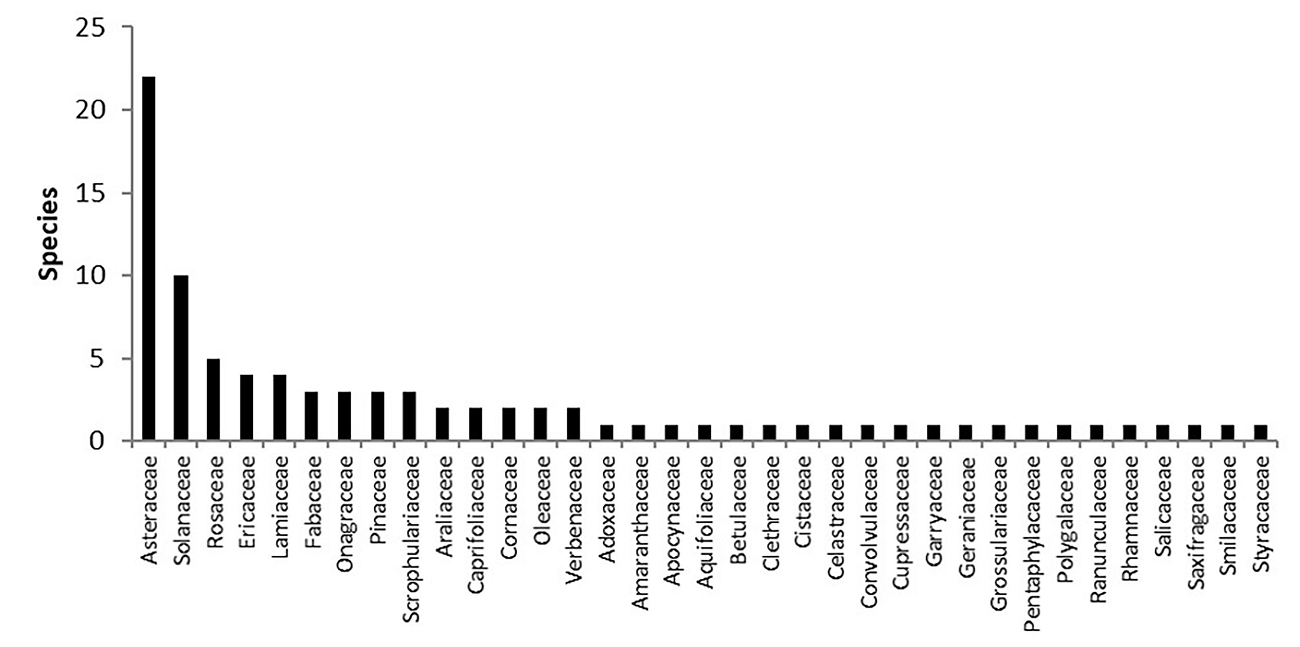
Figure 5 Number of species per family in the Senguio Microbasin, Monarch Butterfly Biosphere Reserve.
We documented the presence of species that indicate disturbance, such as Baccharis conferta Kunth (Asteraceae), present in all management units at abundances agreeing with the management and conservation gradient (CCZ: 5 individuals, CBZ: 32, CIA: 23, DBZ: 40, DIA: 111), being among the top five species due to its abundance and frequency throughout the riparian vegetation of the microbasin. We also registered species that indicate preserved sites, such as Chimaphila umbellata (L.) Nutt. (Ericaceae), restricted to the CCZ; rare species in mid-western Mexico, like Sageretia minutiflora (Michx.) C. Mohr (Rhamnaceae), considered as vulnerable to extinction in the area (Fernández-Nava 1996) (CIA: 23, DBZ: 1, DIA: 91); and species listed in the International Union for Conservation of Nature (IUCN) Red List as vulnerable to extinction, Oreopanax echinops (Schltdl. & Cham.) Decne. & Planch. (CIA:1) (World Conservation Monitoring Centre 1998).
Based on the floristic composition, the main vegetation types in the protection and management gradient were fir forest mixed with pine-oak forest (CCZ, CBZ), oak forest (CIA, DBZ), and secondary vegetation (DIA). However, the presence of Cornus disciflora, Oreopanax xalapensis, O. echinops, and Ilex discolor in the riparian vegetation showed an affinity with the mountain cloud forest, one of the most endangered types of vegetation in Mexico.
We observed changes in the composition of the vegetation along the altitudinal and management gradients. In the highest and most well-preserved area, Abies religiosa (Pinaceae) has the highest IVI among tree species. There, Quercus rugosa (Fagaceae) and Alnus acuminata (Betulaceae) have the highest IVI. In the lowest part outside the reserve, Fraxinus uhdei (Oleaceae) and Salix paradoxa (Salicaceae) are the dominant tree species along with some fruit tree species such as Prunus serotina and Crataegus mexicana species, which suggest human intervention in riparian vegetation composition (Figure 7).
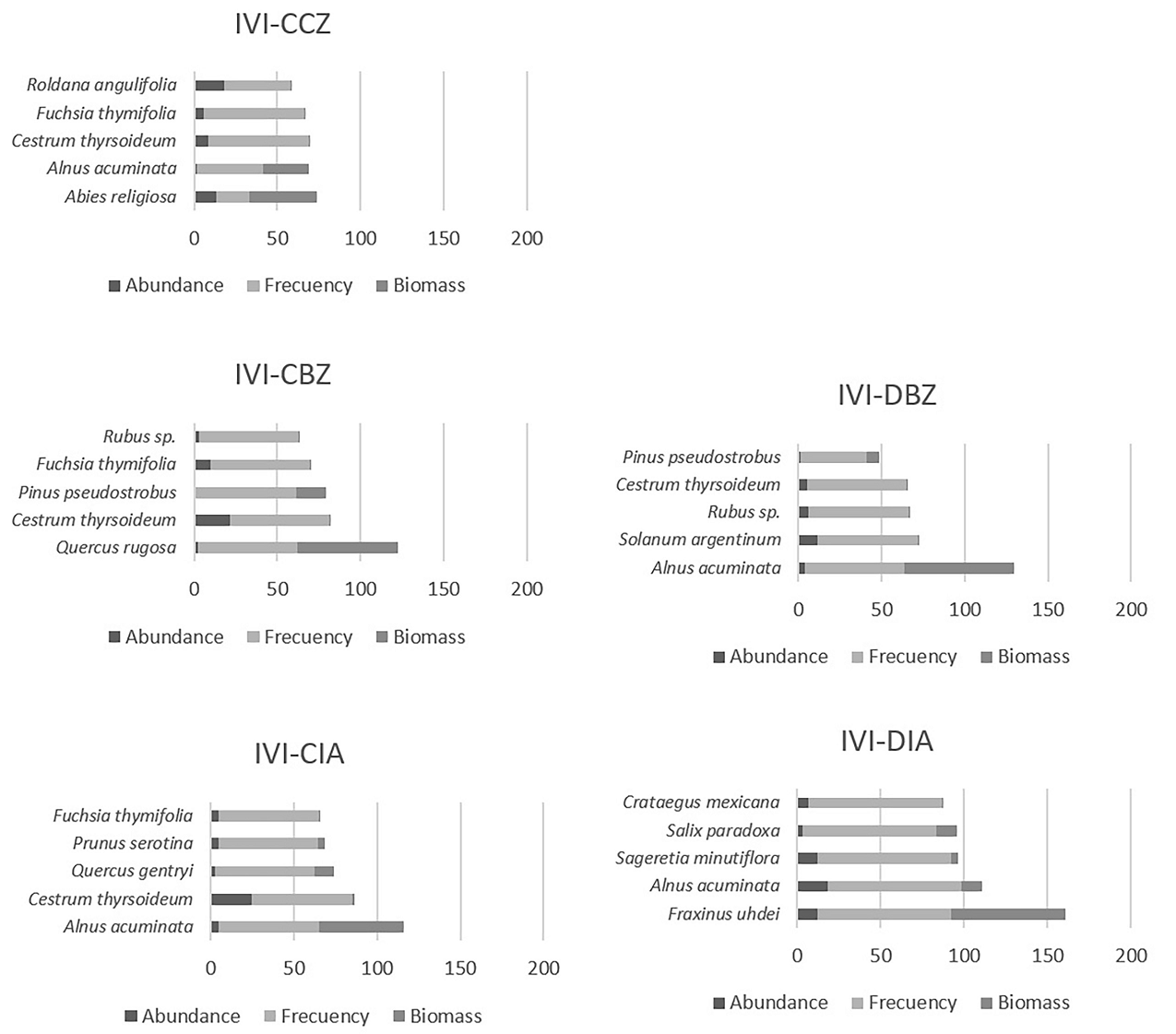
Figure 7 Importance Value Index (IVI) for each protection and management category. Conserved forest in the core zone (CCZ), conserved forest in the buffer zone (CBZ), highly disturbed forest, or farmland in the buffer zone (DBZ), conserved forest in the influence area outside the reserve (CIA), and highly disturbed forest, or farmland in the influence area outside the reserve (DIA).
We observed no significant difference among management categories when analyzing the richness, diversity, and structure among sampling sites and considering all the tree and shrub individuals because they varied widely (Figure 8). The sampling sites within the core zone (CCZ) did not have the highest number of species because of the dominance of Abies religiosa. We recorded there three sites with 32, 27, and 9 species, respectively. The site with the highest species richness was within the conserved buffer zone (CBZ) with 35 species.
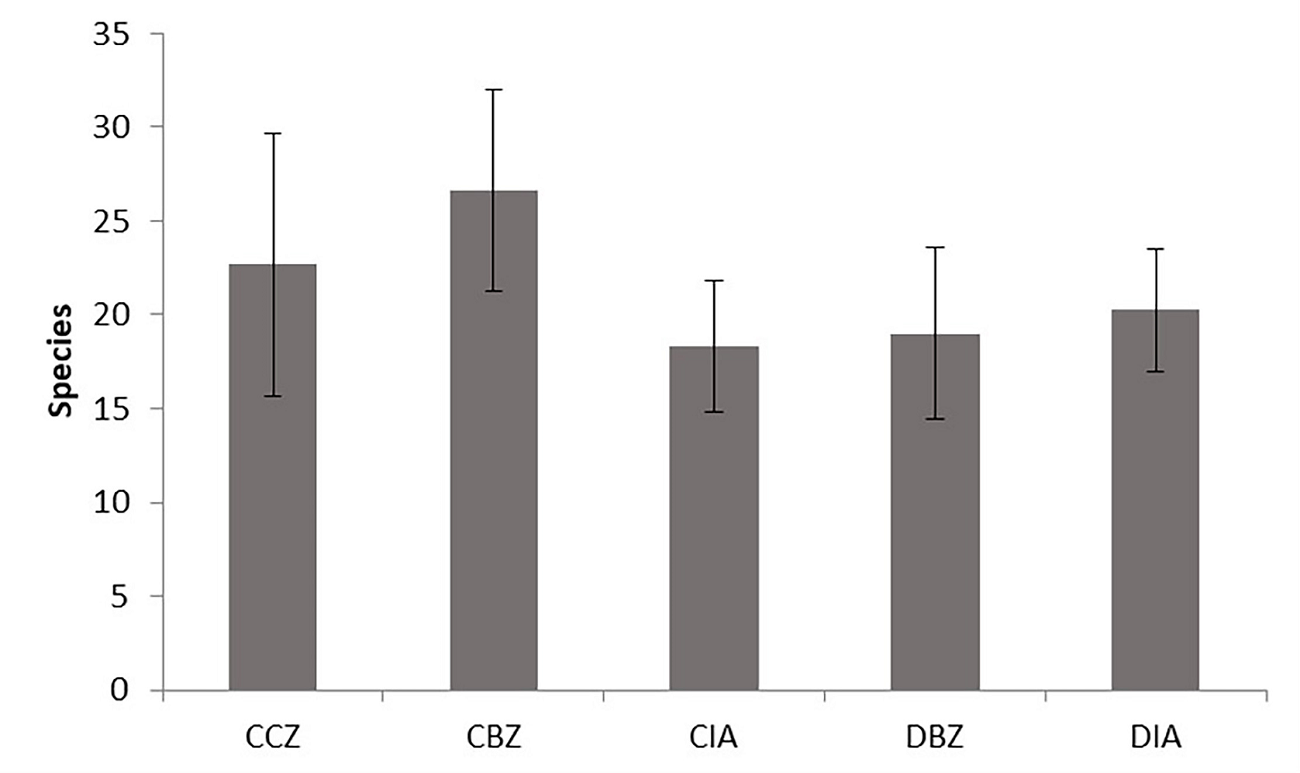
Figure 8 Average species richness for each protection and management category. Conserved forest in the core zone (CCZ), conserved forest in the buffer zone (CBZ), conserved forest in the influence area outside the reserve (CIA), highly disturbed forest, or farmland in the buffer zone (DBZ), and highly disturbed forest, or farmland in the influence area outside the reserve (DIA).
We recorded a total of 1,109 individuals of 35 tree species and 2,190 individuals of 73 shrub species. Both the taxonomic metrics of α-diversity and the values of the number of individuals fluctuated amply within the same category; therefore, the differences were not significant (Table 2, Figure 9). For the tree community, the DIA category showed the lowest value of species richness ( 0 D), common ( 1 D) and dominant ( 2 D) species. However, this category with CCZ presented the highest number of individuals (Table 2) and was statistically different from the other categories. For the shrub community, the number of individuals in the CBZ and CCZ categories was higher than in the other three protection categories. We found no differences in ‘real diversity’ metrics among the protection and land use categories (Figure 9).
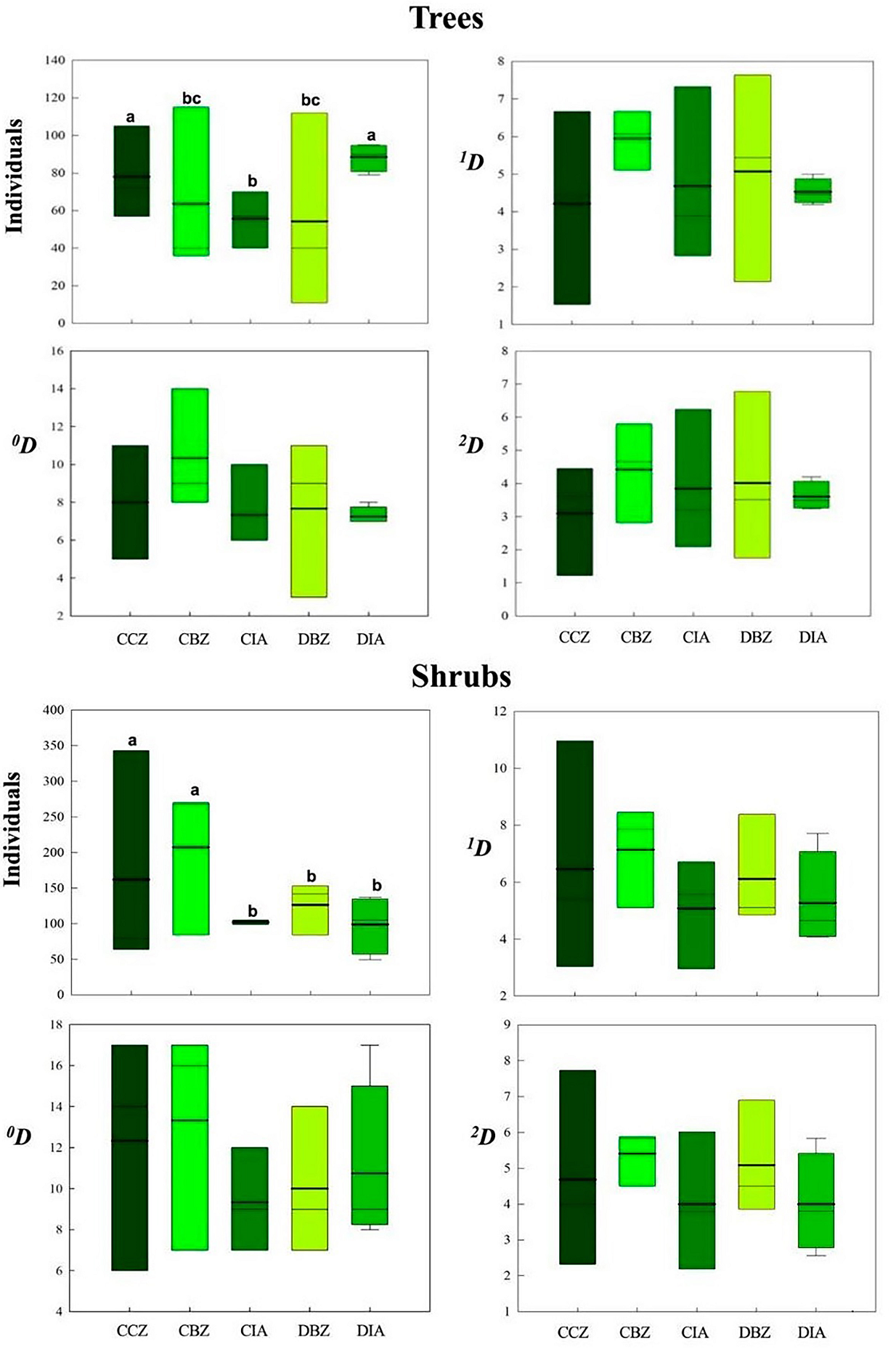
Figure 9 Trees and shrubs 0D, 1D, and 2D α-diversity values and number of individuals for each preservation and management category. Conserved forest in the core zone (CCZ), conserved forest in the buffer zone (CBZ), conserved forest in the influence area outside the reserve (CIA), highly disturbed forest, or farmland in the buffer zone (DBZ), and highly disturbed forest, or farmland in the influence area outside the reserve (DIA)
The α-diversity values we observed indicating the addition of the individuals and species in each category- showed the highest variation in the DBZ category, in which we recorded a site with less than 20 individuals and another one with over 100 individuals. The α-diversity values in the DIA category were the most consistent, every site having the same number of individuals and species richness values. Regarding the total number of species, the CBZ category showed the highest number of species, followed by the CCZ category. Since the averages in trees or shrubs were similar, there were no significant differences between them.
Regarding the β-diversity values, we found significant differences only in the tree species. The taxonomic β-diversity values among the five protection and management categories were less than two, showing a low species turnover. However, when estimating the β-diversity for the arboreal communities, the DIA turned out to be the least varied, which means that it had the most consistent composition regardless of the diversity, as it substantially decreased in 1 D and 2 D (Figure 10a). The sites in the CIA and DIA categories located outside the MBBR had a lower species turnover-both for common and dominant species- than those in sites within the core and buffer zones of the reserve, which means that the communities in the external sites were similar among sites per category (Figure 10b and c). It is important to mention that the sites in the CCZ category had a higher species turnover for the three diversity orders ( 0 D, 1 D, and 2 D). Regarding the community of bushes, the β-diversity values we observed were similar among categories regardless of the diversity order; however, the sites in the CCZ category showed a higher β-diversity in arboreal communities (Figure 10d, f).
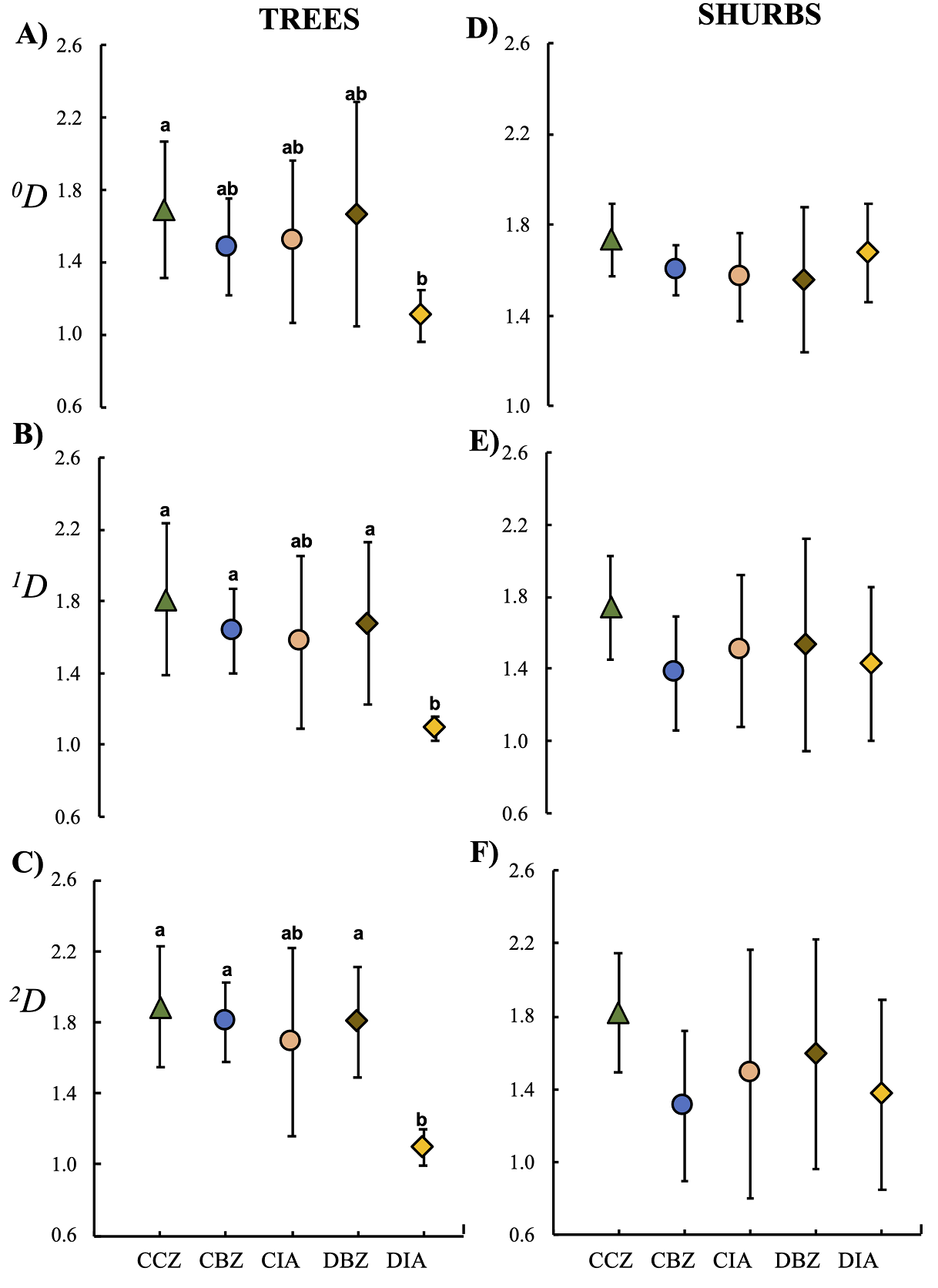
Figure 10 β-diversity (species turnover) of trees (A, B, C) and shrubs (D, E, F) among the five preservation and management categories. Conserved forest in the core zone (CCZ), conserved forest in the buffer zone (CBZ), conserved forest in the influence area outside the reserve (CIA), highly disturbed forest, or farmland in the buffer zone (DBZ), and highly disturbed forest, or farmland in the influence area outside the reserve (DIA).
Based on our β-diversity results, we can state that the sites in the CCZ category were more heterogeneous and showed a higher species turnover both for the common and dominant species. In contrast, the zones outside the reserve and with an important anthropic presence were more homogeneous. When evaluating total composition and species relative abundance, also known as the community structure, we observed that the DIA category differed from the other categories. The Adonis function indicated that those differences were statistically significant for trees (F 4 = 1.94; P < 0.05; Figure 11a) and shrubs (F 4 = 2.94; P = < 0.05; Figure 11b). On this matter, the trees and shrubs community structure in the CCZ category sites was similar to those in the CIA category and the two buffer-zone categories (Figure 11). Analyzing α- and β-diversity separately, we observed no significant differences in bushes. Still, when analyzing the total community structure, the observed pattern indicated that the sites in the DIA category were significantly different, meaning that this category is more homogeneous and separates itself from the remaining categories.
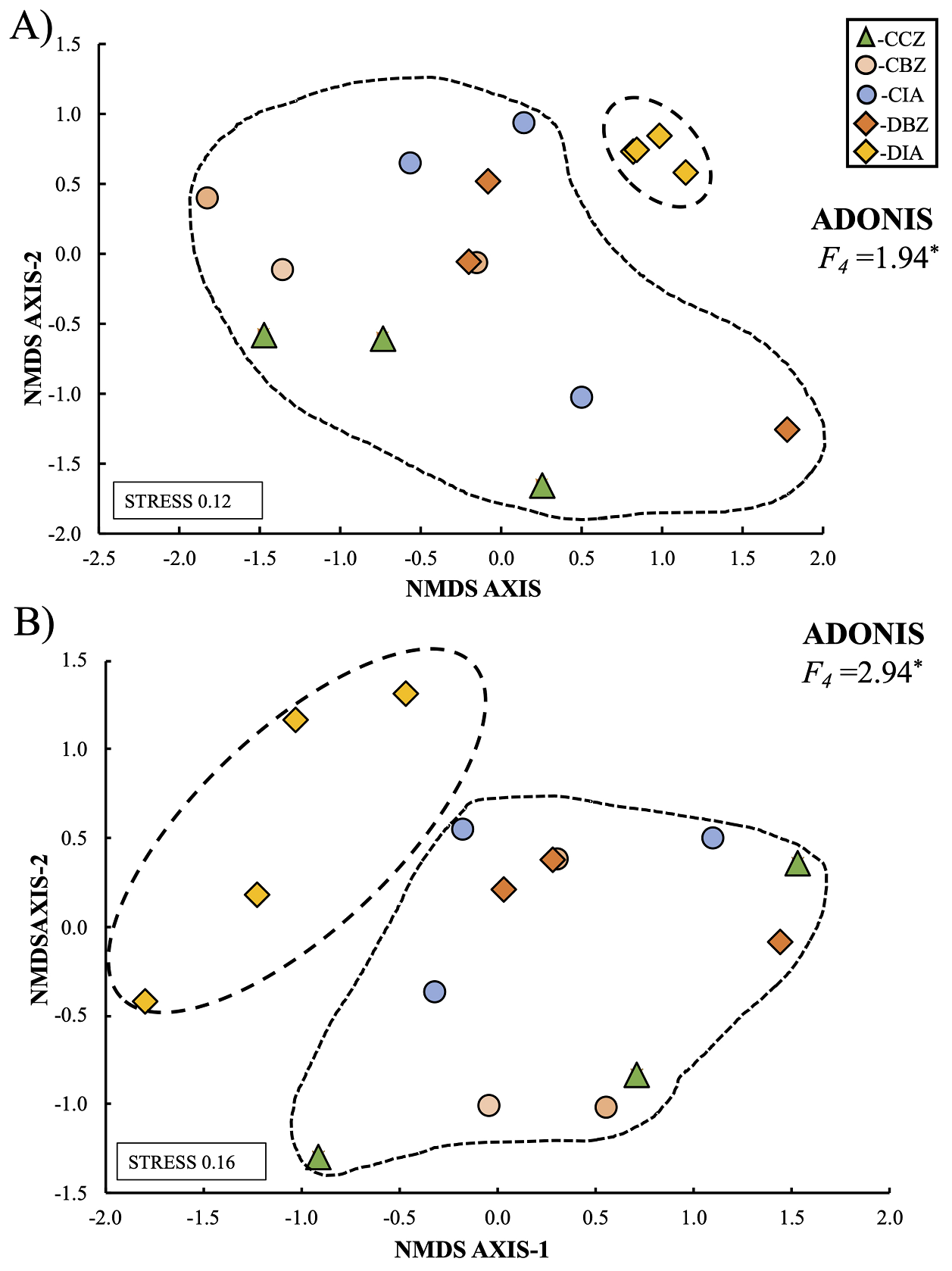
Figure 11 Community structure of trees (A) and shrubs (B) among the five preservation and management categories. Dashed lines indicate different groups in terms of community structure. Asterisks (*) means the level of significance: * = P < 0.05, ** = P < 0.01, *** = P < 0.001. Conserved forest in the core zone (CCZ), conserved forest in the buffer zone (CBZ), conserved forest in the influence area outside the reserve (CIA), highly disturbed forest, or farmland in the buffer zone (DBZ), and highly disturbed forest, or farmland in the influence area outside the reserve (DIA).
Considering richness and number of individuals parameters, we found no significant difference among treatments, which means that riparian vegetation of Senguio Microbasin functions as a well-preserved corridor that connects core and buffer zones with the influence area outside the reserve. Thus, despite anthropic activities, the riparian vegetation maintains high levels of species richness and abundance, vital parameters of ecosystem health. However, it is also essential to consider species diversity and community composition, which show remarkable differences between the preserved sites and the sites modified by anthropic activities, substantially differentiating the DBZ category with lower β-diversity values and more homogeneous structure from the remaining categories.
Discussion
The riparian vegetation of the Senguio Microbasin in the MBBR is in a good state of conservation, maintaining similar species richness throughout the altitudinal and management gradients. This confirms the relevance of this type of vegetation to preserve important fragments of the regional biodiversity in a relatively reduced space (Nilsson 1992, 2002, Hernández-Dávila et al. 2020, Parker et al. 2016).
According to CONANP (2001), the riparian vegetation of the Senguio Microbasin conserves 20 % of the total flora of the MBBR. Comparing different riparian communities is difficult because their richness and species composition is strongly related to the characteristics of the surrounding vegetation, hydrology, geomorphology, and the presence of anthropic pressures. For example, in studies of riparian vegetation associated with temperate forests, in northern México, Graciano-Ávila et al. (2017) identified 12 arboreal species; in West Georgia, USA, Burton et al. (2005) found 65 trees; and, in Canada, Mallik & Richardson (2009) recorded 166 species in all vegetation strata; a similar number found in riparian vegetation of an anthropic landscape in the cloud forest of Veracruz, México (Hernández-Dávila et al. 2020). Burton et al. (2005), Zermeño-Hernández et al. (2020), and Mata-Balderas et al. (2020) documented that the losses in quantity and quality of the vegetation are directly related to the land-use intensity. Also, while we found only one exotic species in the riparian vegetation of Senguio microbasin, Liendo et al. (2015) found 115 exotic species in the 18 microbasins of the Cantabrian watershed in Spain.
The species richness, abundance, and diversity in the riparian vegetation fluctuated widely along the sampled rivers. Some sites outside the reserve that correspond to water sources for domestic purposes have high levels of species richness and diversity. Therefore, they are well-preserved due to the management decisions carried out by local inhabitants (Ryan et al. 2003, Valdivia & Poulos 2008, Flores-Díaz et al. 2014).
We found a statistically significant variation in the riparian vegetation of the disturbed forests outside the reserve (DIA). In this area, the dominant land uses are agriculture, free-range cattle raising, and human settlements. The sample sites in this protection and land use category had a high number of individuals but low species richness, one of the lowest β-diversity values, and the most homogeneous composition. This corresponds to a pattern identified as homogenization by anthropic effects, where the species that are either resistant to disturbance or favored by the ecological conditions of open areas tend to become dominant and homogenize the composition of the plant community (Mesa-Sierra et al. 2020, Arroyo‐Rodríguez et al. 2013). Thus, the use of riparian vegetation can reduce plant abundance, cause the extinction of the most sensitive local species, or favor generalist or invasive species (Cabette et al. 2017).
Our results of the analysis of vegetation structure (species composition and abundance) corroborated this pattern. The DIA appears as the most different category of protection mainly because of the predominance of the tree Fraxinus uhdei and the presence of useful species (fruit trees), a common pattern in areas modified by human activities aimed at searching for direct benefits, such as the spreading and care of valuable plants along the riverbanks (Doradea Monterrosa et al. 2006).
The sites of the CCZ and DBZ categories had the highest numbers of individuals and the highest α-diversity. Therefore, it suggests that the protection status plays a positive role in maintaining this ecosystem’s integrity and working as a biodiversity shelter where anthropic activities are restricted (Arroyo et al. 2013).
Analyzing the riparian vegetation along the whole altitudinal and management gradients, we observed the same pattern reported by Bennett et al. (2014): a structure of corridors of natural or semi-natural vegetation connecting conserved and disturbed landscapes. Thus, the riparian vegetation preserves plant diversity even in modified landscapes like croplands, urban and suburban areas, tree plantations, and managed forests. The riparian vegetation is essential to maintain the composition of plant and animal associations, and its interaction with adjacent anthropic spaces (Zermeño-Hernández et al. 2020).
Casas et al. (2008, 2015) and Clement et al. (2021) documented the dominance of highest culturally important and useful species in the vegetation of areas with strong human presence. In the riparian vegetation of our study area, we observed a higher frequency of fruit trees, trees used for timber, and medicinal plants near urban and productive areas.
Therefore, we conclude that: (1) riparian vegetation is crucial when considering conservation strategies; it is also necessary to consider it to be a part of both the forest and river systems, thus being an essential resource for the ecosystem inhabitants of the region. (2) The integration of strategies for preservation and restrictions within areas in the reserve’s core zone that are moderately used can be successful. The degree of conservation inside the core zone was notably higher than in the buffer zone outside the reserve. (3) In every conservation strategy, it is necessary to consider that the permanence of the system will depend on how the inhabitants of the area use its resources. The riparian vegetation may show human-induced modifications like species homogeneity; however, it still preserves other important components like ground coverage, species richness, and individuals’ abundance, maintaining the ecosystem’s health.











 nueva página del texto (beta)
nueva página del texto (beta)