The study of reproductive biology helps to understand the mechanisms that promote reproduction (Barrett 1998, 2002). On this basis, the reproductive biology of invasive species is essential to know the mechanisms that promote its rapid colonization and find out strategies to control their populational growth (Radosevich et al. 1997). The maintenance of weed populations will depend on the success of the asexual or sexual reproductive strategies (Barrett 2011). In weeds, asexual reproduction can occur by a clonal production of apomictic seeds or vegetative propagation by propagules (León-Martínez & Vielle-Calzada 2019, Zhang et al. 2021). Meanwhile, sexual reproduction involves fertilization, and the strategies are based on the occurrence of the mating system, whether xenogamy (outcrossing), autogamy (selfing), or both (Van Etten et al. 2017). Mating systems are also fundamental mechanisms in the life history of plants, as pollen dispersal patterns and the origin of pollen arriving at the stigmas can influence the fitness of individuals (Barrett 1995, 2002, 2014, Hidalgo & Gleissberg 2010, Whitehead et al. 2018). Many hermaphroditic invasive species present mixed mating systems, which produce seeds through autogamy and xenogamy (Goodwillie et al. 2005, Ruan et al. 2009). Mixed mating systems have been considered oscillatory systems because they have characteristics that promote both outcrossing (xenogamy) and a certain level of reproductive success through autogamy (Carrió & Güemes 2013). However, the most common strategies in invasive weeds are those related to autogamy. Autogamy occurs when the species are hermaphrodites and lack a self-incompatibility system (Van Etten et al. 2017). In these cases, autogamy might be facilitated by the movement of pollinators that cause the deposition of pollen on the same-flower stigma (Yun et al. 2015). However, flowers also display strategies that facilitate autogamy in the absence of pollinators named mechanisms of reproductive assurance (Barrett 1998, Godinez-Álvarez et al. 2008, Busch & Delph 2012, Bretagnolle & Gaba 2015, Cheptou 2019). For instance, the flower movements (Güemes & Boscaiu 2001), the nyctinasty (the movements of petals at the nighttime closure of flowers) (Kwiatkowska et al. 2019), and cleistogamy (where the flowers never open) (Lord 1981, Sauquet 2021) can promote self-fertilization. Nevertheless, if autogamy occurs repeatedly, populations can undergo inbreeding depression (Kephart et al. 1999, Goodwillie et al. 2005).
Papaveraceae offers a wide diversity of mechanisms and strategies concerning sexual reproduction (de Nettancourt 1977, Ohara & Higashi 1994, Paape et al. 2011, Bilinski & Kohn 2012). In the family, there are self-compatible species that show reproductive assurance mechanisms as the facilitated selfing by pollinators (Yun et al. 2015), self-pollination given by petal movements (Yang et al. 2019), or cleistogamy (Ruiz de Clavijo & Jiménez 1993). On the contrary, other species exhibit an obligate xenogamy given by the spatial and temporary separation of sexual functions, despite its self-compatibility as in Sarcocapnos pulcherrima Morales & Romero (Salinas & Suárez 2003). The obligate xenogamy is also given by the presence of a self-incompatibility system (Lundqvist 1964, McClure et al. 2011) as the widely studied in Papaver rhoeas L. (de Nettancourt 1977, 2001). In Papaveraceae, some species present a set of traits that promote both self-fertilization and outcrossing, having a mixed mating system (Lyon 1992, Tepedino et al. 2014, Yang et al. 2019). Nevertheless, some species are called partially self-compatible because more seeds are produced by outcrossing than by self-fertilization (Arredondo-Núñez 2011, Anic et al. 2015, Xiao et al. 2016). Besides, the partial self-compatibility or semi-compatibility systems can also occur in cases where some flowers show a barrier that prevents the growth of pollen tubes through style in autogamous mates as in Argemone munita Durand & Hilg. (Paape et al. 2011).
The genus Argemone L. is a native American group that belongs to the Papaveraceae family and has about 24 species with a wide distribution range (Schwarzbach & Kadereit 1999). The species of genus Argemone are known to have “high-value biological active compounds” used in traditional medicine, pharmaceutics, agriculture, and biofuels (Martínez-Delgado et al. 2022). Although they have a high potential for exploitation in diverse industries, some species of the genus act as highly invasive weeds (Kumar & Rohatgi 1999), and there is not enough information about their reproduction. That is the case of Argemone ochroleuca, known as the Mexican poppy, which is a species with a high pharmacological and ethnobotanical value (Reyes et al. 2011, Martínez-Delgado et al. 2022), but is the species most reported as an invasive weed within the genus (Calderón de Rzedowski 1991, Karlsson et al. 2003, Assaeed et al. 2020). Argemone ochroleuca has been reported growing in all continents, and it has allelopathic effects that increase its invasiveness by causing a reduction in germination rate on native species (Dar et al. 2017). This weed has a successful growth in worldwide disturbed environments (Calderón de Rzedowski 1991), but the strategies that favor seed formation are still unknown. Knowledge about seed formation in A. ochroleuca can serve as a basis to build adequate management in the control of its invasiveness, but also to develop strategies to create potential crops to reproduce A. ochroleuca for its exploitation in bioenergetics, pharmaceutics, and agriculture. Based on the reports of its high invasiveness and the presence of a partial self-incompatibility system in its relative A. munita we expected A. ochroleuca exhibits different sexual strategies that enhance the production of seeds like in other species of the genus or the family. Thus, this study aims 1) to describe the flower cycle (from pre-anthetic buds to seed dispersal), 2) to explore if it has a self-incompatibility system (as occurs in the other studied species of the family) by following the growth of pollen tubes through gynoecium in self-pollinated flowers, and 3) to know the mating system of A. ochroleuca through controlled pollination treatments showing the path by which this weed produces abundant seeds and testing if the studied population exhibits inbreeding depression.
Materials and methods
Study species. Argemone ochroleuca is a native species from Mexico, although it is invasive in disturbed areas and crop fields (Calderón de Rzedowski 1991). This species is an annual plant blooming from November to June, with a flowering peak from February to May (S. Rios-Carrasco, personal observations). The plants are herbaceous with glaucous stems and leaves, both covered with spines. The flowers are solitary and bisexual. The calix has three sepals, horned, fused apically and laterally to each other. The petals vary from 4 to 6, and they are yellowish or creamy. The fruits are dehiscent capsules (Martínez-Delgado 2022).
Study site. The fieldwork was carried out from 2013, 2014, and 2017, in the locality of Tecomitl, in Mexico City at 19° 12’ 16.53’’ N, 98° 58’ 49.32’’ W; 2,250 m asl. The study site is located in an agricultural area surrounded by an urban area with temperate sub-humid weather, following the climate charts of Instituto Nacional de Estadística y Geografía (INEGI 2021). A. ochroleuca is very common in the site as an invasive species, and it is growing at the side of the road, between the crops of prickly pear Opuntia ficus-indica (L.) Mill., on fallow lands, living sympatrically with Reseda luteola L. (Resedaceae), also an invasive weed. We work on two crop fields of Opuntia ficus-indica of 500 m2 each, with approximately > 300 flowering plants of A. ochroleuca between the two fields.
Flower cycle. Anthesis duration and the position of floral parts before, during, and after the anthesis (including the anther dehiscence) were monitored in a total of 60 pre-anthetic flower buds (the sepals of pre-anthetic buds are purple, while young flower buds are green) from two patches (15 plants per patch and two flowers per plant) from 7:00 to 18:00 hr during seven consecutive days in April in the three years of study. We labeled the pre-anthetic flower buds to follow the lifespan of flowers. Additionally, we monitored the population weekly for a month to know the flower changes from flower bud to fruit formation.
Stigmatic receptivity. In 2013, 50 pre-anthetic flowers from different individuals (two flowers per plant) were emasculated and covered to prevent pollen contamination. We placed a few drops of hydrogen peroxide with a syringe on the stigmas to detect the peroxidase activity that indicates receptivity (Galen & Plowright 1987). Ten flowers were used to analyze stigmatic receptivity at each time: pre-anthesis, early anthesis, the second, third, and fourth day of anthesis.
Self-incompatibility system and pollen tube growth. We performed 15 self-pollinations at early anthesis from 15 randomly selected individuals to determine if the studied population exhibits a self-incompatibility system. We placed pollen from a flower on its stigma, and then the flowers were bagged to avoid contamination with foreign pollen. The treated flowers were collected two, four, and six hr after pollination (5 per time), and they were fixed in Farmer’s fixative (100 % ethanol and acetic acid in a 3:1 v/v) for 24 hr. Afterward, they were placed in a sodium sulfite (NaSO3) solution to soften the tissue; then, in 0.1 % (w/v) decolorized aniline blue solution in K3PO4 buffer for 24 hr. The samples were observed under a fluorescence microscope (Olympus Provis AX750) to show the callose wall of the growing pollen tubes (Martin 1959). To describe the pollen tubes path, ten Paraplast embedded self-pollinated gynoecia were cut transversely with a rotary microtome at 8 μm thick to locate transmitting tissue and pollen tubes with fluorescence microscopy. Samples of gynoecia were processed and analyzed by scanning electron microscopy (JSM-5310LV JEOL). The fluorescence, light, and scanning electron microscopy samples were processed using standard protocols (Márquez et al. 2016).
Mating system. In order to know the mating system of A. ochroleuca, we use two methods. The first is the number of gametes by calculating the pollen/ ovule ratio (Cruden 1977). The second includes comparing the number of seeds from xenogamous and autogamous pollination treatments. The procedure for both methods is described in detail below.
P/O ratio.- According to Cruden (1977), we calculated the pollen/ovule (P/O) ratio to estimate the mating system. The P/O ratio was obtained by dividing the number of pollen grains by the number of ovules per flower. The pollen grains were obtained from indehiscent anthers of 30 flowers from different plants (one flower per plant). We collected two anthers per flower, placed them in separated microcentrifuge tubes of 1.5 ml with 100 μl of distilled water and detergent. The anthers were carefully broken with a dissecting needle and vigorously shaken to homogenize the mixture. Afterward, we took a 10 µl aliquot, placed it on a Neubauer chamber to count the number of pollen grains. The results were multiplied by the dilution factor to obtain the number of pollen grains per anther, multiplied by the number of anthers per flower to obtain the total number of pollen grains per flower. To count the number of ovules, we dissected the ovaries to expose all their ovules under a stereomicroscope. We obtained the average and the standard error of the mean (SEM) for the number of pollen grains, ovules, and P/O ratio.
Pollination treatments.- To know which sexual path (xenogamy or autogamy) produces more seeds in A. ochroleuca, five treatments of controlled pollination were carried out from February to May during 2013 and 2014. The pollination treatments were the following: 1) control, in which the flowers were not handled and were exposed to open pollination; 2) cross-pollination, in which a mixture of pollen from at least ten flowers from ten different individuals (from a different patch 1 km away) was applied on stigmas of previously (at pre-anthesis) emasculated flowers from different plants; 3) autonomous self-pollination, non-manipulated flowers were covered before the flower opening; 4) hand self-pollination, pollen from a newly-opened flower was deposited on its stigma; 5) apomixis, flower buds were emasculated during pre-anthesis to avoid contact between pollen and stigma to test if the weed has seed formation through an asexual path. Each treatment consisted of at least 30 flowers (see Table 1) from different individuals (one flower per plant per treatment). Flowers in every treatment were labeled and bagged except in the control. Ripe fruits were collected after 15 days, before the dehiscence of the capsules, to avoid the loss of seeds, to count the number of formed seeds. As we found developed but collapsed seeds, we also counted this type of seeds. Each treatment was subjected to the Shapiro-Wilk normality test, which resulted in a non-normal distribution, except for the control (W = 0.988, P = 0.895). Therefore, the non-parametric Kruskal-Wallis and Dunn tests were performed to determine differences in the number of seeds per fruit among pollination treatments and for multiple comparisons, respectively. The tests were evaluated with a 95 % confidence level using the package rstatix (Kassambara 2020) in the software R v. 4.0.2. (R Core Team 2019).
Table 1 Percentage of formed fruits and number of seeds per pollination treatment, including the cleistogamous flowers as a sixth treatment of Argemone ochroleuca, in the locality of Tecomitl (Milpa Alta, Mexico City, Mexico).
| Treatment | n | Formed fruits (%) | Formed seeds per fruit (Mean ± SEM) | Colapsed seeds per fruit (Mean ± SEM) |
|---|---|---|---|---|
| Control | 50 | 50 (100) | 377 ± 18.1a | 0 |
| Cross-pollination | 32 | 32 (100) | 304 ± 22.6a | 0 |
| Autonomous self-pollination | 57 | 33(57.89) | 84 ± 8.28c | 32.46 ± 9.99 |
| Hand self-pollination | 46 | 46(100) | 205 ± 24.7b | 83. 78 ± 11.84 |
| Apomixis | 32 | 0 | 0 | 0 |
| Cleistogamous flowers | 33 | 26 (78.78) | 60 ± 11.9c | 0 |
Note: Different superscripts denote which treatments differ from each other from the multiple comparison test at P < 0.05
Pseudocleistogamy. During the fieldwork in 2013, we noticed that some flowers from certain individuals never opened. In this sense, individuals can have chasmogamous and rarely cleistogamous flowers where the sepals detach from the receptacle but remain as a hood, preventing the flower opening. We bagged 33 flowers (each from a different plant) that we found with this condition to know if they could form seeds and were considered a sixth pollination treatment (named cleistogamous flowers) that was included in the comparison analysis among pollination treatments described before.
Inbreeding depression. With the number of seeds per fruit from hand self-pollination and cross-pollination treatments, the inbreeding depression was calculated using the equation: δ = 1- (w s /w o ) of Lande & Schemske (1985). With w s as the result of the self-fertilization and w o as the result of the cross-fertilization. We also considered the fruit and seed formation, and we obtained an accumulative result by the following equation: δ = 1- [(w sa /w oa ) × (w sb /w ob )] where δ is the value of inbreeding depression, w sa is the number of developed fruits by selfing, w oa is the number of developed fruits by outcrossing, w sb is the number of seed formed by selfing, and w ob is the number of formed seeds by outcrossing (Husband & Schemske 1996).
Results
Flower cycle. The flowers of A. ochroleuca start the process of anthesis around 9:00 hr. The flower bud comprises three completely fused sepals, each one characterized by a spine at the apex (Figure 1A). During anthesis, the sepals detach from the base (Figure 1B), and the petals are progressively extended; the whole hooded calyx moves towards the apex of the flower until it falls (Figure 1C-F). The maximum aperture of the petals is around 12:00 hr, allowing the stamens to extend and create distance from each other and the gynoecium (Figure 1G). Finally, the flowers are closed between 15:00-18:00 hr (Figure 1H-I); on sunny days, the closing was at 18:00 hr, while on cloudy days, the closing of the flowers occurred earlier. The longevity of the flowers is three days; on the fourth day, the petals dry out and detach from the receptacle (Figure 1J). The fruits (capsules) develop and mature 15-20 days after pollination (Figure 1K), they dehydrate, and the apical region of the dehiscent valves is extended towards the outer region, forming basket-like structures. The wind promotes the dispersion of seeds (Figure 1L) by shaking the capsules from side to side. The test of receptivity with hydrogen peroxide proved that the stigma is receptive a few moments after anthesis initiation. While in the flower bud, the stigma shows a pink color, and it is not receptive; it turns burgundy when receptive and lasts for three days. When the stigma receptivity is over, it turns brown. Regarding the anthers, they open moments after the anthesis has begun. Therefore, both male and female sexual functions are simultaneous within the same flower.
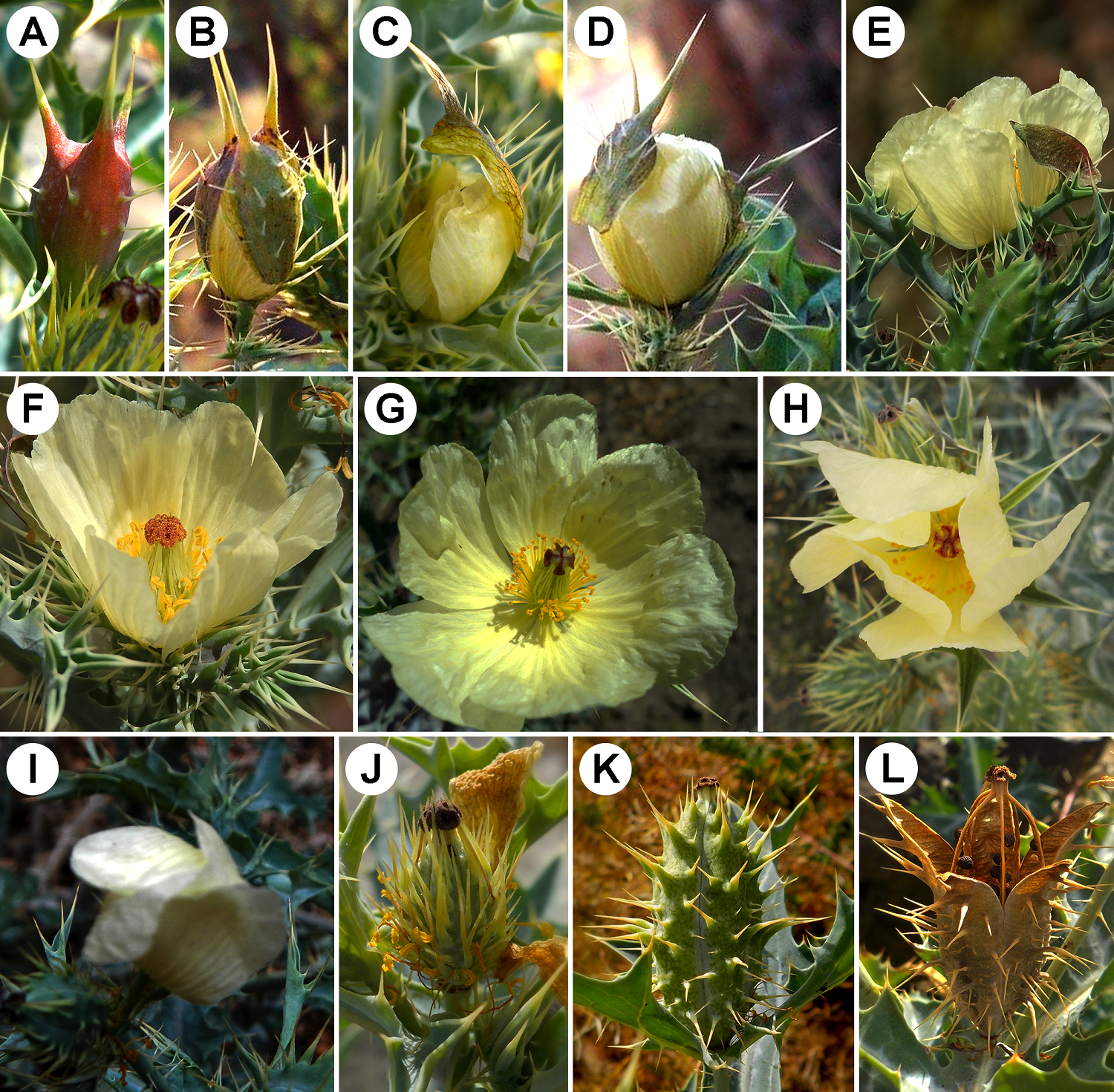
Figure 1 Floral phenology of A ochroleuca. (A) Young floral bud. (B) Earlier anthesis. Sepals detaching from the receptacle. (C) Sepals forming a hood at the apex. (D) Petals extending, causing the fall of the sepals. (E-F) Flowers during anthesis. (G) Maximum floral opening with fully extended petals. (H) Flower about to close. (I) Closed flower. (J) Beginning of fruit formation. (K) Ripe fruit. (L) Valved capsule with exposed seeds.
Self-incompatibility system and pollen tube growth. Pollen tubes grow toward the ovules in the self-pollinated flowers, showing no prezygotic barriers for seed development, proving that the species is self-compatible. The pollen tube route through the style-less gynoecium (Figure 2A) begins with the germination of pollen grains. During the first two hours, they adhere to papillae and hydrate; the pollen tubes emerge through one of the colpi, which initiates the growth phase of the pollen tube (Figure 2B). It is followed by the invasion phase, in which the pollen tube surrounds the stigmatic papillae before entering the stigmatic channels (Figure 2C). After four hours, the pollen tubes pass through the papillae to reach the stigmatic channels (Figure 2D-E). Due to the gynoecium lacks style, the pollen tubes immediately reach the ovary. Once inside the ovary, the pollen tubes grow on the papillae of the placenta (Figure 2F), so they can reach the ovules to fertilize them by porogamy four to six hours after pollination (Figure 2G).
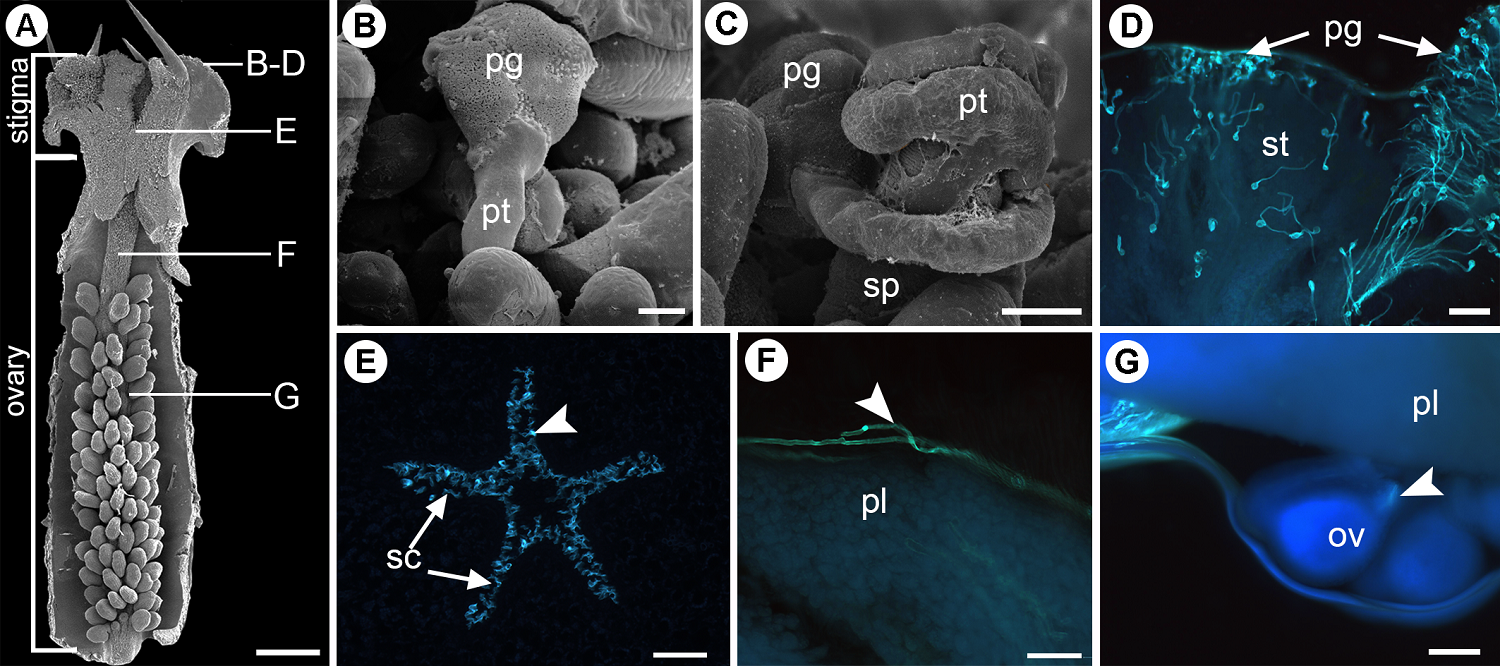
Figure 2 Pollen tube growth in self-pollinated flowers of A. ochroleuca. (A) Dissected gynoecium. Capital letters corresponds to the following figures indicating the level at which each event occurs. (B) Germinating pollen grain. (C) Pollen tube surrounding a stigmatic papilla from the stigma. (D) Germinating pollen grains traversing stigmatic papillae seen under fluorescence microscopy. (E) Pollen tubes (arrowhead) growing through stigmatic channels towards the ovary. (F) Pollen tubes arriving at the ovary passing through the placenta. (G) Pollen tubes penetrating the ovules by the micropyle (arrowhead). ov, ovule; pg, pollen grain; pl, placenta; pt, pollen tube; sc, stigmatic channel; sp, stigmatic papillae; st, stigma. Scale bars: A = 1 mm; B = 20 μm; C-E = 100 μm; F = 200 μm.
Mating system and inbreeding depression. The number of ovules per flower is 360.52 ± 16.2 (Mean ± SEM), the number of pollen grains per flower is 118,335.3 ± 19,766. Thus, the P/O ratio is 384.9 ± 55.86, placing A. ochroleuca between the categories of facultative autogamy and facultative xenogamy, according to Cruden (1976, 1977). Fruit and seeds were present in every treatment, except in apomixis (Table 1). Thus, we discard seed formation by an asexual path. All xenogamous crosses resulted in the formation of fruits, contrary to natural-autogamous flowers (autonomous self-pollination and cleistogamous flowers). The number of seeds differs statistically among pollination treatments (H = 86.25, df = 4, P < 0.0001), and the xenogamous path is by which more seeds are produced, while cleistogamous flowers are the ones that produce the least in the studied population of A. ochroleuca (Table 1). The cleistogamous flowers were scarce in the population and were found in plants with chasmogamous flowers. The level of inbreeding depression was δ = 0.34 for fruits, δ = 0.68 for seeds, and δ = 0.77 as the accumulative value. As δ > 0.5, the population presents inbreeding depression.
Discussion
Self-compatibility in A. ochroleuca. Our results show that A. ochroleuca flowers do not present barriers in the stigma that prevent pollen tubes grow to the ovary. The lack of style in the gynoecium of A. ochroleuca is a feature that favors quick fertilization, as the distance that the pollen tubes must cover is shorter. Although pollen tubes were observed in the self-pollination treatment, fewer seeds were formed by this way than in outcrossed flowers, which might resemble the “weak self-incompatibility” system reported in A. munita (Paape et al. 2011). It consists of a semi-compatible system, where the S genes are described as “weak” or “non-functional” that allow the growth of pollen tubes in some individuals and the formation of scarce seeds (Allen & Hiscock 2008, Paape et al. 2011). Our results of hand self-pollinating flowers show that fruits and seeds are formed in this way. Besides, several developing seeds (fertilized ovules) did not complete their development or collapsed before reaching maturity. In this sense, the low seed production may be due to inbreeding depression rather than a weak incompatibility system. This idea is supported by reports of the presence of self-sterility, which causes a low seed production by autogamy due to inbreeding depression rather than a self-incompatibility system (Allen & Hiscock 2008). Self-sterility has been correlated to the presence of hollow styles or the absence of a true style (Allen & Hiscock 2008, Paape et al. 2011), like in A. ochroleuca. However, a weak self-incompatibility system such as that described for A. munita, cannot be discarded for A. ochroleuca until future work in genetics is carried out concerning S genes.
Reproductive strategies. A. ochroleuca fit the pattern of invasive weeds, mostly hermaphrodites and self-compatible, which can partly explain its high invasiveness (Van Etten et al. 2017). Secondly, the mixed mating system in A. ochroleuca shows strategies that allow cross and self-fertilization (Goodwillie et al. 2005). An example of this is the flower opening and the duration of anthesis of A. ochroleuca, which lasts three days and is the time when the stigmas have the opportunity to receive pollen from other plants (van Doorn & van Meeteren 2003). In addition, the large amounts of pollen grains also promote outcrossing, inciting the exchange of pollen, maximizing fertilization (Mitchell 1997). Besides, this weed exhibits nyctinasty, the temporary closure of flowers (Kwiatkowska et al. 2019), which in turn can promote selfing through petal movements as occurs in Sanguinaria canadensis L. (Lyon 1992), Hypecoum erectum L. (Yang et al. 2019), and Corydalis sheareri S. Moore (Weiping & Sheng-Xiang 1997), also species of Papaveraceae. Thus, the floral movements in chasmogamous flowers can also serve as a strategy that favors the pollen deposition on the stigma (Carrió & Güemes 2013).
Results of autonomous self-pollination treatment demonstrate that A. ochroleuca flowers produce fruits and seeds naturally by self-pollination, and the flower movements during closure could cause the pollen deposition on the stigma. This strategy works more than half of the time, as not all naturally self-pollinated flowers of A. ochroleuca resulted in fruit formation (see Table 1). However, this serves as a reproductive assurance mechanism since the formation of seeds is guaranteed at least in half of the flowers in case there are no pollinators (Busch & Delph 2012). In addition, the low number of seeds in autonomous self-pollinated flowers compared to the hand self-pollinated flowers also demonstrates that pollen transporting vectors appear to be essential to maximize the number of produced seeds.
Pseudocleistogamy.- Another reproductive assurance strategy is the presence of cleistogamous flowers in the studied population of A. ochroleuca. As the chasmogamous and cleistogamous flowers have the same morphology, this is a pseudocleistogamous population, according to Lord (1981). Although it is a rare condition within the population, it resulted in the formation of seeds. These flowers produced a number significantly similar to autonomous self-pollinated flowers; however, the cleistogamous form more fruits than the naturally self-pollinated ones. This fruit formation can occur because the petals never spread out, so neither do the stamens, staying close to the stigma favoring the pollen deposition on the stigma. On the contrary, the chasmogamous flowers extend petals and stamens, and when the flowers close, the contact of the dehiscent anthers with the stigma can be imprecise.
Within Papaveraceae, other species present cleistogamous flowers, but unlike A. ochroleuca, Ceratocapnos heterocarpa Durieu is a species that has cleistogamous and chasmogamous flowers that do not coincide on the same plant. In addition, both types of flowers are different; the cleistogamous ones are smaller and produce less pollen than chasmogamous ones (Ruiz de Clavijo & Jiménez 1993). Moreover, it has been reported that cleistogamy arises in response to unfavorable environmental conditions in other angiosperms as a reproductive assurance mechanism. An example is in Murdannia nudiflora (L.) Brenan (Commelinaceae), where cleistogamous flowers positively correlate with rain (Veena & Nampy 2019). The cleistogamous flowers in the A. ochroleuca population appear to have no pattern at the individual or population level. Thus, further studies are needed to know if pseudocleistogamy is caused by environmental stimuli that prevent the enlargement of the petals.
Mixed mating system and inbreeding depression.- The seed formation of A. ochroleuca is carried out only by the sexual path. The P/O ratio places A. ochroleuca between the facultative autogamy and facultative xenogamy proposed by Cruden (1976, 1977). Thus, it presents a mixed mating system, which is consistent with the results of pollination treatments and is the most common system recorded for weeds (Shivanna 2014). Mixed mating systems are advantageous because plants can produce seeds by outcrossing, which increases genetic variability between individuals and populations; however, they are also characterized by having a reproductive assurance mechanism given by autogamy (Goodwillie et al. 2005).
The advantages of the mixed mating system can be reflected in A. ochroleuca. Naturally, A. ochroleuca does not show pollen limitation regarding quantity as the number of seeds in the control and cross-pollination treatments are comparable, and similar to the ovule number. The latter indicates that pollen transport is sufficient to form the maximum number of seeds (Knight et al. 2005) either by selfing or outcrossing. Although A. ochroleuca produces more seeds through outcrossing, autogamy also plays a significant role in developing seeds, especially when pollinators are scarce or absent. Thus, the mixed mating system enables A. ochroleuca to populate new areas where pollinators may or may not interact immediately with the flowers of this weed and ensure reproductive success (Kalisz et al. 2004). Reproductive assurance strategies through autogamy are common in weedy plants (Shivanna 2014, Van Etten et al. 2017). Although these mechanisms can evolve in a few generations and represent an advantage for the colonization of new sites in the short term, they can be a disadvantage in the long term by presenting inbreeding depression (Cheptou 2019).
The inbreeding depression is a common feature among populations of weeds with mixed mating systems (Carr & Dudash 1996, Herlihy & Eckert 2002, Goodwillie et al. 2005, Ruan et al. 2009, 2011, Busch et al. 2010, Carrió & Güemes 2013, Munoz et al. 2016). Inbreeding depression can be seen in the low quantity of seeds and fruits formed by autogamy (Lande & Schemske 1985, Husband & Schemske 1996) as in A. ochroleuca. This information has also been reported for other species of Papaveraceae with mixed mating systems, where autogamy is a reproductive assurance mechanism in settings where pollinators are scarce (Anic et al. 2015, Yang et al. 2019). In the case of A. ochroleuca, inbreeding depression is present in the population. The results show that if the stigmas receive large amounts of self-pollen, the number of seeds formed will be affected. Although the pollen grains reach the ovules, the seeds can collapse during their development, as shown in the results of self-pollinated crosses.
The presence of autogamy and xenogamy in A. ochroleuca maximizes the formation of seeds. In addition, the dispersion favored by the wind makes the species successful in the cultivated lands where it lives. Moreover, inbreeding depression can be alleviated as long as outcrossing increases. The oscillations between autogamy and xenogamy can be observed within weed populations, preserving and favoring the maintaining of mixed mating systems (Goodwillie et al. 2005, Porcher & Lande 2005, Winn et al. 2011). However, if interest arises in cultivating the species to extract active compounds (Reyes et al. 2011, Martínez-Delgado et al. 2022), the consequences of reproducing the species by autogamy must be considered.
A. ochroleuca is a wide-world invasive weed and a precious species for its compounds of great interest in different industries. Here, we demonstrate that the studied species is self-compatible, and the seeds can be formed either by autogamy or xenogamy, indicating a mixed mating system. A. ochroleuca displays different strategies that increase its potential for invasiveness in the study site. These mechanisms favor the production of seeds by autogamy. The first occurs in chasmogamous flowers, which are the majority of flowers, where the movement of petals favors self-pollination during flower closure. The second one occurs in cleistogamous flowers, a rare floral type in the population, where pollination is accomplished in flowers that never open. These are mechanisms of reproductive assurance that A. ochroleuca exhibits as a backup way to form seeds in case the pollinators are scarce or null, a common strategy in invasive weeds. Although flowers reached the maximum number of formed seeds per fruit, the inbreeding depression is present in the studied population. That indicates that the number of seeds will decrease if several self-pollination cycles are carried out. Finally, this work shows some reproductive aspects of A. ochroleuca that can be considered to create plans against its invasiveness or for its propagation for pharmacological or agricultural purposes.











 nueva página del texto (beta)
nueva página del texto (beta)



