Mezcal production is increasing in the last decade in México, Guanajuato state have been looking for an inclusion of norther municipalities in mezcal origin appellation. During the processes of tequila and mezcal production, the agave leaves are discarded and become a crop residue. To date, efforts have been made to exploit this residue as bioethanol and in the production of enzymes. Several studies have been focused on the recovery of fermentable sugars by chemical, enzymatic, and physical treatments followed by the use of sugars recovered for bioethanol or the production of enzymes (Close et al. 2017, Avila-Gaxiola et al. 2018, Contreras-Hernández et al. 2018, González-Llanes et al. 2018). In these cases, soluble carbohydrates extracted from leaves were hydrolyzed by thermal or enzymatic processes and transformed into fermentable reducing sugars. Fructans can also be obtained from agave leaves (Praznik et al. 2013). The agave fructans recovered from Agave salmiana leaves are a potentially valuable product.
Agave fructans are branched fructose polymers, their molecular structure is composed of fructose-fructose glycosidic bonds containing β(2-1) and β(2-6) linkages, with internal or external glucose units (López et al. 2003). Fructans are a soluble fiber that can act as a prebiotic in the gut microbiota. Fructans are a non-digestible fiber and are not hydrolyzed by human enzymes. Instead, fructans are used as a carbon source by microorganisms in the colon (Gomez et al. 2010). Besides their prebiotic effects, agave fructans have been reported to be immune system activators, body weight reducers, mineral absorption promoters, and microencapsulation agents (García-Vieyra et al. 2014, Moreno-Vilet et al. 2014, Ortiz-Basurto et al. 2017, Castillo-Andrade et al. 2018).
The degree of polymerization and the molecular weight distribution have an effect on the biological properties of agave fructans. For example, low- and medium-molecular-weight fructans extracted from Agave salmiana and A. tequilana presented greater prebiotic bacteria growth than high-molecular-weight fructans (García-Gamboa et al. 2018). The growth of some probiotic strains was greater when agave fructans with a low polymerization degree were used as the carbon source (Mueller et al. 2016). Moreover, agave fructans with a low degree of polymerization reduced the body weight and fat accumulation in mice fed high-fat diets (Márquez-Aguirre et al. 2013). Furthermore, the addition of low-polymerization-degree A. tequilana fructans reduced liver steatosis and hyperglycemia in mice fed high-fat diets (Márquez-Aguirre et al. 2016).
Agave tequilana fructans have been extensively studied because commercial agave fructans are extracted from this agave heads. However, few studies on A. salmiana fructans have since been published. The species A. salmiana is characterized by its broad, strong, and succulent leaves featuring long acuminate and sigmoid apices, short and solid stems with rosette shapes, and heights ranging from 1.5 to 3.4 m. This species has several different variants (Mora-López et al. 2011), including A. salmiana subspecies crassispina, which is characterized by its green color and a more developed apical spine compared to the A. salmiana subspecies salmiana, which has a larger size, wider leaves, and a cinder color. Studies on A. salmiana fructans focused on purification of the fructans by nanofiltration to remove low-molecular-weight sugars, thus enabling better drying (Reynoso-Ponce et al. 2017). On the other hand, Agave salmiana fructans have been tested in vitro as prebiotics and immune system activators (Moreno-Vilet et al. 2014, Martinez-Gutierrez et al. 2017) and as dietary supplement in rats (Castillo-Andrade et al. 2018).
With the aim to describe the molecular weight distribution of soluble carbohydrates contained in the leaves of Agave salmiana species, we extracted soluble carbohydrates and characterized them by size exclusion chromatography (SEC).
Materials and methods
Plant materials. The bases of the leaves close to the stems of Agave salmiana ssp. salmiana and Agave salmiana ssp. crassispina plants, approximately six years old, were harvested from northern Guanajuato in México (Figure 1). Twelve municipalities, that could be included in the appellation of origin Mezcal, were sampled: Ocampo (O), San Felipe (SF), San Diego de la Unión (SDU), Victoria (V), Xichú (X), Atarjea (A), Dolores Hidalgo (DH), Doctor Mora (DM), Santa Catarina (SC), Tierra Blanca (TB), San Miguel Allende (SMA), and Comonfort (C). Two agaves of each specie were sampled in each locality. The tissues were weighed before and after the cuticle and upper epidermis of the leaf were removed and the tissues were then fragmented into small pieces, washed with distilled water, and maintained at 4 °C for further use.
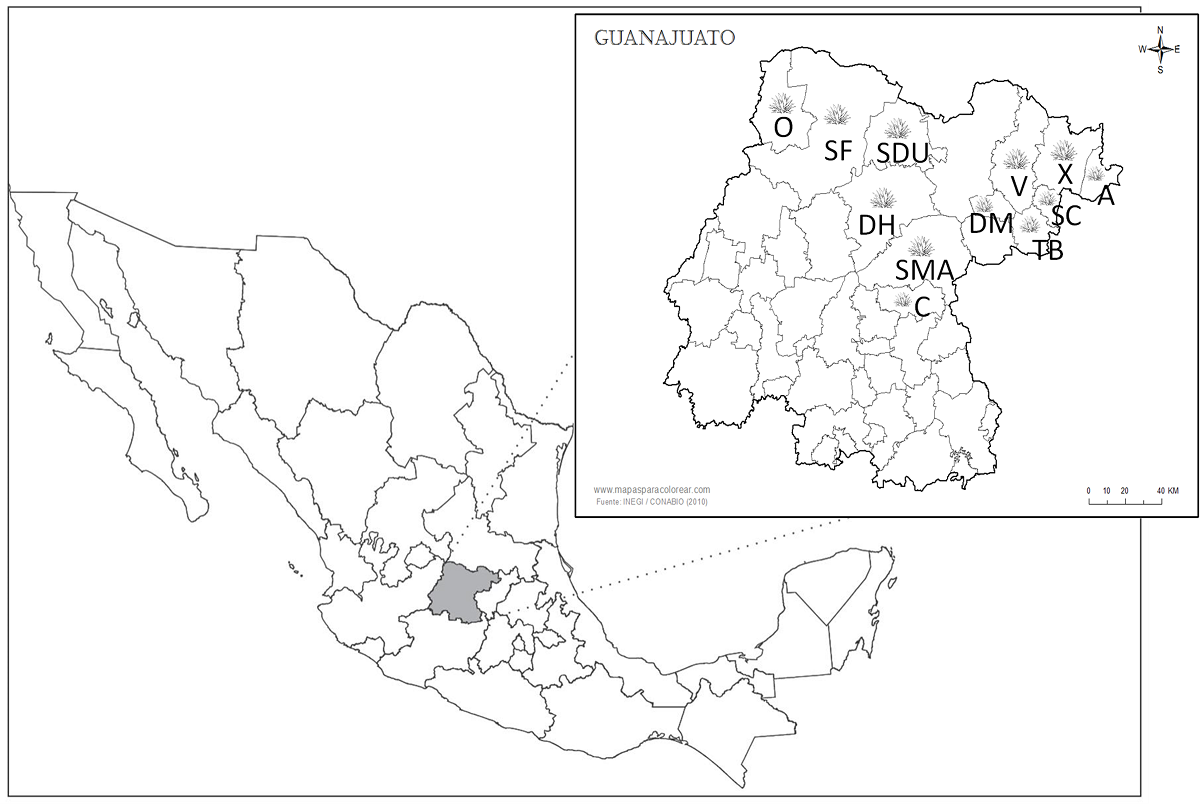
Fuente: INEGI / CONABIO (2010)
Figure 1 Map of sampled sites in Guanajuato, México. O: Ocampo; SF: San Felipe; SDU: San Diego de la Unión; V: Victoria; X: Xichú; A: Atarjea; DH: Dolores Hidalgo; DM: Doctor Mora; SC: Santa Catarina; TB: Tierra Blanca; SMA: San Miguel Allende; C: Comonfort.
Reagents. Analytical standards were used for the HP-SEC analysis. Maltopentaose DP5, maltohexaose DP6, maltoheptaose DP7, nystose DP4, 1-kestose DP3, sucrose, fructose and blue dextran were obtained from Sigma-Aldrich (Sigma-Aldrich, Saint Louis, Misuri, USA). Dextran 5000 Da and Dextran 12000 Da were obtained from Fluka Analytical (Fluka, Buchs, Switzerland). Commercial Agave tequilana fructans (Olifructine®) were obtained from Nutriagaves (Nutriagaves, Ayotlan, Jalisco, México). Degasified and distilled water pH 5.4, filtered through a 0.45 µm polyethersulfone (PES) membrane was used as the mobile phase, and 10 % acetonitrile was used as washing solution.
Soluble carbohydrate extraction. Extraction of concentrated agave juice from plant tissues was performed using a CARVER hydraulic press (Carver, Inc. Wabash, Indiana, USA). The fragmented leaf tissues were subjected once to a pressure of up to 20 tonnes until all juice was extracted. Once the agave juice was recovered, soluble solids (°Bx) were measured with a digital refractometer (Atago, Tokyo, Japan). To avoid enzymatic activity and hydrolysis of the agave fructans, the juice was subjected to heating at 70 °C on an IKA (Ika, Cincinnati, Ohio, USA) stirring plate at 800 rpm, for one hour. To obtain a clear liquid without sediments, the juices were centrifuged (Eppendorf 5810R, Hamburg, Germany) at 4,000 rpm for 10 minutes at 4 °C. The soluble carbohydrates were measured again after centrifugation. Extracts were then maintained at -80 °C for further use.
Sample conservation by spray drying. After extraction, enzymatic inactivation, and centrifugation, the samples were filtered through an 80-micron mesh and then spray dried. The spray dryer (Yamato model ADL311S, Yamato, Tokyo, Japan) was stabilized with sterile distilled water running for 10 minutes under the following conditions: pressure of 0.2 MPa and an inlet temperature of 150 °C. The agave juice was then diluted with distilled water in a 1:1 ratio and passed through the spray dryer. The resulting whitish powder was passed through the cyclone system and collected at the bottom of a container. Subsequently, the white powder recovered with a spatula was deposited in 50 mL centrifuge tubes and stored in a drying chamber for further analysis. Some samples were not susceptible to being dried and were instead frozen until their characterization.
Molecular weight distribution of agave fructans. High Performance Size Exclusion Chromatography (HP-SEC) was used to estimate the molecular weight distribution of the extracted agave fructans. The pulverized samples were diluted to 25 g/L in distilled water in triplicate within volumetric flasks of 5 and 10 mL and then filtered through a polyethersulfone membrane (PES) of 0.45 μm. Next, the solution was placed in 1.5 mL vials. The HP-SEC system consisted of a high-resolution liquid chromatograph, Waters model E2695, coupled with a refractive index detector. An Ultrahydrogel DP column (7.8 mm d.i. x 300 mm, Waters, Milford, MA, USA) was used as the stationary phase. The mobile phase was prepared with tridistilled water and filtered on a polyethersulfone membrane (PES) of 0.45 μm; then, the pH was adjusted to 4.5-5. The conditions were as follows: Pump A, 100 % tridistilled water, pH = 4.5; mobile phase at flow: 0.36 mL/min; temperature of the column: 61.7 °C; IR detector temperature: 50 °C, running time: 32 min; and amount of injected sample: 20 µL. A calibration curve was then made, with sugars ranging from 180 Da to 12,000 Da used as the standards. The calibration curve and number average molecular weight (Mn), weight average molecular weight (Mw), dispersity index (D), number average degree of polymerization (DPn), and weight average degree of polymerization (DPw) were determined; mono and disaccharides were not considered in calculations (Moreno-Vilet et al. 2017). The equations used to calculate these parameters are classical for polymers and are described below:
Statistical analysis. Statistical analysis was performed by one way ANOVA using the Fisher LSD method for means comparison at 95 % of confidence level. Minitab software was used for calculations.
Results
Sampling of Agave salmiana leaves. We performed a sampling of Agave salmiana leaves from twelve municipalities in the north of Guanajuato (Figure 1). Two subspecies of A. salmiana were sampled in each municipality: A. salmiana spp. crassispina (known locally as agave “verde”) and A. salmiana spp. salmiana (known as agave “cenizo”). The site with the greatest diversity of agave species was Victoria. However, in Atarjea and Xichu, A. salmiana spp. crassispina was more abundant than A. salmiana spp. salmiana. Meanwhile, in Ocampo and San Francisco, A. salmiana spp. salmiana was more abundant than A. salmiana spp. crassispina.
Water-soluble carbohydrate extraction. Water-soluble carbohydrates were extracted from the bottoms of the leaves (near the stems). Between 0.32 % and 2.22 % of the total leaf weight (wet basis) corresponded to water-soluble carbohydrates. The carbohydrate content was related to the place from which the samples were collected. Agaves collected from Doctor Mora (DM) had the greatest soluble sugar content, as shown in Figure 2. Meanwhile, agaves collected from Atarjea (A) contained six times fewer carbohydrates than agaves from DM (Figure 2). Notably, in almost all sampled sites, A. salmiana spp. crassispina contained a slightly greater content of soluble carbohydrates (0.74 %) compared to the A. salmiana spp. salmiana subspecies (0.68 %).
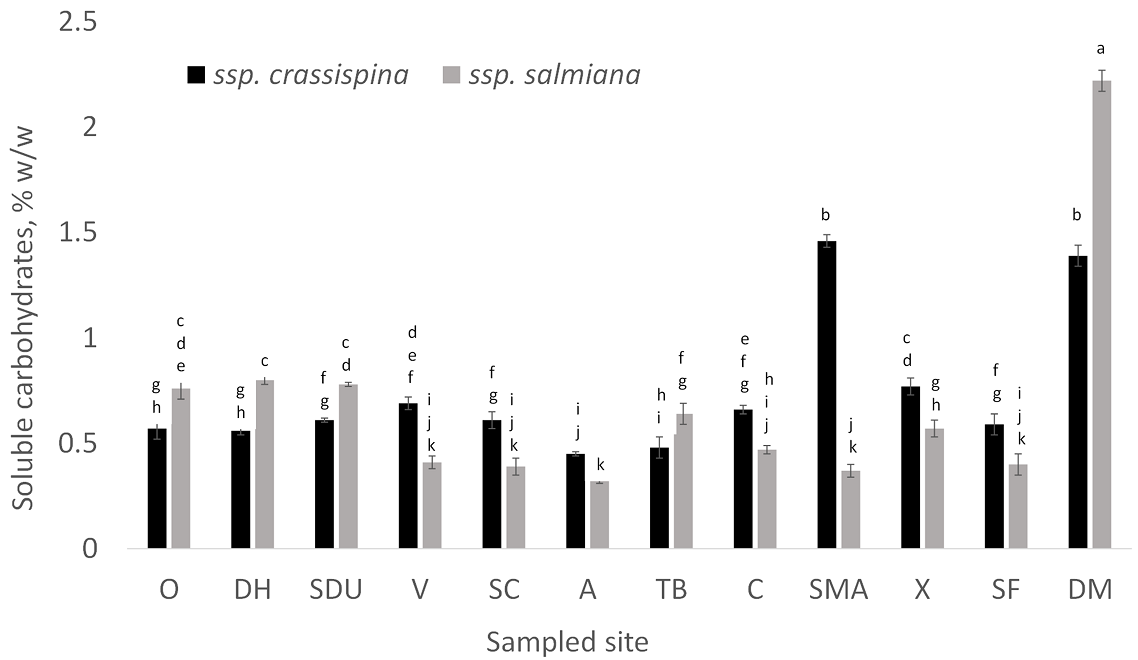
Figure 2 Content of water-soluble carbohydrates. The percentage refers to the grams of carbohydrate per 100 grams of sample (wet basis). The results are the average of three replicate samples. O: Ocampo; DH: Dolores Hidalgo; SDU: San Diego de la Unión; V: Victoria; SC: Santa Catarina; A: Atarjea; TB: Tierra Blanca; C: Comonfort; SMA: San Miguel Allende; X: Xichú; SF: San Felipe; DM: Doctor Mora. Means comparison was performed using Fisher LSD. Lower case letters: means that do not share a letter are significantly different.
Molecular weight distribution of agave fructans. The molecular weight distribution of fructans extracted from Agave salmiana was determined by HP-SEC, as described in the Materials and Methods section (Table 1). The average molecular weight in number (Mn) fluctuated from 1,156 to 4,933 g/mol, with an average of 3,209 g/mol. The average molecular weight in weight ranged from 1,975 to 6,432 g/mol, with an average of 5,046 g/mol. The number average degree of polymerization (DPn) fluctuated between 7 and 30, with an average of 19. The weight average degree of polymerization (DPw) ranged from 12 to 39, with an average of 30. In general, A, salmiana spp. salmiana presented a lower molecular weight and DP than A, salmiana spp. crassispina. The content of lower degree of polymerization fructans was also greater in A, salmiana spp. salmiana (55 %) than in A, salmiana spp. crassispina (47 %) (Figure 3).
Table 1 Molecular weight distribution of fructans extracted from Agave salmiana
| Sampled site | ssp. | Mn g/mol |
Mw g/mol |
DPn | DPw | D | DP>10 (%) |
DP<10 (%) |
|---|---|---|---|---|---|---|---|---|
| O | salmiana | 4620 ± 52ab | 5652 ± 5cdefg | 28 ± 1ab | 34 ± 1bcdef | 1.2 ± 0.1k | 80 ± 1b | 20 ± 1j |
| crassispina | 4568 ± 45abc | 6432 ± 27a | 28 ± 1abc | 39 ± 1a | 1.4 ± 0.1ij | 64 ± 1cd | 36 ± 1hi | |
| DH | salmiana | 2078 ± 23op | 4703 ± 8jk | 13 ± 1m | 29 ± 1jk | 2.2 ± 0.1cd | 29 ± 1i | 73 ± 2c |
| crassispina | 3933 ± 52efg | 6074 ± 57abcde | 24 ± 1efgh | 37 ± 1abcd | 1.5 ± 0.1ghi | 55 ± 1efg | 45 ± 1fg | |
| SDU | salmiana | 4096 ± 23de | 4765 ± 21jk | 25 ± 1de | 29 ± 1jk | 1.1 ± 0.1k | 88 ± 1a | 12 ± k |
| crassispina | 2627 ± 238mn | 5533 ± 282efgh | 16 ± 2kl | 34 ± 2defg | 2.1 ± 0.1d | 32 ± 3i | 68 ± 3c | |
| TB | salmina | 1293 ± 15q | 3436 ± 17l | 8 ± 1n | 21 ± 11 | 26 ± 0.1a | 10 ± 1k | 90 ± 1a |
| crassispina | 3551 ± 50ghi | 5492 ± 23fgh | 22 ± 1hi | 34 ± 2efgh | 1.5 ± 0.1ghi | 58 ± 3de | 42 ± 3gh | |
| A | salmiana | 3286 ± 15ijk | 5552 ± 14defgh | 20 ± 1ij | 34 ± 1cdefg | 1.7 ± 0.1efgh | 45 ± 1h | 55 ± 1de |
| crassispina | 4065 ± 262de | 4802 ± 229ijk | 25 ± 2def | 30 ± 2ijk | 1.1 ± 0.2k | 84 ± 7ab | 16 ± 7jk | |
| SC | salmiana | 2344 ± 36no | 3489 ± 141l | 14 ± 1lm | 21 ± 1l | 1.4 ± 0.1ij | 43 ± 1h | 57 ± 1de |
| crassispina | 4348 ± 225bcd | 5007 ± 5hij | 27 ± 2bcd | 31 ± 1ghijk | 1.1 ± 0.1k | 86 ± 4ab | 17 ± 1jk | |
| C | salmiana | 1902 ± 9p | 4448 ± 180k | 12 ± 1m | 27 ± 1k | 2.3 ± 0.1bc | 20 ± 2j | 80 ± 2b |
| crassispina | 3002 ± 533klm | 5363 ± 663fghi | 18 ± 3jk | 33 ± 4efghi | 1.7 ± 0.1ef | 43 ± 4h | 58 ± 4d | |
| SMA | salmiana | 4933 ± 248a | 5868 ± 285bcdef | 30 ± 2a | 36 ± 2abcde | 1.1 ± 0.1k | 82 ± 1ab | 18 ± 1jk |
| crassispina | 3683 ± 1fgh | 6120 ± 51abc | 23 ± 1fghi | 37 ± 1abcd | 1.6 ± 0.1ghi | 50 ± 1fgh | 50 ± 1ef | |
| X | salmiana | 3623 ± 114fghi | 6208 ± 174ab | 22 ± 1ghi | 38 ± 1ab | 1.7 ± 0.1efg | 50 ± 2fgh | 50 ± 1de |
| crassispina | 4226 ± 12cde | 6122 ± 41abc | 26 ± 1cde | 38 ± 1abc | 1.4 ± 0.1i | 62 ± 1de | 39 ± 1gh | |
| SF | salmiana | 2036 ± 133op | 4895 ± 217ijk | 12 ± 1m | 30 ± 2hijk | 2.4 ± 0.1ab | 21 ± 2j | 79 ± 2b |
| crassispina | 3404 ± 161hij | 5766 ± 138bcdef | 21 ± 1ij | 35 ± 1bcdef | 1.6 ± 0.1efgh | 47 ± 2h | 53 ± 2de | |
| V | salmiana | 3981 ± 42def | 6100 ± 187abcd | 24 ± 1defg | 38 ± 1abcd | 1.5 ± 0.1hi | 57 ± 2ef | 44 ± 2fg |
| crassispina | 3060 ± 252jkl | 5209 ± 11ghij | 19 ± 2j | 32 ± 1fghij | 1.7 ± 0.1efg | 48 ± 5gh | 54 ± 2de | |
| DM | salmiana | 1156 ± 21q | 2113 ± 25m | 7 ± 1n | 13 ± 1m | 1.8 ± 0.1e | 8 ± 1k | 92 ± 1a |
| crassispina | 1211 ± 42q | 1975 ± 187m | 7 ± 1n | 12 ± 1m | 1.6 ± 0.1fgh | 8 ± 1k | 92 ± 1a | |
| R | A. tequilana | 2701 ± 200lmn | 3416 ± 184l | 16 ± 1kl | 20 ± 1l | 1.26 ± 0.1jk | 70 ± 3c | 30 ± 2i |
Mono and disaccharides are not considered in calculations. O: Ocampo; DH: Dolores Hidalgo; SDU: San Diego de la Unión; V: Victoria; SC: Santa Catarina; A: Atarjea; TB: Tierra Blanca; C: Comonfort; SMA: San Miguel Allende; X: Xichú; SF: San Felipe; DM: Doctor Mora; R: commercial agave fructans from Agave tequilana; Mn: number average molecular weight; Mw: weight average molecular weight; D: dispersity index; DPn: number average degree of polymerization; DPw: weight average degree of polymerization; DP > 10: fructans with higher polymerization degree; DP < 10: fructans with lower polymerization degree. Means comparison was performed using Fisher LSD. Means that do not share a letter are significantly different.
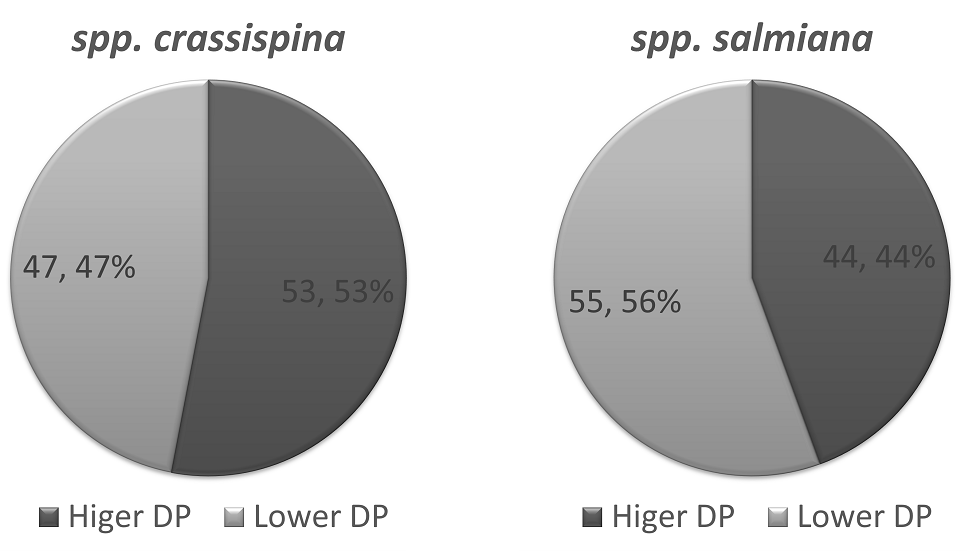
Figure 3 Distribution of agave fructans with a lower and higher degree of polymerization (DP) according to the agave subspecies.
The molecular weight distributions of the fructans extracted from A, salmiana and commercial agave fructans were also compared. Ten samples of commercial fructans (Olifructine®) extracted from A, tequilana were analyzed (Table 1, sample R). The molecular mass and degree of polymerization was calculated for the ten samples, and the averages obtained were as follows: Mn: 2,701 g/mol; Mw: 3,416 g/mol; DPn: 16; DPw: 20; DP > 10: 70; and DP < 10: 30. The molecular weight distribution averages from the A, salmiana fructans were as follows: Mn: 3,209 g/mol; Mw: 5,046 g/mol; DPn: 19; DPw: 30; DP > 10: 48; and DP < 10: 52. We observed a higher molecular weight and average degree of polymerization in the A, salmiana fructans. Analyzing the abundance of fructans according to their polymerization degree revealed a greater abundance of A, salmiana fructans of between 23 and 32 DP, whereas A, tequilana fructans had a lower abundance between 4 and 20 DP (Figure 4). On the other hand, the proportion of fructans with lower and higher degrees of polymerization were remarkably different between A. salmiana and A. tequilana. Fructans extracted from the leaves of A. salmiana featured a greater proportion of fructans with a lower degree of polymerization than that of the commercial fructans extracted from A. tequilana stems (Table 1).
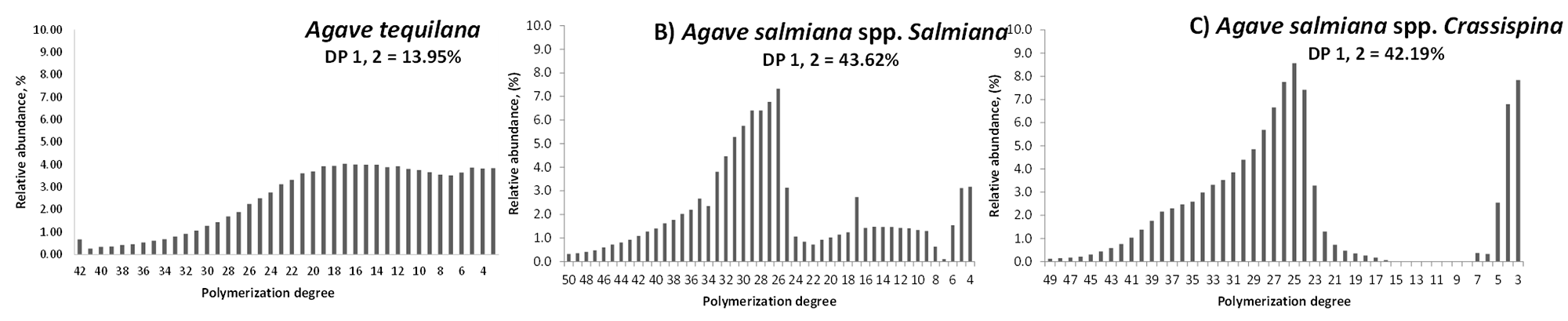
Figure 4 Molecular weight distribution of agave fructans. Relative abundance of fructans according to their polymerization degree. A: commercial agave fructans from Agave tequilana Olifructine®; B: fructans from Agave salmiana spp. salmiana leaves; C: fructans from Agave salmiana spp. crassispina leaves. DP 1, 2 %: content of mono and disaccharides in the samples.
Discussion
Agave leaves are a type of crop waste from the mezcal and tequila industries. In the last five years, research has focused on exploring options to exploit these leaves to produce high-value products. However, these studies have focused primarily on the recovery of fermentable sugars from agave leaves for use as carbon sources in the production of biofuels and enzymes (Close et al. 2017, Avila-Gaxiola et al. 2018, Contreras-Hernández et al. 2018, González-Llanes et al. 2018). However, agave leaves synthesize fructans that have been shown to offer digestive health benefits (Gomez et al. 2010, Praznik et al. 2013). Therefore, the extraction of fructans from leaves is of great interest especially fructans from agaves such as A. salmiana, which have been rarely studied. On the other hand, municipalities of northern Guanajuato are applying for Denomination of Origin of Mezcal (DOM) status, and an increase in agave crop residue generation is expected (Capilla-Vilchis 2017). Thus, the different potential processes for exploiting fructans represents a compelling avenue of research.
In this study, we observed great differences between soluble carbohydrate content in sampled agave leaves, ranging between 0.32 and 2.22 % (Atarjea and Doctor Mora, respectively). These differences indicated a relationship with the place of sampling. It was previously reported that soil composition, climate, and other factors, such as agave age, are related to the carbohydrate composition and fructan structure in agave plants (Arrizon et al. 2010, Mellado-Mojica & López 2012). Although the agaves sampled were all A. salmiana, the climate differed greatly between the sampled locations. Atarjea is located in the “Sierra Gorda” of Guanajuato and contains several microclimates (tropical, warm-humid, and semi-warm), while the Doctor Mora climate is predominantly temperate-dry.
The fructan content in the agave leaves of some agave species has been determined. The content of fructans in A. durangensis leaves was reported as 8.9 % (Contreras-Hernández et al. 2018), which is greater than the 2.22 % from the A. salmiana leaves tested in the present study. The powder obtained from A. tequilana leaves presented fructan content of 37.5 % (dry matter) (Avila-Gaxiola et al. 2018). A recent study noted that the fructan content in agave leaves decreases according to the distance between the leaves and the stem, with higher levels observed closer to the bases of the leaves near the stem (González-Llanes et al. 2018). The fructan content at the bases of the A. salmiana leaves measured in this work was lower than that observed in the different agave species tested by Gonzalez-Llanes: A. salmiana spp. salmiana (6.8 %), A. salmiana spp. crassispina (6 %), and A. americana (3 %) (González-Llanes et al. 2018). The leaves collected from two-year-old Agave tequilana plants were dried, and agave fructans were extracted, detecting 70.9 g of fructans/kg of agave (dry weight) (Close et al. 2017).
It is known that the properties of fructans depend on their degree of polymerization. For example, fructooligosaccharides (DP < 10) and mildly polymerized fructans are effective as prebiotics (Biedrzycka & Bielecka 2004). Agave fructans with low and medium degrees of polymerization perform well as prebiotics (Mueller et al. 2016, García-Gamboa et al. 2018). On the other hand, agave fructans with large degrees of polymerization have been reported to function as fat replacers in baked products (Santiago-García et al. 2017). The fructans extracted from Agave salmiana leaves showed diverse average polymerization degrees ranging from 7 to 30, with an average DPn of 19 (mono and disaccharides were not considered in calculations). This diversity could be attributed to the soil composition and climate of the sampling locations, in addition to the ages of the agaves. In the present study, the ages of the samples were estimated to be six years old; however, the ages of wild agaves remain uncertain. It was previously reported that young agaves contain fructans with a lower degree of polymerization, while mature agaves feature fructans with a higher degree of polymerization. In addition, the branching of agave fructans increases with age (Arrizon et al. 2010, Mellado-Mojica & López 2012).
We next compared fructans extracted from A. salmiana leaves with commercial fructans. Commercial fructans showed the greatest proportion of high-molecular-weight fructans. On the other hand, fructans from A. salmiana leaves presented almost equal proportion of low- and high-molecular-weight fructans; indeed, some samples contained more than 90 % low-molecular-weight fructans. Agave tequilana synthesizes low-molecular-weight fructans in its leaves. Later, these fructans are transported to the stem and transformed into high-molecular-weight fructans (Praznik et al. 2013).
According to the results, all sampled agave leaves have the potential to be exploited, but the agave leaves collected from DM presented the greatest quantity of soluble carbohydrates, and the largest amount of short-chain agave fructans DP < 10. These characteristics make DM agave leaves an interesting subject for future studies.
The technical feasibility to obtain fructans from A. salmiana leaves was demonstrated in this study. Water-soluble carbohydrates were recovered from the bases of the leaves near the stem, and fructans represented 0.7 % of leaf weight on a wet basis. Fructans content in the leaves is low but it is proposed by other authors the use of the leaves in different applications, if we think in a biorefinery scheme where more than one single product is obtained the agave leaves exploitation could be successful. The fructans extracted were susceptible to be dried. Moreover, we determined the molecular weight distribution of the fructans extracted from the leaves. We found great diversity in molecular weight distributions, but, in general, the leaves of A. salmiana contained a low proportion of high-molecular-weight fructans compared to commercial agave fructans. However, the average DPn of high-molecular-weight fructans (DPn = 19) in the samples tested was greater than that of commercial fructans (DPn 16). Ultimately, leaves from Agave salmiana are a good source of fructans, but the commercial feasibility of these leaves must be explored further.











 nueva página del texto (beta)
nueva página del texto (beta)



