Shrublands constitute the most characteristic plant communities of arid and semiarid zones in Mexico (González-Medrano 2003, Estrada-Castillón et al. 2012). Although the plants that make up the xerophytic scrublands have a physiognomy, a way of life and particular characteristics adapted to drought conditions, there are variants according to the conditions of the terrain or their distribution (Rzedowski 2006). Among the plant associations frequently included in this group are the piedmont scrublands, desert scrublands (rosetophilous and microphilous), thorn scrublands, thornless scrublands and semi-thorn scrublands. The thornless scrublands typical of the Chihuahuan desert are parvi-perennifoliate and are dominated by Larrea tridentata (Miranda & Hernández-X. 1963). However, in northeastern Mexico and southern Texas (Tamaulipan Biotic Province), there is a more deciduous scrub usually predominated by Leucophyllum frutescens and associated with thorny elements such as Vachellia rigidula forming semi-thorn communities (Diamond et al. 1987). The latter are common in northern Tamaulipas, where they are mixed with Tamaulipan thornscrub (MET, for its acronym in Spanish), which is more widely distributed in the Northeastern Coastal Plain (SPP 1983).
Plant communities in southern Texas and northeastern Mexico are often classified and encompassed as MET (Graciano-Ávila et al. 2018). However, new hierarchical classification systems consider that not all these plant associations are equal. Even these new systems consider these shrublands not as a community but as a macrogroup (Tamaulipan Scrub and Grassland) grouped according to their biogeographic similarities (Faber-Langendoen et al. 2018). Thus, other as yet unidentified and/or undescribed Tamaulipan grassland and shrubland associations may exist in this region.
Currently, semi-thorn shrublands and others are strongly diminished and fragmented by changes in land use for agricultural and livestock purposes (Alanís-Rodríguez et al. 2013, Jiménez-Pérez et al. 2009). In fact, northern Tamaulipas is one of the areas with the greatest loss of vegetation cover since the creation of the Bajo Río Bravo and Bajo Río San Juan irrigation districts in the 1940s (Herrera-Pérez 1998). Additionally, recent shale gas extraction activities in the Burgos Basin are a further threat to the shrublands of northeastern Mexico (Wood et al. 2011).
For all these reasons, it is important to conduct ecological studies of the shrubland communities distributed in the Tamaulipan Biotic Province or northeastern Mexico, so that they can serve as the basis for a sustainable management plan that guarantees their maintenance. Unfortunately, any existing work on scrubland in this part of Mexico is scarce or outdated (González-Medrano 1972) and, with the exception of the Laguna Madre, there are no natural areas that protect its biological diversity (SEMARNAT 2015). In this sense, the general objective of this study was to describe the structure and composition of a fraction of low shrubland in the north of the state of Tamaulipas.
Materials and methods
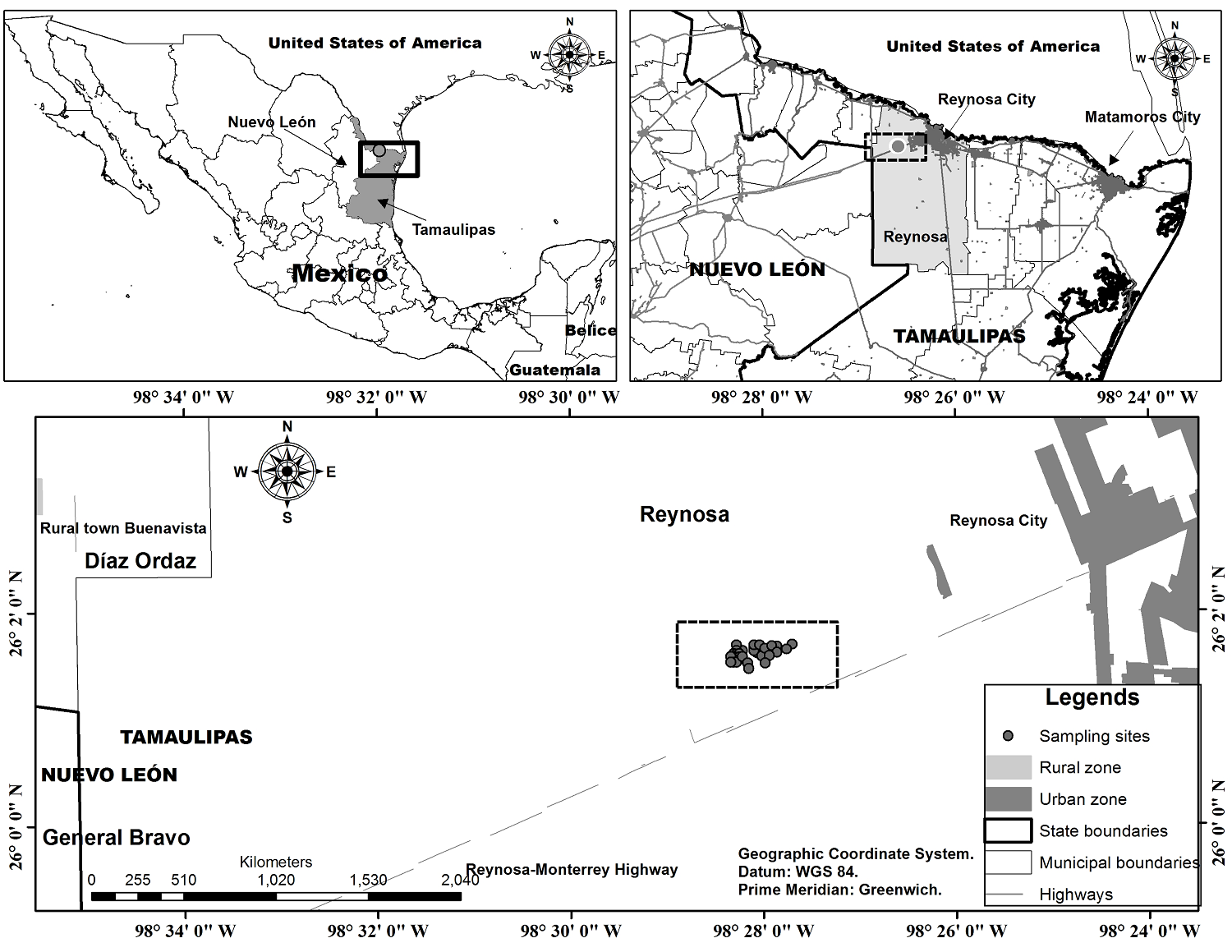
Study area. The study area was located in Northeastern Mexico (INEGI 2019) (Figure 1). The climate is semiarid, warm, with a mean annual temperature higher than 22 °C, temperature of the coldest month higher than 18 °C, rainfall distributed throughout the year, and percentage of winter rainfall higher than 18 % of the annual total BS1(h')(x') (Cuervo-Robayo et al. 2019). The sites presented two shallow soil types, predominantly rendzinas (E) / medium textured, petrocalcic phase and to a lesser extent luvic xerosol (Xl) / medium textured, gravelly phase. These soils are established on caliche-type Tertiary sedimentary rock (Batjes et al. 2020). The sampling sites are located in an elevational interval between 111 and 126 m asl, with a slope of 0-22° (INEGI 2013). The community studied within the region is distributed in the highest portion of the low hills. The environmental conditions in the area allow the establishment of vegetation continuums, being mostly Tamaulipan thornscrub and swathes of Texas sage known as cenizales, with which the studied vegetation borders. These scrublands are fragmented due to increasing urban sprawl and the establishment of induced pastures intended for livestock activities, such as cattle ranching and sorghum cultivation (INEGI 2016).
Field work. The samplings were carried out between August 2018 and July 2019 in a continuum of low semi-thorn shrubland over an area of 30 ha, considering the shrub and tree strata. While the herbaceous stratum, considering succulent plants within it, was registered and measured in sub-squares of 1 m2. The sampling was conducted on 33 randomly distributed plots of 10 × 10 m (100 m2). The sample size was selected considering obtaining the largest possible number of plots within the reduced extension of this vegetation fragment, maintaining between these both representativeness (more than 30 plots) and statistical independence (minimum distance between plots greater than 20 meters) (Schreuder et al. 2006). Plants were recorded within each quadrat, where all individuals were measured for height and average canopy diameter. One individual of each species was used to take botanical samples, which were pressed and herborized according to Lot & Chiang (1986). The material was identified through specialized literature (Correll & Johnston 1970, Mora-Olivo & Martínez-Ávalos 2012, Estrada-Castillón et al. 2014, Molina-Guerra et al. 2019) and subsequently deposited in the herbarium of the Instituto de Ecología Aplicada, Universidad Autónoma de Tamaulipas (UAT).
Data analysis. Nomenclature was standardized using the Taxonstand v. 2.1 package (Cayuela et al. 2019) of the R programming language v. 4.0.3 (R Core Team 2017), which uses ThePlantList (www.theplantlist.org) database. The horizontal structure was determined using the Importance Value Index (IVI). The IVI considers the sum of the relative abundance (through the number of individuals per hectare, N/ha), the relative frequency (number of quadrats where each species occurs) and the relative dominance of the species occupied by the average cover of each individual (Mora-Donjuán et al. 2014). Using the IVI, it was possible to determine the structural importance of each species within the community. Meanwhile, the vertical structure was analyzed through the Pretzsch index (A), which is a modification of the Shannon-Wiener index, where three strata are proportionally represented according to the maximum height recorded (Mora-Donjuán et al. 2014). Given that the A index was designed to analyze the structure of temperate forests, which are different in structure from low shrublands, we decided to consider four strata: stratum I with height zones ranging from 75-100 % of the height of the tallest tree (3.38-4.50 m), stratum II with height zones of 50-75 % (2.25- 3.37 m), stratum III with height zones of 25-50 % (1.13-2.24 m) and stratum IV with height zones of 0-25 % (0.00-1.12 m). We used the A index to derive the Amax, which corresponds to the maximum value of A, given by the number of species and height zones; and the Arel, which is the standardization in percentage of the A index (Mora-Donjuán et al. 2014). It is advisable to mention whether a comparison was performed of the strata between study sites or of diameter structures and height (DBH) of the arboreal and shrub strata. Additionally, alpha diversity indexes were estimated to describe the dominance (Simpson index, λ), the evenness (Shannon-Wiener index, H´), and the influence of most abundant species (Berger-Parker index) (Moreno 2001). The alpha diversity indexes were estimated used PAST v. 4.04 software (Hammer 2021).
Results
Floristic composition. The sampled community consisted of a total of 5,580 individuals, belonging to 55 species, distributed within 54 genera, 25 families, 16 orders, three classes and two divisions (Supplementary material, Table S1). The only genera represented with two species was Croton, the rest were represented by a single species. The most represented family was Fabaceae (nine species), followed by Poaceae, Asteraceae, Cactaceae, and Euphorbiaceae (four species). On the other hand, the families Verbenaceae, Malvaceae, Rhamnaceae and Rutaceae were represented by three species, while Boraginaceae and Celastraceae had two species and the remaining families presented only one species within the community. Most of the species were concentrated in the class Magnoliopsida (49 species), the class Liliopsida presented five species and the class Gnetopsida, as well as the division Gnetophyta were represented only by Ephedra antisyphilitica.
Horizontal structure. The smallest shrub species presented the highest importance index values (IVI), based on their abundance, frequency and dominance. These species were Lippia graveolens (30.65), Calliandra conferta (28.68), Leucophyllum frutescens (23.64), Turnera diffusa (22.99), Vachellia rigidula (21.39), Krameria ramosissima (21.25) and Senegalia berlandieri (21.16). All the species with the highest importance values, except V. rigidula and S. berlandieri, are characterized by being non thorny plants, in addition to showing the highest relative density (Supplementary material, Table S2). Similarly, S. berlandieri, V. rigidula and L. frutescens share the characteristic of being shrubs with heights between 1.2-2.5 m, the other four species being shrubs with a height of less than 1 m. The absolute density on which the relative density is based is the result of the quotient between the number of individuals of the same species and the area sampled. The most frequent species were S. berlandieri (6.77 %), L. frutescens (6.55 %), V. rigidula (6.13 %), K. ramosissima (5.71 %), L. graveolens (5.71 %), T. diffusa (5.71 %), C. conferta (5.50 %), Rhamnus huboldtiana (5.50 %) and Croton incanus (5.29 %). Relative dominance gives the proportion of land occupied by the perpendicular projection of the aerial parts of the individuals of the species considered. The species with the highest dominance were Echinocactus texensis (19.38 %), V. rigidula (11.39 %), C. conferta (10.52 %), S. berlandieri (9.99 %), L. frutescens (8.47 %) and L. graveolens (6.13 %).
Vertical structure. The vertical structure of the community is represented by four well-defined strata, some constituted by a high number of species and individuals (50 % restricted to one stratum and the other equally distributed in all strata) and other underrepresented strata (A = 2.86, Amax = 5.39, Arel = 52.98 %). The lowest stratum concentrates the majority of species and individuals, while the tallest stratum is poorly represented (Supplementary material, Table S3). This indicates that the most of components of this scrub are small dimensions shrubs, being few individuals of tall arboreal size. The species with the highest proportional density, considering the total number of individuals were L. graveolens (18.90 %), T. diffusa (13.90 %), C. conferta (12.73 %), K. ramosissima (10.69 %) and L. frutescens (8.61 %), highlighting that, except for L. frutescens, these species are distributed only in stratum IV. The species that were represented in the three lowest strata were Nahuatlea hypoleuca and Sideroxylon celastrinum, while Senegalia berlandieri, V. rigidula, C. torreyanus and Leucophyllum frutescens were mostly distributed in stratum III.
Stratum IV (0 - 1.12 m in height) was represented by 32 species, with a proportion of the total density of 81.16 %, of which L. graveolens (18.90 %), T. diffusa (13.90 %), C. conferta (12.73 %) and K. ramosissima (10.69 %) were the most abundant. Stratum III (1.13-2.24 m in height) was represented by 27 species, with a proportion of the total density of 18.72 %, of which L. frutescens (6.53 %), C. torreyanus (3.43 %), S. berlandieri (2.24 %) and V. rigidula (2.60 %) were the most abundant. Stratum II (2.25 - 3.37 m in height) was represented by three equally represented species, which accounted for 0.11 % of the total proportion. Stratum I (3.38 - 4.5 m high) was represented only by Prosopis glandulosa, with a total density of 0.02 %.
Alpha diversity. The specific richness was 55 species, whose distribution of abundance was presented in a complex way. The entropy within the community was high (H´ = 2.69) and the dominance value was low (λ = 0.09), indicating overall an equitable trend in the distribution of abundance between species. However, in contrast, the abundance of the most dominant species showed a high influence on this distribution (Berger-Parker = 0.18), which could be considered as a reflection of the vertical and horizontal structures, corroborating these results.
Discussion
The structure analyzed in the present study indicated a greater dominance of small shrub species (height less than 1 m), highly branched, mostly devoid of thorns, with small leaves and some deciduous species. These results were corroborated with the Berger Parker index, which indicated a high influence of the dominant species (L. graveolens) in the distribution of abundance among the community components. These characteristics conform to the structure typical of a low semithorn shrubland (Miranda & Hernández-X. 1963, Treviño-Carreón & Valiente-Banuet 2005, Estrada-Castillón et al. 2012). Although this type of community structure can often be encompassed within the group of piedmont shrublands characteristic of the region, the species composition differs considerably. An example of this is the absence of dominant medium shrub species (height greater than 2 m) such as Helietta parvifolia, Fraxinus greggii, Rhus virens and Neopringlea integrifolia, which are frequently indicators and representatives of this type of associations (García-Hernández & Jurado 2008, Canizales-Velázquez et al. 2009, Estrada-Castillón et al. 2012, Alanís-Rodríguez et al. 2015b). In addition, the high dominance of Lippia graveolens, Calliandra conferta, Turnera diffusa and Krameria ramosissima, as well as a lower cover occupied by Leucophylum frutescens, keeps it distant from the thornless shrubland association commonly referred to as cenizal (González-Medrano 2003, Treviño-Carreón & Valiente-Banuet 2005). On the other hand, most of the species documented in the present analysis are endemic to northeastern Mexico, the Gulf of Mexico region, North America and the regions proposed by Rzedowski (1991), referred to as Megaméxico I and Megaméxico III. However, some of the species Calliandra conferta, Randia obcordata, Lantana camara, T. diffusa, Hibiscus martianus, Celtis pallida and Zanthoxylum fagara present a neotropical distribution (www.naturalista.mx), thus being similar in composition to the associations commonly referred to as Tamaulipan thornscrub (González-Medrano 2003, Mora-Donjuán et al. 2013a, Molina-Guerra et al. 2019). Considering the structure and species composition, the studied community can be grouped or at least related to the classification system proposed by Faber-Langendoen et al. (2018) in the Texas Barometerbush - Shrubby Blue Sage - Mexican Oregano Shrubland association, within the Tamaulipan Calcareous Thornscrub alliance, of the Tamaulipan Dry Mesquite & Thornscrub group, in the Tamaulipan Scrub & Grassland macrogroup (NatureServe 2021).
The low portions of this community could be associated with successional stages of the Tamaulipan thornscrub, and could be considered as Tamaulipan thornscrub or secondary vegetation in the mapping, as in the case of this study area (INEGI 2016). However, species composition and distribution of abundance does not correspond with that documented in various localities and derived from different disturbances. The main common characteristic of the disturbed areas is the high dominance of Vachellia farnesiana, Prosopis glandulosa, Vachellia rigidula and V. schaffneri species, both in disturbed areas and in regeneration by livestock activity (Alanís-Rodríguez et al. 2008, Pequeño-Ledezma et al. 2012, Alanís-Rodríguez et al. 2013, Jiménez-Pérez et al. 2013, Molina-Guerra et al. 2013, Mora-Donjuán et al. 2013b, Leal-Elizondo et al. 2018), agriculture (Alanís-Rodríguez et al. 2008, Jiménez-Pérez et al. 2013), mining (Marroquín-Castillo et al. 2016), and land clearing (Alanís-Rodríguez et al. 2008, Jiménez-Pérez et al. 2013). In addition, disturbed surfaces or those in a secondary succession stage are characterized by the presence and sometimes high dominance of invasive species (Dyderski & Jagodziński 2019). However, the studied surface only presented the species Cenchrus ciliaris and Abutilon theophrasti in very low densities. Thus, the option that this is a regenerating area could be ruled out. It has been mentioned that there is great heterogeneity in the scrublands of northeastern Mexico, where the areas of hillsides, with somewhat steep slopes, greater aridity and caliche soils are related to the presence of C. conferta, Croton torreyanus, J. dioica and K. ramossisima as well as other species of succulents such as Coryphantha runyonii, Mammillaria heyderii, Opuntia spp. and Cylindropuntia spp. (Reid et al. 1990). In addition, most of the thornless species tend to be concentrated in the areas of low hills (Pequeño-Ledezma et al. 2017). Considering the existing affinity between piedmont scrub and MET (Alanís-Rodríguez et al. 2015a), and the physical-biological characteristics found in the present investigation, it could be concluded that the plant association studied corresponds to a transitional plant community between MET and piedmont scrub.
The Berger-Parker index with the horizontal and vertical structure indicates a greater importance of small shrubs in the structural composition of the community. However, both the Shannon and Simpson indices showed equity in the distribution of abundance between species. This can be explained by the existence of species present in different strata, as well as the presence of many abundant species, such as L. frutescens, S. berlandieri and V. rigidula, in a similar way to those reported for MET (Alanís-Rodríguez et al. 2018). The sensitivity of these indices to this community characteristic has been documented not only in plant communities, but also in other biological groups, for which their use is frequently recommended in conjunction with other measures of diversity (Moreno 2001).
The low semi-thorn shrubland communities of northeastern Mexico and southern Texas can be considered a priority for conservation due to their restricted and discontinuous distribution compared to other types of shrubland, as well as the presence of threatened species such as some cacti and herbs such as Manihot walkerae (Garza et al. 2020, NatureServe 2021). Within the species composition of the studied community, individuals of Echinocereus poselgeri were found, one of the priority species for conservation listed under the category of Special Protection (Pr) in the NOM-059-SEMARNAT-2010 (SEMARNAT 2019). Furthermore, this type of association could possibly provide habitat for species such as Amoreuxia wrightii (Soto-Mata et al. 2018) and Frankenia johnstonii (NatureServe 2021), which are categorized as Endangered (P), and Manfreda longiflora (Foroughbakhch et al. 2013) listed as Threatened (A), which have been recorded in semi-thorn scrub in nearby localities. Similarly, Manihot walkerae and Ayenia limitaris, protected species in Texas, have been recorded (Garza et al. 2020, USFWS 2014, NatureServe 2021). In addition, the dominant species L. graveolens, T. diffusa, C. conferta, K. ramosissima, L. frutescens, Ephedra antisyphilitica, C. torreyanus, J. dioica, S. ballotiflora and Aloysia macrostachya, are used as food and for medicinal purposes (Hernández-Sandoval et al. 1991, Nicholson & Arzeni 1993, Guerra-Pérez 2016), for which their potential use in the fight against cancer is currently being analyzed (Avelino-Flores et al. 2015, Campos-Xolalpa et al. 2017). Therefore, further analysis of the conservation status of this type of shrubland is needed in order to design, if necessary, measures to ensure its preservation.
The Pretzsch index is very useful in the analysis of vertical structure, which is why it has been used in the study of Mexican shrublands (Mora-Donjuán et al. 2014). However, its design was developed based on studies of temperate forest trees that are considerably taller (Pretzsch 2009). For this reason, it was decided to use this design in a manner adjusted to the structural characteristics of the community studied, which allowed the strata that composed it to be favorably identified. The statistics of Pretzsch index to this study was similar to the results of original method to estimate Pretzch index, and the same similarity was observed in the calculation for tallest strata to MET reported in others investigations (Mora-Donjuán et al. 2014). It is also important to note that in both studies the calculations indicate the significance of the delimitation used to calculate the strata (for four strata in the case of the present work and for three strata in the consulted antecedents). However, in order to carry out a deep comparative analysis between both studies, it is necessary to consider the limiting height between the strata, in order to detect possible biases due to the influence of the most abundant species, but this data is frequently omitted in the report of the results. This is because the Pretzsch index is based on the Shannon index, which, along with other indices such as Simpson's, is susceptible to highly abundant species bias due to its logarithmic nature (Moreno et al. 2011). Likewise, the structure commonly reported in the MET tends to present a markedly homogeneous abundance distribution among individuals with heights between 0.00 to 2.00 m, being different for this semi-thorn shrub. Although this adjustment was useful in this research, since the presence of the low shrub stratum was evident to the naked eye, a later application would require the statistical determination of the height classes. Thus, it is recommended to incorporate this type of adjusted quantitative descriptor in vegetation studies, as well as to explore hierarchical and quantitative classification proposals such as those presented by Faber-Langendoen et al. (2018).
The presence of this plant association in this region, where land use change and vegetation cover loss is rapidly increasing, highlights the need to study the current status of shrublands. Tools such as niche modelers and remote sensing could be used to estimate the original potential distribution of these communities, the current area they cover, and project their fate considering different scenarios of habitat loss and climate change (Wang et al. 2017, Çoban et al. 2020). In addition, it is necessary to continue exploring the different shrublands of the Tamaulipan Biotic Province, incorporating both these analyses and diversity estimators and spectral signature determinations to preserve the plant communities of the region.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2970
Supplementary material, Table S1-S3











 text new page (beta)
text new page (beta)