Agastache foeniculum (Pursh) Kunze, commonly known as anise hyssop is a perennial herbaceous plant belonging to the Lamiaceae family. This aromatic plant is native to South America and also grows in the rocky hills of the Mediterranean region as well as in northern and Western Europe (Zielińska & Matkowski 2014). Aerial parts of A. foeniculum contain essential oil (EO) (1.4 to 2 %), which has antibacterial and antifungal properties. Also, the active ingredients of this plant are used in the food, pharmaceutical, cosmetics and also in ice cream, and beverage industries (Omidbaigi & Sefidkon 2003).
One of the most important factors affecting the growth and yield of crops is accessibility to water. Water stress reduces the water potential, negatively affecting physiological, biochemical processes and productivity in plant species (Naghizadeh et al. 2019). Adaptation and tolerance to water-deficit are the results of physiological and biochemical responses that lead to the conservation of water in the tissue, maintenance of chloroplasts and homeostasis ions (Rai et al. 2012). Therefore, knowing the response of plants to moisture deficit is necessary to improve our understanding of how drought affects plant ecology and plant functional traits, as this will impact agricultural productivity and vegetation management (Guo et al. 2006).
To survive the water-deficit-mediated oxidative stress, plants use either non-enzymatic anti-oxidative system such as flavonoids, phenols compounds, and anthocyanins are the most important antioxidants that scavenge both free radicals and their excessive production. Also, regulation of antioxidant compounds by exogenous materials can mediate crop tolerance to environmental stress (Arnao & Hernandez-Ruiz 2014, Shi et al. 2007).
Melatonin (Mel) is an amphipathic derivative of an important amino acid that can freely cross cell membranes and distribute to any aqueous compartment including the nucleus, cytosol, and mitochondria (Menendez‐Pelaez et al. 1993). Mel has emerged as a vital multifunctional signaling molecule, is ubiquitously distributed in different parts of a plant that regulates plant responses and tolerance to environmental stresses (Arnao & Hernandez-Ruiz 2019, Wei et al. 2018). It is known to be an endogenous free radical scavenger and a broad-spectrum antioxidant which protects plants against environmental stresses and increases crop production (Tan et al. 2007, 2012). For example, it has been reported to stimulate the growth of coleoptiles in wheat, oats, and barley (Hernández‐Ruiz et al. 2005). Studies also show that Mel could improve the plant production (Manchester et al. 2015). Thus, Mel-rich plants or plants to which Mel has been applied exogenously have a higher potential for enhanced growth and tolerance to stress such as drought, salinity, high temperature, heavy metals, and pathogen attack (Manchester et al. 2000, Naghizadeh et al. 2019, Manchester et al. 2015).
Sustaining crop yield is an important task in current agriculture, and to produce stress-tolerant crop plants, water stress is the major limiting factor for oasis-desert agricultural production of crops. It is necessary to improve A. foeniculum for drought tolerance and minimize drought-related crop losses. Because secondary metabolites derived from medicinal plants are unique sources for pharmaceuticals, food additives, flavors, and industrially important biochemical, furthermore accumulation of such metabolites often increases in plants under drought stress conditions (Akula & Ravishankar 2011). As a result, by applying water deficit while saving water, it is possible to achieve an acceptable performance of metabolites (Selmar & Kleinwächter 2013). Mel also has a significant effect on plant growth and defense mechanism against environmental factors and stresses (Tan et al. 2012).
Therefore, the goal of the present study was to evaluate the effect of different concentrations of Mel on the growth and phytochemical responses of A. foeniculum under water-deficit stress conditions. Deciphering the Mel-mediated mechanisms in the plant protection responses and metabolic pathways under unfavorable conditions is required to gain insight into their potential, it will open new approaches to exploit Mel as a plant growth regulators tool against drought in sustainability and food security.
Materials and methods
The present study was performed as a factorial experiment based on the randomized completely block design, with three replications, in the research greenhouse at the Azarbaijan Shahid Madani University, Tabriz, Iran in 2019. The first factor was water-deficit with three levels (i.e., withholding watering up to severe water-deficit stress (30 % of field capacity (FC), moderate water-deficit stress (60 % FC) as well as normal irrigation (as control)) and the second factor was foliar spraying melatonin (Mel) i.e., 0, 50 and 100 μM. In the current study, Mel powder (purity: ≥ 98 %) was purchased from Sigma-Aldrich Company. The characteristics of the pots soil are presented in Table 1. Then, six uniform seeds of A. foeniculum were sown in each plastic pot that contained 10 kg soil (25 cm in length and 30 cm in diameter). The seedlings were thinned later and only three plants were kept after emergence in each pot. The plants were kept in the greenhouse with day lengths of 16 hr at 25 °C and night lengths of 8 hr at 17 °C. Exertion of water-deficit stress treatments and foliar application of Mel was done before the plant flowering stage. Execution timeline in the different steps of the experiment was regarded as: seed planting date: 2019-7-27, plants well irrigated up to stem elongation phase until 2019-10-25, then treatment application performed in three continues weeks until 2019-11-16 (October 25-November 16), from 2019 November 17 treatment application stopped, finally samples harvested at full flowering at 2019-12-21. For water deficit, all pots were then weighed and kept well-watered until the stem elongation stage, from the before flowering, when water-deficit was imposed by withholding watering until the soil moisture content was approximately 30 and 60 % of field capacity. However, non-stressed plants were kept at soil moisture content to about 85 % and continually irrigated normally through the entire experimental period. Pots were weighed every 1-2 days to monitor water use and adjust watering as plant demand increased. Time Domain Reflectometer (TDR) moisture meter was used to measure the soil moisture of each pot for applying the water-deficit treatment. Foliar spraying of Mel was done on the plants by hand atomizer at sunset for three consecutive weeks until the solution started to drip from the leaves. Control pots (without Mel foliar application) sprayed just with distilled water. Tween-20 at 0.05 %was used as a surfactant in foliar application.
Table 1 Physical and chemical characteristics of the studied soil
| pH | EC (dSm-1) | Organic Matter (%) | Clay (%) | Silt (%) | Sand (%) | Texture |
|---|---|---|---|---|---|---|
| 7.20 | 2.20 | 2.76 | 29.18 | 40 | 30.82 | Clay loam |
During the complete flowering stage (date: 2019-12-21), the plants were harvested for each treatment and then, the above-ground tissue was separated, and transferred to the laboratory to be dried in shade conditions. Total phenol, total flavonoids, total anthocyanins content, essential oil EO) content, and EO compounds were measured.
Plant extraction was done by the ultrasonic method using Powersonic 405 ultrasonic device (South Korea's Haushin Technology Company) (Baskan et al. 20017). Ten ml of 80 % methanol were added to 0.4 g finely grounded dried plant in a 25 ml Erlenmeyer and placed in an ultrasonic bath for 30 minutes. The contents of the Erlenmeyer flask were then passed through a filter paper and the pure extract was stored in small dark glass jars at 4 °C until the future analyses.
Content of total chlorophyll. Shoot fresh tissues (0.1 g each sample) were grounded in 5 mL of acetone (80 % V/V) to extract photosynthetic pigments. The samples absorbance was measured at 645, and 663nm in a T80+ UV-Vis spectrophotometer (PG Instrument Ltd., UK) (Lichtenthaler &Wellburn 1983).
Content of total phenols. Two ml of sodium carbonate (2 %), 2.8 ml of distilled water, and 100 μl of Folin-Ciocalteu reagent (50 %) were added to 100μl of extract. After 30 min, the absorption by the spectrophotometer was read at 725 nm. Gallic acid was used as the standard; the total phenol content was calculated based on the mg of gallic acid per g of dry weight (mg GAE g-1) (McDonald et al.2001).
Content of total flavonoids. This assay was performed based on Chang et al. (2002). Thus, 0.1 ml of 10 % aluminum chloride was added to 0.5 ml of extract, and then the mixture was mixed with 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. Thirty minutes elapsed at laboratory condition, their adsorption was measured at 415 nm and the standard curve was plotted based on different concentrations of quercetin. The amount of flavonoid was determined to milligram equivalent of quercetin per gram of dried plant powder.
Content of total anthocyanin. The pH differential method, consisting of KCl buffer (0.025 M, pH 1.0) and CH3COONa buffer (0.4 M, pH 4.5), was applied to measure the content of total anthocyanin of the extract (Sutharut & Sudarat 2012).1 mL of the extract was mixed with 4 ml of the buffers and kept for 15 min at 28 ºC thus that the solution could equilibrate. The absorbance was measured at 510 and 700 nm using deionized water as a blank. The result was converted to mg of cyanidin-3-glucoside equivalents (CGE) per g dry weight.
Essential oil content. The EO was extracted by hydro-distillation (25 g each sample) in Clevenger-type apparatus for three h according to the method suggested by European Pharmacopoeia (British Pharmacopoeia 1998). The EO was dried over Na2SO4and kept at 4 °C until analyzed.
Composition of essential oils. TRACE MS gas chromatography equipped with a DB-5 fused silica column (30m × 0.25mm, film thickness 0.25µm) was used to perform GC analyses. For 4 min oven temperature was kept at 60°C which then reached to 250 °C at the rate of 4 °C/min. Nitrogen was used as carrier gas with flow rate: 1.1 ml/min while the temperature of the injector and detector (FID) held at 280 °C; calculation of percentages were done by electronic integration of FID peak areas where no response factor correction were applied. In case of GC-MS analyses, quadrupole system equipped with a DB-5 fused silica column (30m × 0.25mm, film thickness 0.25µm) were used; helium applied as carrier gas with a linear velocity of 31.5cm/s, split ratio 1/100, ionization energy 70 ev with the scan time 0.4s and mass range 40-460 amu where the oven temperature program was set as 60-250 °C at a rate of 5 °C/min and transfer line temperature was 250 °C. The same experimental conditions were set for the calculation of the retention indices (RI) that was based on the retention times of injected n-alkenes (C6-C40). These RIs were compared to identify the EO compounds. To obtain the compound mass spectra, Xcalibur (2.07), with the installed libraries was used. Finally, the area normalization method, was applied for the determination of compounds contents (%) without considering the response factors (Adams 2007).
Statistical analysis. The data were analyzed with SAS v. 9.1 program and the comparison of means test was done using Duncan's test and the diagrams were plotted by Excel 2013.
Results
Plant shoot dry weight. Plant shoot dry weight was affected by the interaction of water-deficit stress and Mel (Table 2). The irrigation at 60 % FC increased the shoot dry weight up to 20 % compared to the well-watered treatment, but under severe water-deficit stress, the plant dry weight decreased up to 44 % compared to well-watered treatment. On the other hand, Mel increased the plant shoot dry weight by 26 and 54 % (at 50 and 100 µM Mel, respectively) over the respective control plant under well-watered conditions. Moreover, shoot dry weight in A. foeniculum was increased by 42 % under moderate water-deficit stress with both concentration of applied Mel treatment compared to control. However, the application of Mel (at 50 and 100 µM) recorded an increase of 25 and 27 %, respectively, over the untreated plants under water deficit stress conditions (Table 3).
Table 2 Analysis of variance for studied traits in Agastache foeniculum plants with or without melatonin foliar spray under different irrigation regimes.
| Source of variation (S.O.V) | df | Mean squares | |||||
|---|---|---|---|---|---|---|---|
| Total dry matter | Total chlorophyll content | Total phenol | Total anthocyanin | Total flavonoid | Essential oil content | ||
| Block | 2 | 3.434 ns | 0.132 | 20.014ns | 0.011ns | 10.325** | 0.139ns |
| Factor a (Water regimes) | 2 | 73.639** | 0.173** | 107.956** | 0.535** | 32.041** | 0.369** |
| Factor b ( Melatonin application) | 2 | 15.838** | 0.017** | 62.506** | 0.1656** | 4.903** | 0.056** |
| Interaction a×b | 4 | 2.175** | 0.001 ns | 4.254** | 0.028** | 0.255** | 0.028** |
| Error | 16 | 0.307 | 0.003 | 0.746 | 0.001 | 0.150 | 0.005 |
*, **: significantly different at the 5 and 1 % probability level, respectively, ns: non-significant.
Table 3 Effect of melatonin foliar spray on some traits of Agastache foeniculum plants under different irrigation regimes.
| Water regimes | Melatonin application |
Total chlorophyll content(mg g-1) |
Total dry matter (g plant-1) |
Phenol (mg GAE g-1) |
Anthocyanin (mg CGE g-1) |
Flavonoid (mg QUE g-1) |
Essential oil content (w/w%) |
|---|---|---|---|---|---|---|---|
| Well-watered | 0 (distilled water) | 0.94 ab ±0.07 | 6.30d±0.51 | 21.88f±0.51 | 0.06i±0.03 | 8.56d±0.34 | 0.33d±0.08 |
| 50 µM | 0.97 ab ±0.06 | 7.97c±0.47 | 22.80f±0.48 | 0.15h±0.03 | 9.09d±0.52 | 0.37d±0.07 | |
| 100 µM | 1.03a±0.05 | 9.75b±0.50 | 24.71e±0.66 | 0.18g±0.03 | 9.64d±0.69 | 0.37d±0.06 | |
| Severe water-deficit stress (30 %) FC | 0 (distilled water) | 1.09a±0.05 | 7.57c±0.45 | 27.01cd±1.41 | 0.23f±0.02 | 10.99c±0.70 | 0.57c±0.05 |
| 50 µM | 1.10a±0.04 | 10.81a±0.42 | 28.74b±1.52 | 0.56d±0.02 | 11.65c±0.66 | 0.55c±0.07 | |
| 100 µM | 1.19a±0.07 | 10.78a±0.41 | 32.18a±1.48 | 0.71a±0.03 | 12.20c±0.68 | 0.64c±0.06 | |
| Moderate water-deficit stress (60 % FC) | 0 (distilled water) | 0.82ab±0.10 | 3.52e±0.38 | 25.67de±0.42 | 0.49e±0.02 | 11.72c±0.71 | 0.57c±0.12 |
| 50 µM | 0.85ab±0.08 | 4.40e±0.47 | 27.97bc±0.46 | 0.61c±0.02 | 12.77b±0.78 | 0.78b±0.11 | |
| 100 µM | 0.88ab±0.09 | 4.48e±0.43 | 33.14a±0.51 | 0.68b±0.02 | 13.87a±0.81 | 0.94a±0.01 |
*: Means followed by the same letter(s) in each column are not significantly different based on Duncan’s Multiple Range Test (n = 3).
Essential oils content and composition. The EO content of A. foeniculum was affected by the interaction of water-deficit and Mel foliar application (Table 2). The maximum EO content (0.94 %) was obtained under severe water-deficit stress and100 µM Mel was used (Table 3). In general, water-deficit and exogenous application of high concentration Mel could increase the content of EO.
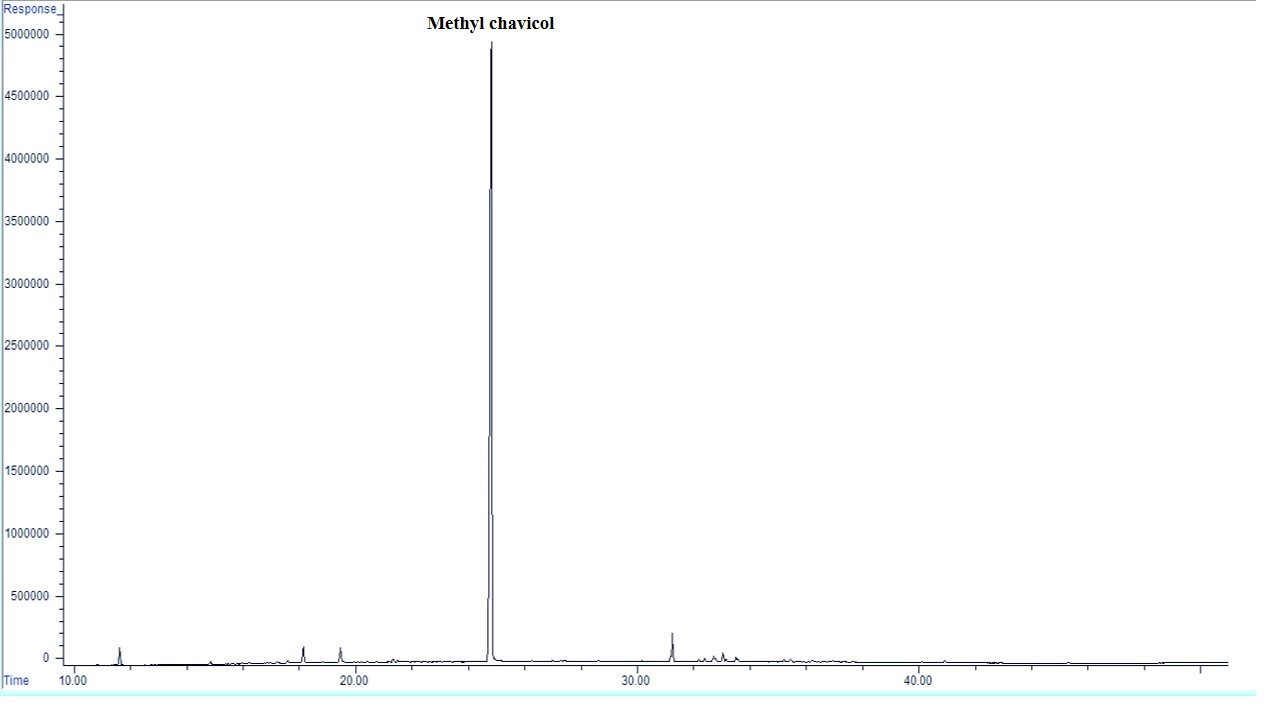
There is a difference in the EO composition of the treated plants (Figure 1, Table 4). Based on GC/MS analysis, a total of 10 chemical compounds (98.35-99.43 %) were identified in A. foeniculum EO. Compounds obtained from all samples consist of essentially hydrocarbon monoterpenes (limonene and β-ocimene), oxygenated monoterpenes (pulegone, geraniol, trans-carvone oxide, bornyl acetate and carvyl acetate), oxygenated sesquiterpene (spathulenol and caryophyllene oxide) and phenylpropene (methyl chavicol). Hydrocarbon monoterpenes ranged from 0.84 to 6.64 % of the total, of which limonene accounted for 0.75-3.74 % and β-ocimene accounted for 0.25-3.6 5 %. Oxygenated monoterpenes also including 2.45-15.85 % of EO compounds, pulegone, 1.03-3.07 %, geraniol 0.08-5.58 %, trans-carvone oxide 0.08-2.46 %. Bornyl acetate contained 0.03-11.42 % and carvyl acetate 0.05-1.54 % of EO, respectively. Oxygenated sesquiterpene also accounted for 1.55-6.37 percent of the EO compounds, of which spathulenol0.11-5.16 % and caryophyllene oxide 0.12-2.46 %EO compounds were included under the studied treatments. The main component of A. foeniculum EO was methyl chavicol (74.90-92.73 %) with increasing the intensity of water stress and foliar application of Mel, its content increased, the highest content was obtained under severe water-deficit stress and application of Mel at 100 µM, compared with the control treatment was 19 % higher.
Table 4 Composition of essential oils in Agastache foeniculum plants with or without melatonin foliar spray under different irrigation regimes.
| Compounds | RI | LRI | Well-watered | Moderate water-deficit stress (60 % FC) | Severe water-deficit stress (30 %) FC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (distilled water) | 50 µM melatonin |
100 µM melatonin |
0 (distilled water) | 50 µM melatonin |
100 µM melatonin |
0 (distilled water) | 50 µM melatonin |
100 µM melatonin |
|||
| Limonene | 1,031 | 1,029 | 2.99 | 1.72 | 3.75 | 3.11 | 2.09 | 0.75 | 2.09 | 1.68 | 1.23 |
| β-Ocimene | 1,058 | 1,050 | 3.65 | 1.35 | 1.16 | 2.06 | 1.67 | 0.10 | 1.69 | 0.34 | 0.26 |
| Methyl chavicol | 1,205 | 1,195 | 74.90 | 79.19 | 79.11 | 82.29 | 87.69 | 88.02 | 83.45 | 90.22 | 92.73 |
| Pulegone | 1,234 | 1,233 | 1.04 | 1.11 | 1.13 | 2.08 | 1.11 | 1.73 | 3.07 | 1.11 | 1.09 |
| Geraniol | 1,249 | 1,249 | 2.13 | 5.58 | 1.06 | 1.12 | 2.37 | 0.18 | 1.10 | 1.60 | 0.09 |
| Carvone oxide <trans-> | 1,269 | 1,273 | 1.02 | 1.59 | 0.74 | 1.05 | 0.11 | 1.56 | 2.03 | 2.46 | 0.08 |
| Bornyl acetate | 1,282 | 1,284 | 11.42 | 0.16 | 5.77 | 0.03 | 1.61 | 1.83 | 1.58 | 0.32 | 1.12 |
| Carvyl acetate | 1,335 | 1,365 | 0.25 | 1.27 | 0.51 | 1.54 | 0.05 | 0.19 | 0.14 | 0.29 | 0.06 |
| Spathulenol | 1,542 | 1,577 | 0.32 | 5.17 | 3.43 | 3.17 | 0.13 | 2.21 | 4.14 | 0.11 | 0.23 |
| Caryophyllene oxide | 1,581 | 1,582 | 1.23 | 1.21 | 2.47 | 2.24 | 2.14 | 2.32 | 0.12 | 1.21 | 2.133 |
| Total of compounds identified (%) | 98.95 | 98.35 | 98.95 | 98.35 | 99.14 | 98.69 | 99.00 | 98.89 | 99.43 | ||
| Classes of constituents | |||||||||||
| Monoterpene hydrocarbons | 6.64 | 3.07 | 4.91 | 5.17 | 3.76 | 0.85 | 3.78 | 2.02 | 1.49 | ||
| Oxygenated monoterpenes | 15.86 | 9.71 | 9.22 | 5.82 | 5.27 | 5.50 | 7.93 | 5.79 | 2.45 | ||
| Oxygenated Sesquiterpene | 1.55 | 6.38 | 5.90 | 5.40 | 2.27 | 4.52 | 4.26 | 1.32 | 2.37 | ||
| phenylpropene | 74.90 | 79.19 | 79.11 | 82.29 | 87.69 | 88.02 | 83.45 | 90.22 | 92.73 | ||
Content of total chlorophyll. The chlorophyll content was affected by water-deficit stress and Mel application (Table 2). The total chlorophyll content decreased under water-deficit stress and increased with foliar application of Mel. The result shows that 60 % FC increased the total chlorophyll content with increasing Mel concentration. The highest content of total chlorophyll (1.19 mg g-1) was obtained at 60 % FC, and application of 100 μM Mel (1.04) (Table 3).
Content of total phenol and flavonoid. Total phenol content was affected by the interaction of water-deficit stress and Mel application; flavonoids biosynthesis can be affected by water-deficit (Table 2). The result shows that 60 % FC increased the total phenol content with increasing Mel concentration. The highest content of total phenol (32.18 and 33.14 mg GAE g-1) was obtained at moderate water-deficit stress (60 % FC) and severe water-deficit stress, respectively and application of 100 μM Mel (Table 3). In the present research, water-deficit stress at 60 % FC and severe water-deficit stress increased flavonoids by 28.4 and 36.9 %, respectively (Table 3). However, application of Mel (at 50 and 100 µM) increased, over the untreated plants.
Total anthocyanin contents. The anthocyanin content was affected by the interaction of water-deficit stress and Mel application (Table 2). The total anthocyanin content increased underwater-deficit stress with foliar application of Mel. The highest total anthocyanin content was obtained with 100 µM Mel in moderate water-deficit stress and severe water-deficit stress (0.71 and 0.68 mg CGE g-1, respectively) (Table 3).
Discussion
The positive role of exogenous application of Mel as growth regulators on plant growth and development has been previously reported by many researchers. Moreover, Mel due to their protecting properties against abiotic stress, as well as for their antioxidant functions is also another alternative for help the stress tolerance in crops (Farouk & Al-Amri 2019, Reiter et al. 2015, Xalxo & Keshavkant 2019). The highest and the lowest shoot dry weight were obtained with Mel application (at 50 and 100 µM) under water-deficit stress at 60 % FC and severe water-deficit stress without Mel, respectively. Our results showed that 60 % FC may maintain and/or improve plant dry weight by improving traits such as photosynthetic pigments (Table 3). However, severe water-deficit stress pigments, cell turgor, plant growth as well as plant dry weight decrease. One of the reasons for the reduction of these traits under drought stress conditions could be related to less water uptake, photosynthesis disruption, decreased production of hormones and the activity of enzymes. On the other hand, Mel can improve plant dry weight by reducing lipid peroxidation and reactive oxygen species and maintaining membrane stability (Xalxo & Keshavkant 2019). Among different strategies which were applied to cope with drought stress, the foliar application has proven to be an excellent technique and this approach has recently been used to overcome drought stress conditions. So, foliar spraying of Mel, especially 100 µM concentration, could mitigate the adverse effects of drought stress. A previous study clarified that the maintenance of high turgor potential and relative water content in plants that were treated with Mel compared with untreated plants prevented the reduction of the shoot and root growth under drought stress (Naghizadeh et al. 2019). In plants, Mel affected plants metabolites and stimulated biosynthesis of phytohormones, facilitated nutrients absorption, stimulating root and shoot growth and finally led to enhancement of the quality and quantity of production. Also, Mel regulated rooting through induction of auxin and caused an increase in shoot growth via the increment of cytokinin production (Arnao & Hernandez-Ruiz 2014).
Synthesis of secondary metabolites in plants is affected by several factors including genetic and environmental factors, plant species type, growth stages, specific seasonal conditions, nutrient supply, among which, stress conditions are the most important factor affecting the biosynthesis of secondary metabolites (Liang et al. 2019). Therefore, in this experiment, the EO content of A. foeniculum under water-deficit stress was higher than the normal conditions. Also, the EO content of A. foeniculum in foliar application with Mel improved under severe water-deficit stress (at 30 % FC) conditions (Table 3). Studies show that drought stress can increase EO of aromatic plants by affecting the biosynthetic pathway of secondary metabolites since these compounds play a defensive role for plants (Bistgani et al. 2017). It has been stated that Mel application increases the content of EOs in the aromatic plants (Farouk & Al-Amri 2019). It seems that under stress conditions, the density of glands containing EOs was increased and/or photo-assimilates may be more partitioned to terpenes biosynthesis rather than to the ordinary cellular biological processes. Ultimately, forgoing changes would improve the plant ability to tolerate the stressors (Turtola et al. 2003), since these compounds may also have several biological activities (Mazza & Kiehn 1992). Drought stress triggers the production of the EOs in medicinal plants since these compounds preferably prevent cellular oxidation under stress condition. Studies show that application of Mel ameliorated the chromium-induced plant senescence by activating antioxidant defense, ultra-structural change, and osmolyte accumulation and boosted the percentage and yield of EOs in the marjoram plant (Farouk & Al-Amri 2019). Similar findings were found by Turtola et al. (2003) who, reported that the use of Mel significantly improved the content and yield of EOs due to a higher density of oil glands (Turtola et al. 2003).
The most abundant EO compounds of A. foeniculum was methyl chavicol, which among the treatments, ranged from 74.90-92.73 % of the EO compounds (Table 4). The highest composition of methyl chavicol (92.73 %) was obtained when the plants were under severe water-deficit stress and sprayed with Mel at a concentration of 100 μM (Table 4). Our results are similar to other studies that have reported that only 10 compunds accounted for more than 0.1 % and methyl chavicol constituted 95-98 % of the A.foeniculum EO (Mazza & Kiehn 1992). Also, Omid Beigi & Mahmoudi Sourestani (2010) in their study on the effect of water availability on the composition of A. foeniculum EO reported that drought stress did not have a significant effect on the content of methyl chavicol (Omid Beigi & Mahmoudi Sourestani 2010), which is inconsistent with our results, which may be due to the effects of drought stress, especially severe stress, affects the activity of enzymes and metabolic processes involved in the synthesis of secondary metabolites and increases the synthesis of these compounds (Zheljazkov et al. 2006). Abdollahi Mandoulakani et al. 2017, reported that the transcripts of phenylpropanoid genes were differentially expressed during progressive drought. CVOMT enzyme, catalyze the final step to convert chavicol, to methyl chavicol using S-adenosyl methionine (SAM) as the methyl donor. They showed that the expression levels of CVOMT increased under drought stress conditions. In agreement with these results, our findings showed methyl chavicol content increased under severe water deficit. On the other hand, the results for the first time in this plant showed that Mel caused a significant increase in EO compounds, especially methyl chavicol, at all stress levels, especially severe water-deficit stress. Studies show that external application of Mel prevents oxidative damage induced by drought stress by regulating secondary metabolites and increasing the activity of phenylalanine ammonialyase (PAL) and polyphenol oxidase (PPO) enzymes (Naghizadeh et al. 2019).
The current study, the total phenol content of water deficit-stressed A. foeniculum plants was higher than those cultivated under well-watered conditions. Also, total phenol content increased with foliar spraying of Mel (Table 3). Increased phenol content in drought stress can be due to disruption of various metabolic processes in plant cells due to stress, which leads to an increase in phenolic compounds (Keutgen & Pawelzik 2009). The production of ROS in plants is intensified by increasing the intensity of drought stress, building up the biosynthesis of carbon-based secondary metabolites, mainly phenolic compounds. In addition, the role of Mel inducer in phenolic contents is due to the induction of different metabolic pathways and causes the formation of various compounds, especially under stress. Many other studies have reported the effect of Mel on increasing phenolic compounds on various plant species (Sadak et al. 2020). The accumulation of several phenolic compounds under the water stress situations can act as a signal to cause chains of other reactions that finally lead to improved stress tolerance (Andersen & Markham 2005). It has been stated that the total phenolic content can help as antioxidant in the cell by giving electrons to the peroxidase-type enzymes and the H2O2 detoxification (Sakihama et al. 2002). Salehi et al. (2016) described in Mentha spicata that total phenol content increases under the water stress (Salehi et al. 2016).
Abiotic stresses increase the content of ROS and if they are not scavenged, they may cause serious oxidative damage to macromolecules. Tolerant plants usually have effective defense strategies to balance oxidants and antioxidants (Mittler 2002). Antioxidant systems are a combination of enzymatic antioxidants (such as peroxidase, superoxide dismutase and catalase) and non-enzymatic antioxidants (such as ascorbic acid, glutathione, vitamin E, phenolic compounds, flavonoids, and anthocyanins) (Xalxo & Keshavkant 2019). Anthocyanins are water-soluble compounds that accumulate significantly under stress conditions and can exhibit different biological roles (Khoo et al. 2017).In this investigation, the anthocyanin contents increased significantly upon exposure to water deficit stress. However, the foliar spray of Mel (at both concentrations) improved the contents of anthocyanin (Table 3). Current results agree with Landi et al. (2015), who stated that stress conditions increased anthocyanin content and could scavenge activated oxygen species and reduced sensitivity to photoinhibition (Landi et al. 2015). It is also possible that increasing antioxidant capacity and increasing the production of anthocyanins can lead to improved plant dry weight and reduced oxidants such as H2O2 (Xu et al. 2018).
According to the result, the flavonoid content of A. foeniculum was increased under water deficit stress (at 60 and 30% FC) than normal conditions (Table 3). Flavonoids are the most common secondary metabolites in higher plants and can directly scavenge superoxide radicals, hydroxyl radicals and hydrogen peroxide. This is apparently due to the involvement of flavonoids in the catabolism of hydrogen peroxide by peroxidase, where flavonoids act as electron donors (Bienert et al. 2006). Also, the content of flavonoids increased with exogenous application of Mel (Table 3). Previous studies showed that Mel could increase non-enzymatic antioxidants such as flavonoids (Xalxo & Keshavkant 2019) which are in line with the results of the present research.
In conclusion, Mel application led to improve in plant growth and phytochemical compositions under water-deficit. Also, our results showed that total phenol, total anthocyanins, and total flavonoids contents as non-enzymatic antioxidants increased in A. foeniculum plants treated with melatonin under both irrigation conditions. Moreover, exogenously applied melatonin augmented the EO content and chemical profile of EOs in A. foeniculum under control and water-deficit stress situations. In our study, methyl chavicol was a major compound among of composition of EOs. The highest methyl chavicol was obtained in A. foeniculum plants sprayed with 100 μM Mel under water deficit stress (at 30% FC). Since Mel is a safe and low-cost substance, it can be considered as a practical method to improve the EO content and composition as an effective elicitor in A. foeniculum plant under water-deficit stress.











 nova página do texto(beta)
nova página do texto(beta)