In Mexico, approximately 1,040 species of ferns and lycophytes have been recorded belonging to 143 genera (Tejero-Díez et al. 2016, Villaseñor 2016), which represent a considerable number considering that Mexico is a megadiverse country. Ferns and lycophytes represent approximately 5 % of the total vascular flora of the country and 20 % are endemic (Tejero-Díez et al. 2016). These plants are mainly distributed in humid vegetation types, where mesic conditions enable the reproductive capacity and biological cycle of ferns and lycophytes (Judd et al. 1999, Tejero-Díez et al. 2016). These two plant groups have distinctive evolutionary lineages, but share many features (Judd et al. 1999, Schneider & Schuettpelz 2016); due to this, they have long been collectively treated as ‘pteridophytes’, so it still seems appropriate to address biological studies of both lineages together (Almeida & Salino 2016, PPG I 2016, Schneider & Schuettpelz 2016, Mendoza-Ruiz et al. 2017).
Fern and lycophyte species have been used in only a few biogeographic studies to analyze the distributional patterns of Mexican flora (Sanginés-Franco et al. 2015). These studies include the following: Palacios-Ríos & Gómez-Pompa (1997) in Veracruz, Ramírez-Barahona et al. (2009) in the Yucatan peninsula, and the regional studies of Sanginés-Franco et al. (2011, 2015) in the Sierra Madre Oriental and the Mexican Transition Zone, respectively. Other studies have been focused on specific groups, e.g., Ramírez-Barahona et al. (2011) for Cyatheaceae and Luna-Vega et al. (2012) for Polypodium, whereas some are focused in species richness related to vegetation types, e.g. Arreguín-Sánchez et al. (2009) and Hernández-Álvarez et al. (2019).
Spatial patterns for the richness and endemism of Mexican pteridoflora are not well known, particularly of those highly biodiverse areas. The state of Oaxaca is considered a highly biodiverse Mexican state (García-Mendoza 2004, Luna-Vega et al. 2013, Villaseñor & Ortiz 2014), where ferns and lycophytes contribute to this biodiversity. From a botanical viewpoint, Oaxaca is one of the Mexican states with the greatest richness of ferns and lycophytes (Mickel & Beitel 1988, García-Mendoza 2004, Tejero-Díez & Mickel 2011, Luna-Vega et al. 2013, Martínez-Salas & Ramos 2014, Mendoza-Ruiz et al. 2017). This richness has been explained by several factors, such as its geographic position, complex topography, presence of almost all Mexican vegetation types, a wide altitudinal gradient from 0 to 3,720 m asl, and different climate types (García-Mendoza 2004, Martínez-Salas & Ramos 2014, Tejero-Díez & Mickel 2011, Trejo 2004). Several taxonomic studies related to ferns and lycophytes have been carried out in Oaxaca (e.g. Mickel & Beitel 1988, Tejero-Díez & Mickel 2004, 2011). Oaxaca has been included in only a few biogeographic analyses, considering only part of its territory (Ramírez-Barahona et al. 2011 Luna-Vega et al. 2012, Sanginés-Franco et al. 2015).
The aim of this study was to analyze the distributional patterns of fern and lycophyte species to detect areas of richness and endemism in Oaxaca using grid cells as area units. We also undertook complementary and beta diversity analyses to check whether this Mexican state is a beta-diverse area. We revised the conservation status of ferns and lycophytes and their distribution in relation to conservation areas, also contributing to the delineation of biodiversity conservation strategies.
Materials and methods
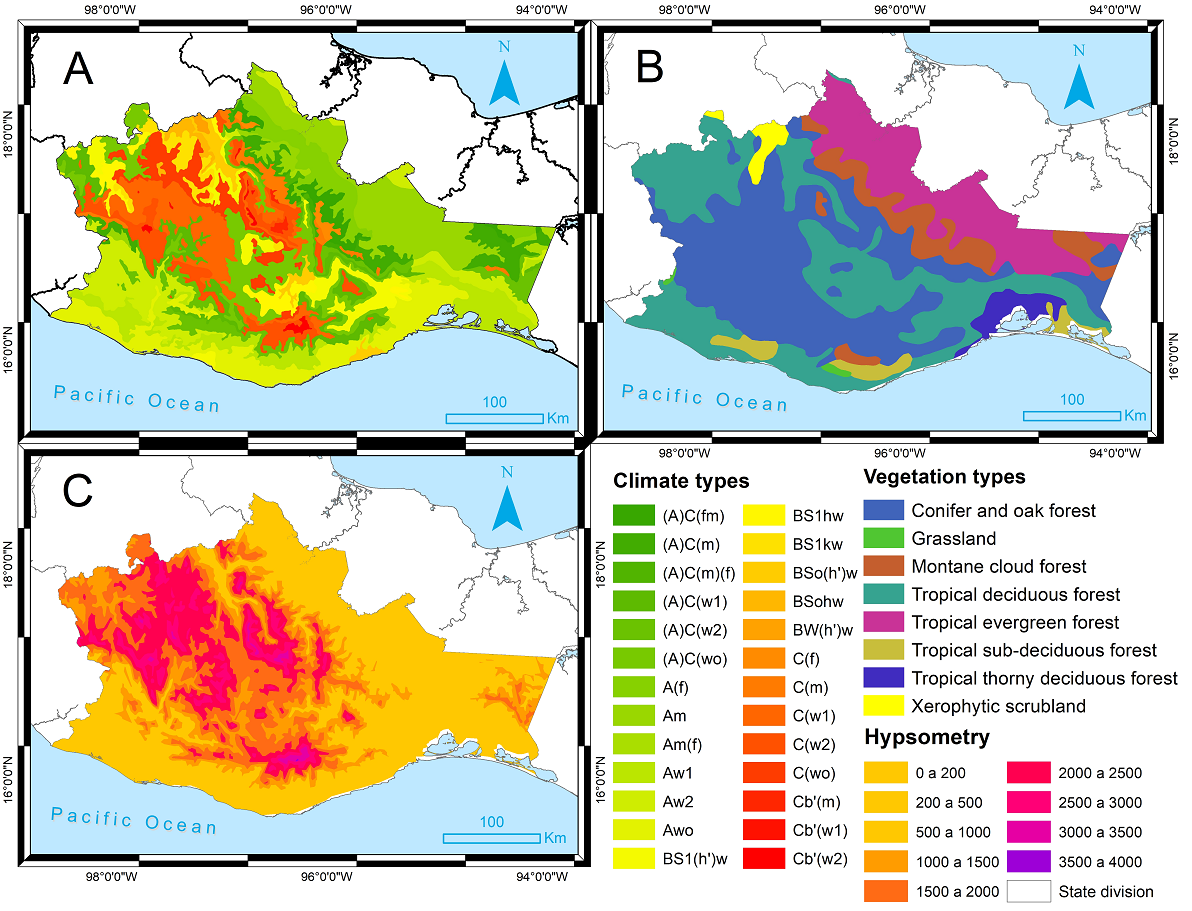
Study Area. The state of Oaxaca is located in southeastern Mexico (Figure 1). This state faces the Mexican Pacific slope but is also influenced by meteorological phenomena occurring in the Gulf of Mexico, mainly in the Istmo, Papaloapan and Cañada regions (Figure 1). The Sierra Sur and Sierra Norte are the two main mountain regions in the state, and the Papaloapan and Istmo regions are at lower elevations, the latter occurring along the Pacific coastal plain. Almost all the climate Mexican types are present in Oaxaca (Trejo 2004). This climatic diversity (Figure 2A) is due to its complex physiographic heterogeneity and frequent meteorological phenomena, such as hurricanes and tropical storms originating in the Pacific and in the Gulf of Mexico (Trejo 2004, Luna-Vega et al. 2012).
Different and diverse vegetation types occur in Oaxaca (Velázquez et al. 2003) (Figure 2B). In the mountain regions, temperate forests prevail; arid environments are present in the northwestern part (Valle de Tehuacán-Cuicatlán); lowland areas are present in the Pacific slope and in the eastern part, known as the Istmo region. La Chinantla, located in the Sierra Norte region, is by far the rainiest area in all the country, with more than 5,000 mm (Meave et al. 2017). The hypsometry is also diverse (Trejo 2004) (Figure 2C) from sea level to 3,750 m above sea level in the highest peak of Oaxaca (Briones-Salas et al. 2016).
Data sources. Tejero-Díez & Mickel (2011) recorded 683 species, whereas Villaseñor (2016) reported 736 species of lycophytes and ferns in the state of Oaxaca. In our study, we had access to the distributional data of 620 species. The remaining 63 species were not included because we did not revised specimens from foreign herbaria. We did not consider varieties nor hybrids in our study because species are the essential taxonomic units for description of natural diversity (Valencia-Ávalos 1991, Villaseñor 2015).
Our distributional data were obtained mainly from an exhaustive revision of hundred specimens held in some of the main Mexican herbaria. The collections revised were: National Herbarium of the Instituto de Biología, Universidad Nacional Autónoma de México (MEXU); Herbarium of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional (ENCB); Herbarium of the Universidad Autónoma Metropolitana Unidad Iztapalapa (UAMIZ); Herbarium of the Centro Interdisciplinario de Investigación Regional, Instituto Politécnico Nacional, Unidad Oaxaca (OAX); Herbarium of the Facultad de Ciencias, Universidad Nacional Autónoma de México (FCME); Herbarium of the Universidad de la Sierra Juárez (UNSIJ); and Herbarium of the Instituto de Historia Natural de Chiapas (CHIP). In addition, we consulted some records from the database of Comisión Nacional para el Uso y Conocimiento de la Biodiversidad (CONABIO). Finally, we carried out five field expeditions in different regions of Oaxaca, specifically in the Cañada, Costa, Mixteca, Sierra Norte and Valles Centrales regions. All plant specimens collected were deposited in the Herbarium of the Escuela de Ciencias, Universidad Autónoma Benito Juárez de Oaxaca (UABJO). From all these information sources, we compiled a database that includes 3,899 georeferenced records.
With the above information, we drew the geographic distribution maps of each species using ArcView GIS (ESRI 2000). Most of the fern and lycophyte species are also present in other Mexican states, and some of them are distributed in neighboring areas such as North America and Central America. Few species are restricted to Oaxaca.
Since the study of Tejero-Díez & Mickel (2011), new combinations (Grusz & Windham 2013, Smith & Tejero-Díez 2014), new genera (Moran et al. 2010, Labiak 2011, Li et al. 2012, Sundue et al. 2012, Sundue 2013), new species (Sundue 2017), and new records (Tejero-Díez et al. 2016, Mendoza-Ruiz et al. 2017, Sundue 2017) have been published for ferns. We included these new records and these new nomenclatural changes to the pteridoflora of Oaxaca in our study, following the proposal of the Pteridophyte Phylogeny Group I (PPG I 2016).
Species richness and endemism analyses. We divided the state of Oaxaca into 81 grid cells of 20 × 20 minutes of latitude and longitude, which were used as units of analysis (Figure 3). We chose grid cells of 20 minutes per side, partially to facilitate the data manipulation and to reduce the effect of sampling artefacts, such as mapping errors and unsampled grid cells in sparsely inhabited areas (Crisp et al. 2001). This scale size was also chosen because similar scales have been tested in previous studies on areography and diversity of different groups of the Mexican biota (Luna-Vega et al. 2006, Suárez-Mota & Villaseñor 2011, Ochoa-Ochoa et al. 2014). The state of Oaxaca has yet some unexplored areas (García-Mendoza & Meave 2011, Ramírez-Toro et al. 2017).
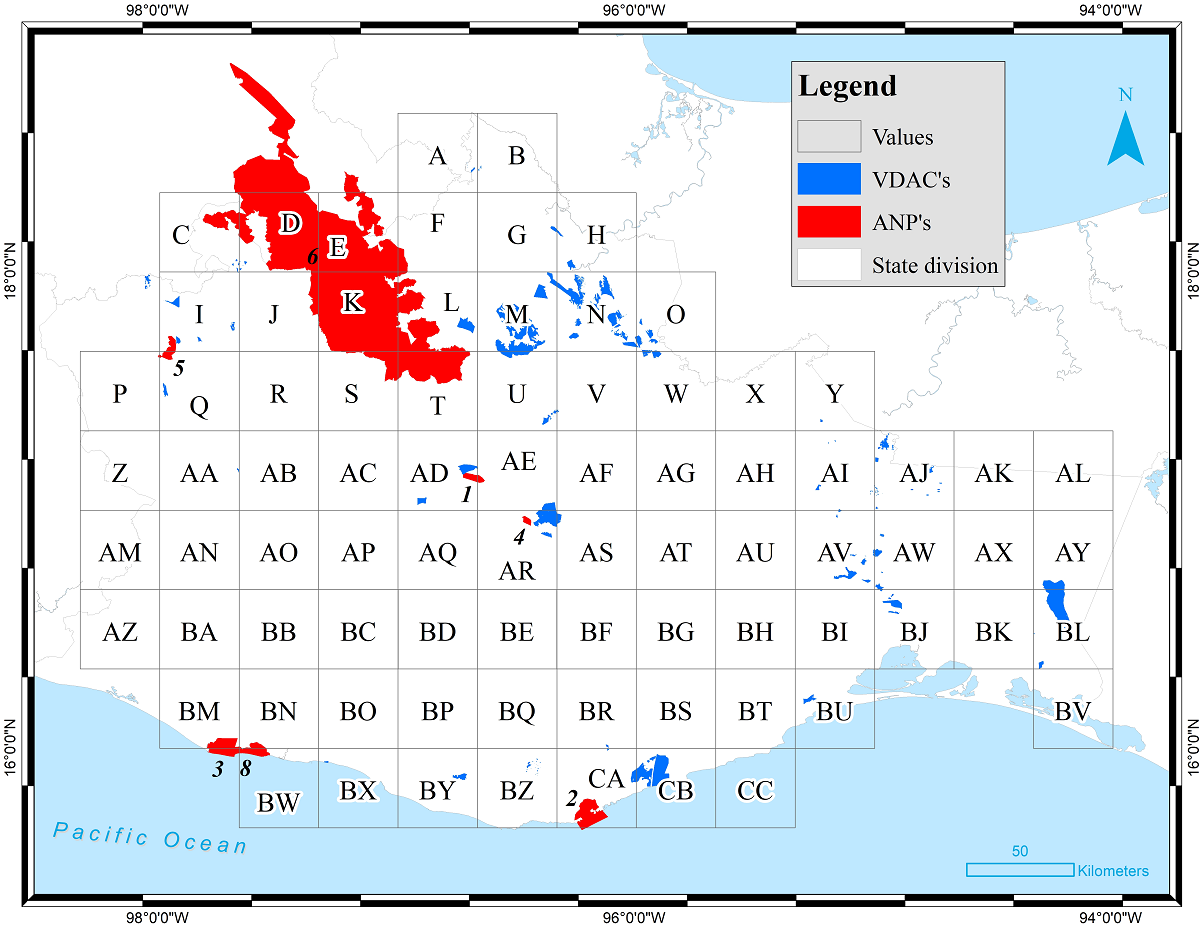
Figure 3 The state of Oaxaca divided into grid cells of 20 × 20 minutes of latitude and longitude. Protected Natural Areas (ANPs) and Areas Voluntarily Designated for Conservation (ADVCs) in Oaxaca. The ANPs are as follows: (1) Benito Juárez National Park, (2) Huatulco National Park, (3) Lagunas de Chacahua National Park, (4) Yagul Natural Monument, (5) Boquerón de Tonalá Flora and Fauna Protection Area, (6) Tehuacán-Cuicatlán Valley Biosphere Reserve, (7) Playa de Escobilla Sanctuary, and (8) Playa de la Bahía de Chacahua Sanctuary.
Species richness, also known as ‘unweighted species richness’, was measured as the total count of species within each grid cell (Crisp et al. 2001, Linder 2001). A first index named ‘weighted endemism’ or WE consists of several steps (Crisp et al. 2001). In the first step, each grid-cell occurrence was divided by the total number of grid cells in which one species occurs. Thus, a species restricted to a single grid cell was scored as ‘1’ for that grid cell, and ‘0’ for all other grid-cells, and a species found in 10 grid-cells was scored as ‘0.1’ for each of the 10 grid-cells, and ‘0’ for all remaining grid-cells; then the sum of all score species values for each grid cell was obtained. A second index, named ‘corrected weighted endemism’ (Crisp et al. 2001, Linder 2001) or CWE, consisted of dividing the weighted endemism index by the total count of species in each grid cell. Those grid-cells with the highest scores in the first index were considered centers of richness, whereas for the second index, the grid cells with highest values are considered centers of endemism (Crisp et al. 2001, Linder 2001). In Mexico, CWE index has been applied to the geographic distribution of ferns in some areas of the country, such as the Sierra Madre Oriental (Sanginés-Franco et al. 2011), the Mexican Mountain Component (Sanginés-Franco et al. 2015), and the Yucatan peninsula (Ramírez-Barahona et al. 2011).
Grid cell analyses, maps and index calculations were performed using the software Biodiverse, version 3.0 (Laffan et al. 2010). Results from the richness and endemism analyses were clustered with this tool and results were plotted on a map according to the groups recovered. Grid cells with zero or one species recorded were not considered in the analysis of CWE (Santa Anna del Conde et al. 2009, González-Ávila et al. 2018), because these grid cells do not include overlapping distributions, which is a basic criterion for the area of endemism concept (Morrone 1994, Aagesen et al. 2013).
The centers of richness or endemism are formed by sets of neighboring grid cells or isolated grid cells with high values in each index (Crisp et al. 2001, Linder 2001, Santa Anna del Conde et al. 2009). Each species was scored as present in a grid cell independently of whether it was recorded once or numerous times in a determined grid cell (Linder 2001, Contreras-Medina & Luna-Vega 2007, Santa Anna del Conde et al. 2009, González-Ávila et al. 2018). The WE index has been considered as a measure sensitive to diversity, this fact implies that patterns of richness resemble partially or totally the WE values (Linder 2001, Santa Anna del Conde et al. 2009). In contrast, the CWE index is not significantly correlated with grid diversity (Linder 2001, Santa Anna del Conde et al. 2009, González-Ávila et al. 2018).
The grid cells with highest values in species richness were superimposed onto the map of federal Protected Natural Areas (CONANP 2016) or ANPs (in Spanish, ‘Áreas Naturales Protegidas’) which are decreed by the Mexican government (Figure 3) and onto the map of Areas Voluntarily Designated for Conservation (Figure 3) or ADVCs (‘Áreas Destinadas Voluntariamente para la Conservación’) (CONANP 2014), areas preserved and protected by indigenous communities in Oaxaca (Martin et al. 2011, Briones-Salas et al. 2016). With this, we evaluated the distribution of ferns and lycophytes in relation to both types of conservation areas.
Diversity and complementarity analyses. We calculated alpha diversity (α) using the software Biodiverse, version 3.0 (Laffan et al. 2010). We defined a grid of 20’ × 20’ latitude-longitude and then we mapped alpha diversity by counting the species in each grid cell (observed alpha); we also calculated and estimated alpha with the coefficient Chao1 (abundance-based). Finally, we assessed the importance of the pteridoflora inventory calculating the undetected species values through the difference between the estimated and observed richness.
Beta diversity is a component of diversity that measures the differences between communities in terms of species composition (Whittaker 1960). Several factors have been proposed that influence species turnover, such as those related to the environment and its heterogeneity, and those inherent to species, such as their vagility and tolerance ranges to different environmental factors (Goettsch & Hernández 2006). Complementary and beta diversity analyses have been applied to other biological groups of Mexican biota (Rodríguez et al. 2003, Aguilar-Aguilar & Salgado-Maldonado 2006, Goettsch & Hernández 2006, González-Ávila et al. 2018). Only a few studies have applied beta diversity analyses to ferns and lycophytes, mainly for the Mexican states of Durango (Montelongo-Landeros et al. 2015) and Hidalgo (Sánchez-González et al. 2016).
We considered alpha diversity (α) as a synonym for fern species richness (number of species in a particular grid cell), whereas beta diversity (β) is a measure of species turnover between grid-cell pairs, reflecting heterogeneity of the different communities regarding their species composition (Magurran 1988, Goettsch & Hernández 2006). In order to calculate the β diversity values, we used Whittaker’s (1960) original formula: β = Sγ/Sα, where Sγ is the number of species considering the whole study area (in this case, the state of Oaxaca) and Sα is mean diversity in the grid cells. The β diversity values range from 1 (without species turnover) to a theoretical maximum equal to Sγ (Rodríguez et al. 2003, Aguilar-Aguilar & Salgado-Maldonado 2006).
To complement the β-diversity analysis, we undertook a cluster analysis to display beta diversity in a spatial frame with the software Biodiverse, version 3.0 (Laffan et al. 2010). We constructed a presence-absence matrix that includes grid cells and species distributions, where plant taxa were coded for their absence (0) or presence (1) in each grid cell; a similarity matrix was obtained using Sorensen’s coefficient. We built the cluster of grid cells by applying the UPGMA method (Unweighted Pair Group Method using Arithmetic averages).
A complementarity analysis of the pteridoflora of Oaxaca was also carried out, which has been used to define priorities to optimize the biodiversity conservation plans in specific areas (Gómez-Hinostrosa & Hernández 2000, Aguilar-Aguilar & Salgado-Maldonado 2006). Colwell & Coddington’s (1994) complementary index was used to calculate the difference values in species composition between grid-cell pairs using the formula: ICAB = (a + b - 2c) / (a + b - c), where a: number of species in a determined area A, b: number of species in a determined area B, and c: number of shared species. The complementarity values go from ‘0’ to ‘1’, where zero indicates similar areas in species composition and ‘1’ represents total complementarity, where any species is shared between areas (Colwell & Coddington 1994, Aguilar-Aguilar & Salgado-Maldonado 2006, González-Ávila et al. 2018). As in the analysis of CWE, those grid-cells with zero or one species recorded were not considered in the complementary analysis, as has been suggested previously (Aguilar-Aguilar & Salgado-Maldonado 2006, González-Ávila et al. 2018).
Risk categories. Finally, we revised Official Mexican Standard NOM-059 (SEMARNAT 2010) to recognize how many fern and lycophyte species of Oaxaca are listed and to verify the corresponding risk category for each species. NOM-059 is the official document generated by the Mexican government that includes threatened species of animals, plants, and fungi.
Results
Species distribution and species richness. Oaxaca was divided into 81 grid cells. From these, 78 grid cells included at least one record and three lack records (BB, BE, and BS); the BB grid cell is located in the Sierra Sur region, whereas BE in the border of the Sierra Sur and the Valles Centrales regions, and finally BS in the limits between the Sierra Sur and Istmo regions. In the present study, we included 3,899 occurrence records for 620 species of pteridoflora belonging to 125 genera and 36 families. From these 54 species, three families and seven genera correspond to lycophytes and the rest correspond to ferns. The taxa found in most grid cells (Figure 4) were Equisetum myriochaetum Schltdl. & Cham. (recorded in 22 grid cells), Phlebodium pseudoaureum (Cav.) Lellinger (21 grid cells), Lygodium venustum Sw. and Pityrogramma calomelanos (L.) Link (both in 17 grid cells). Other well-represented taxa were Adiantum andicola Liebm., Cheilanthes lozanoi (Maxon) R. M. Tryon & A. F. Tryon, Dryopteris wallichiana (Spreng.) Hyl., Niphidium crassifolium (L.) Lellinger, Tectaria heracleifolia (Willd.) Underw., Tectaria mexicana (Fée) C. V. Morton and Selaginella pallescens (C. Presl) Spring, all recorded in 15 grid cells, whereas Blechnum occidentale L. and Pleopeltis polylepis (Roem. ex Kunze) T. Moore were both recorded in 14 grid cells.

Figure 4 Distribution maps of some fern and lycophyte species in Oaxaca. Species located in the largest number of grid cells (Equisetum myriochaetum, Lygodium venustum, Pityrogramma calomelanos and Phlebodium pseudoaureum), some threatened species included in NOM-059 (Asplenium serratum and Cyathea divergens) and endemic species (Asplenium stolonipes, Asplenium yelagagense, Ceradenia sacksii, Hypolepis microchlaena, Lellingeria labiakii, and Selaginella subrugosa).
The 10 grid cells with high scores in species richness (> 68) are shown in Figure 5A and include 69 to 223 species. Among these, grid cells U and AE have the highest number of species (>1 70); these two grid cells are neighbors and the U grid cell is located in the Sierra Norte region, whereas AE in the border of the Sierra Norte and Valles Centrales regions (Figure 5A). These richest 10 grid cells are distributed throughout all the regions of Oaxaca, but four of the 10 high scored (126-223 species per grid cell) were particularly located in the Sierra Norte region. Based on these 10 high scored grid cells, the main center of richness was located in the northern-central portion of Oaxaca, consisting of seven neighboring grid cells (M, N, U, V, AD, AE and AF) that include areas of the Cañada, Papaloapan, Sierra Norte and Valles Centrales regions. The other three grid cells are located in the Mixteca (AA), Costa (BO) and Istmo (AW) regions (Figure 5A).

Figure 5 Values obtained for species richness (A), weighted endemism (B), and corrected weighted endemism (C) from grid cells in Oaxaca.
The observed (Figure 5A) and assessed alpha diversity (Figure 6A) showed similar spatial patterns. The difference between them is that the cluster of observed species richness showed a narrower extension than the assessed one. The seven neighboring grid cells between Papaloapan, Sierra Norte and Valles Centrales regions have the highest values of observed alpha diversity. The assessed alpha diversity also had high levels in the southwestern Mixteca, northern Istmo, and adjoining grid cells between Valles Centrales and Sierra Madre del Sur regions. The map of undetected species (Figure 6B) represents those areas that need to be explored to complete the pteridoflora inventory. Therefore, assessed alpha diversity (Figure 6A) and undetected species richness (Figure 6B) displayed similar spatial tendencies.

Figure 6 Values obtained for Chao 1 (A) and undetected species (B). Map representation of the cluster obtained (C) and tree cluster analysis of grid cells in Oaxaca (D).
In our study, we included near 100 botanical specimens collected by ourselves, most of them collected previously. Grid cell C located in the Mixteca region originally did not contain fern or lycophyte records but with our field work we found the following eight species: Adiantum capillus-veneris L., Argyrochosma formosa (Liebm.) Windham, Astrolepis sinuata (Lag. ex Sw.) D. M. Benham & Windham, Cheilanthes lozanoi (Maxon) R. M. Tryon & A. F. Tryon, Myriopteris myriophylla (Desv.) J. Sm., Notholaena rosei Maxon, Selaginella lepidophylla (Hook. & Grev.) Spring and S. peruviana (Milde) Hieron. Also, our field work in the Costa region allow us to find Bolbitis umbrosa (Liebm.) Ching. in grid cell BZ, considered a new record for the state of Oaxaca.
Endemism indices. The values obtained for weighted endemism and corrected weighted endemism indices of pteridoflora are shown in Figure 5. The highest values obtained for weighted endemism (WE) resembles partially the pattern of species richness. In fact, seven of the 10 grid cells scored with the highest values of species richness were also detected with high WE value. These seven grid cells are AD, AE, AF, M, N, U and V (Figure 5B).
The corrected weighted endemism index (CWE) emphasizes those areas that are not necessarily high in species richness, but that have a high proportion of species with restricted distributions. Based on the 15 grid cells high scored in values of CWE (> 0.37), we recognize three sets of neighboring grid cells. The first set includes grid cells AZ, BA, and BM, which are located mainly in the Pacific slope belonging to the Costa region. Some species located here are listed in Table S2, Area I.
The second area of endemism consists of AK, AW, AX, and AY grid cells, which are in the Istmo region. Some species located in this area are listed in Table S2, Area II.
The eight grid cells that conform the third area of endemism included M and N located in the Papaloapan region, T in the border of the Valles Centrales and Cañada regions, U, V, AE, and AF in the Sierra Norte region and finally AD located in the Valles Centrales. These grid cells, with the exception of T, are considered within the 10 high scored for species richness, whereas all are recorded in the 10 high scored grid cells of WE values. Most of the species endemic to Oaxaca are recorded in this area (Figure 4), especially in the grid cells AE and U, both located in the Sierra Norte region. Some species included in this area are listed in Table S2, Area III.
The Protected Natural Areas (ANPs) of Oaxaca are distributed along limited areas of the Pacific Coast, on the border with Puebla, and in the center of the state near to the city of Oaxaca (Figure 3). A total of 16 grid cells contain an ANP or part of one (Figure 3). This is the case of the Tehuacán-Cuicatlán Valley Biosphere Reserve, which is a largest area and is shared with the state of Puebla, covering seven grid cells (C, D, E, K, L, S, and T). Grid cells AD, AR, BM, BY, and CA included one ANP; two NPAs cover part of two grid-cells, particularly the Boquerón de Tonalá in I and Q, and the Playa de la Bahía de Chacahua in BN and BW (Figure 3). Regarding the ADVCs (Figure 3), grid cell N is the grid cell that contains by far the most ADVCs (18), with 104 species recorded (Figure 5A); other important grid cells in terms of number of ADVCs included are I, M, O, AJ, and AV. The grid cells with the highest number of species (U and AE) include few ADVCs (Figure 3).
Diversity and complementarity analysis. The mean alpha and gamma diversity values were 30.51 and 620 respectively, considering the 78 grid cells with at least one record, whereas beta diversity value was equal to 20.3. The Sierra Norte region and surrounding areas had the highest alpha diversity. The estimated alpha diversity based on abundance (number of species-presence records) showed the same pattern of the observed alpha but with a wider coverage. The undetected species richness is also greater in the borders of the Sierra Norte region, but also in the Mixteca, Sierra Sur, and some grid ells of the Costa and Istmo regions containing a high level of potential fern diversity waiting to be discovered.
The complementarity analysis provided high values in most of the grid-cell pair combinations (> 0.5). Considering all pairs of combinations obtained (2,311), 1,271 had the highest complementarity values (> 0.8) and 693 resulted in total complementarity (= 1), indicating a high complementarity of grid-cell pairs (85 %). The species included in grid cells AA located in the Mixteca region, AW in the Istmo region, BO and BZ in the Costa region, N in the Papaloapan region, and finally AE and U in the Sierra Norte region exhibit 80 % of the diversity represented for the state of Oaxaca.
The clustering results were displayed on a map (Figure 6C) linked to a phenogram (Figure 6D), where nodes represent the clusters of similar groups. We observed different levels of aggregation, but unfortunately, the six groups obtained did not show clear patterns (Figure 6C). Only the grid cells with highest number of species richness are clustered in a group (blue group) and the lack of definite groups based on similarity reflect the lack of floristic studies in several regions of Oaxaca.
Risk categories. Twenty-two species of ferns and two species of lycophytes are included in NOM-059 (SEMARNAT 2010). These species are listed in the Table S1. Of these, seven species are in the threatened category (A), 14 are in the special protection category (Pr) and three species are considered at risk of extinction (P) (Abbreviations of each category correspond to those established in the NOM-059); the latter species are Cyathea costaricensis (Mett. ex Kuhn) Domin, Nephrolepis cordifolia (L.) C. Presl, and Selaginella porphyrospora A. Braun (Figure 4). Most of these endangered species are distributed in temperate forests.
Discussion
Different analyses showed that the beta diversity component is an important element that explains the highest biodiversity of Mexico (Rodríguez et al. 2003, Aguilar-Aguilar & Salgado-Maldonado 2006, Goettsch & Hernández 2006, Godínez-Álvarez & Ortega-Baes 2007, Ochoa-Ochoa et al. 2014), placing it as one of the most biodiverse countries in the world. Within this biodiversity, the Mexican states located in the southeastern region (e.g., Chiapas, Veracruz, and Oaxaca) are considered the most biodiverse states based on different plant groups (Contreras-Medina & Luna-Vega 2007, Luna-Vega et al. 2013, Villaseñor & Ortiz 2014). In this context, the fern and lycophyte richness in Oaxaca has been considered by some authors as a product of its geographical location, geological and ecological complexity, intricate topography, and the presence of different environmental conditions that allowed the establishment of different vegetation types (Mickel & Beitel 1988, Mickel & Smith 2004, García-Mendoza & Meave 2011, Luna-Vega et al. 2013, Tejero-Díez et al. 2016). The great complexity and diversity of ferns and lycophytes of Oaxaca is also due to confluence of the Nearctic and Neotropical biogeographic regions (Luna-Vega et al. 2012, Ochoa-Ochoa et al. 2014).
The northern Oaxaca highlands are known as one of the most diverse areas in Mexico (León-Paniagua & Morrone 2009, Meave et al. 2017). The two grid cells highest scored in species richness are located in the Sierra Norte region (U and AE). This fact was previously identified by Mickel & Beitel (1988) and recently recognized as a fern hunter’s paradise (Sundue 2017), due to the high fern richness and the possibility to find new species and new records. A high level of richness has also been suggested based on oaks (Ramírez-Toro et al. 2017), Asteraceae (Suárez-Mota & Villaseñor 2011), gymnosperms (Contreras-Medina & Luna-Vega 2007), reptiles (Urbina-Cardona & Flores-Villela 2010), and mammals (Koleff et al. 2003). Furthermore, the Sierra Norte region has been identified as an area of convergence for vertebrate species belonging to different assemblages as result of historical processes (León-Paniagua & Morrone 2009).
In the case of ferns and lycophytes, the Sierra Norte region presents several factors that allow the highest richness observed, such as highly complex topography and an elevation ranging from 800 to 3,020 m asl (Meave et al. 2017). This region represents a barrier that blocks the movement of moisture winds from the Gulf of Mexico, rendering the rainiest area in all the country with more than 5,000 mm (Meave et al. 2017). In this region different temperate vegetation types are present, such as fir forest, mountain cloud forest, deciduous forest, pine and oak forests (Torres-Colín 2004). These native forests are more or less structured in altitudinal belts, although this pattern is broken by the presence of slopes with different orientation, exposed ridges and deep ravines (Meave et al. 2017). The Sierra Norte region is also recognized as an area with species belonging to both Nearctic and Neotropical biotas (León-Paniagua & Morrone 2009).
Grid cells with high species richness include families and genera that usually inhabit humid environments (Hernández-Álvarez et al. 2019). Some of these taxa have a wide distribution in the country and, to a lesser extent, some are distributed in the tropical Americas (Mickel & Smith 2004). The presence of some species of certain families, such as Lycopodiaceae, Selaginellaceae, Aspleniaceae, Blechnaceae, Cyatheaceae, Equisetaceae, Hymenophyllaceae, Lomariopsidaceae, Polypodiaceae, Pteridaceae, Thelypteridaceae and Woodsiaceae show the complexity of these grid cells, because these vascular plants are sensitive to environmental changes (Tejero-Díez et al. 2016).
The Istmo region is one of the most interesting areas from a botanical point of view (García-Mendoza 2004, García-Mendoza & Meave 2011). In our study, we recorded 118 species in this region, some of which inhabit tropical deciduous forest, which is the predominant type of vegetation. However, most of the herbaria specimens consulted came from the Chimalapas (mainly grid cells AW, AX, and AY), where exuberant tropical evergreen forests and cloud forests occur (Peterson et al. 2003). The Chimalapas is one of the rainiest areas of Oaxaca, promoting the development of many fern and lycophyte species (García-Mendoza & Meave 2011, Luna-Vega et al. 2012).
Notwithstanding that, the Tehuacán-Cuicatlán Valley and Sierra Norte region seem to be floristically over-sampled (Mickel & Beitel 1988, García-Mendoza & Meave 2011), undetected species estimations indicate paradoxically, that these also deserve to be studied to complete the pteridoflora of Oaxaca. This fact is due to several species present in those areas having narrow distributions. Therefore, in the incidence matrix, they appear only in one or two grid cells.
The WE values obtained are related to species richness, as previously suggested (Crisp et al. 2001, Santa Anna del Conde et al. 2009, Sanginés-Franco et al. 2015). Seven grid cells with highest number of species are replicated in the WE index. In the CWE, we recognized three grid cell sets with higher CWE values. This index highlights those species with restricted distribution, so the values of grid cells for the WE index are different in comparison with the CWE index (Santa Anna del Conde et al. 2009, González-Orozco et al. 2014, Sanginés-Franco et al. 2015). The grid cells with high CWE values that are not replicated in the WE are AW, AY and AK located in the Istmo region, and AZ, BA and BM in the Costa region. The three grid cells sets recognized by the CWE index in terms of both endemism and richness (with more than 100 species) include grid cells M and N located in the Papaloapan region, U, V and AF in the Sierra Norte, whereas AE in the border of Valles Centrales and Sierra Norte regions. Some of the most important grid cells in richness values (U, V, AD, AE, AF, M, and N) are replicated with high values in both WE and CWE indexes, which may due to a high number of species, but also is influenced by the geographic distribution of the species that occur in these grid cells, some of which are endemic to Oaxaca (Tejero-Díez et al. 2016), a condition that heightens CWE values (Crisp et al. 2001, Santa Anna del Conde et al. 2009, Sanginés-Franco et al. 2015). In contrast, two grid cells (AX and T) have high values in both WE and CWE indexes but have less than 40 species (37 and 15 species, respectively).
We consider that the CWE index is a reliable measurement of endemism, due to its independence from species richness (Crisp et al. 2001, Santa Anna del Conde et al. 2009, Sanginés-Franco et al. 2015) and because species endemic to Oaxaca and to Mexican territory are included in our study. We propose that grid cells which are replicated in both WE and CWE indexes should be considered as priority areas for further investigations aimed at the conservation of fern and lycophyte species that inhabit Oaxaca.
Comparing our results with those obtained by Monroy-García (2009) for Oaxaca using mammals as a model (β values between 7.0 to 8.0) and Aguilar-Aguilar & Salgado-Maldonado (2006) in the Lerma and Papaloapan basins with helminthes (β = 3.33 and β = 4.74, respectively), we obtained a higher beta diversity value in our study (β = 20.3). It is possible that this value is overestimated due to the lack of floristic studies in several regions of Oaxaca. A comparison with mammals, helminths and ferns is complicated due to their different biogeographical histories, biological cycles, vagility, and morphological characteristics, notwithstanding the evidence that species turnover occurs between the grid cells of Oaxaca, as result of differences in species composition from region to region within the state and this can contribute to explaining the Oaxaca higher diversity.
When we compared our beta diversity value obtained using ferns as a distributional model with other studies carried out in Mexico, we noted similarities and differences. Rodríguez et al. (2003) observed that the beta diversity values obtained ranged from 1.31 to 2.65 when using mammals as a model and Mexican mastofaunistic province as area units. Amphibians are the vertebrate group with highest beta diversity values in southeastern Mexico (Ochoa-Ochoa et al. 2014). Our results are consistent with these exceptionally high values for Oaxaca.
Despite that Oaxaca has not been fully explored for ferns and lycophytes, we consider that our beta diversity results reflect the high richness that exhibits this Mexican state, as confirmed with other biological groups (e.g. Contreras-Medina & Luna-Vega 2007, Suárez-Mota & Villaseñor 2011, Luna-Vega et al. 2013, Briones-Salas et al. 2016, Villaseñor 2016). This high biodiversity and beta diversity is related to several factors that generate environmental heterogeneity in the state. In Oaxaca, elevation occurs from sea level to 3,750 m (Briones-Salas et al. 2016); precipitation has a wide range, from 500 mm in the Cañada and Mixteca regions to more than 5,000 mm in the Sierra Norte region (Meave et al. 2017). The climatic diversity found in Oaxaca generates main tendencies in vegetation, also related with its geographic position, relief complexity and exposure to the meteorological phenomena developed both, in the Pacific and the Gulf of Mexico (Trejo 2004). Different vegetation types occur in Oaxaca, from tropical forests in lowland areas and temperate forests in mountain regions to dry areas in the northwestern portion with xerophytic vegetation, as well as different associations of aquatic vegetation (Torres-Colín 2004). This mosaic of environmental and biological conditions allows the development of different fern and lycophyte species and explains the high biodiversity in Oaxaca, because environmental heterogeneity is considered a process that drives beta diversity patterns (López-González et al. 2015, Liu et al. 2018, Vega et al. 2020).
Patterns of diversity are scale dependent and beta diversity is not the exception (Whittaker et al. 2001, Ochoa-Ochoa et al. 2014). Our beta diversity value can be related to the size of the grid cells used in the biogeographic analysis. Based on the distribution of Mexican vertebrates, Ochoa-Ochoa et al. (2014) suggested that beta diversity values are higher at the coarsest scales, such as grid cells of 400 × 400, 200 × 200, and 100 × 100 km and tended to decrease at the finest scales, such as 50 × 50, 25 × 25, and 12.5 × 12.5 km. In this study, we used grid-cells with a spatial resolution of approximately 36.75 × 36.75 km (~1,350 km2). However, the beta diversity of each group contributes differentially to the megadiversity of a region (Ochoa-Ochoa et al. 2014). We conclude that ferns and lycophytes are important elements that contribute to the megadiversity of Oaxaca.
The complementarity analysis considering grid cells as units of study has been used previously in order to propose conservation areas in Mexico for different biological groups, such as mammals (Rodríguez et al. 2003), helminths (Aguilar-Aguilar & Salgado-Maldonado 2006), herpetofauna (Ochoa-Ochoa & Flores-Villela 2006), cacti (Godínez-Álvarez & Ortega-Baes 2007), and fungi (González-Ávila et al. 2018). In these studies, the importance of richness and species turnover between grid cells in a megadiverse and beta-diverse country is relevant. Our results agree with these previous studies because it is evident that an assemblage of species from one grid cell is not replicated in another grid cell in Oaxaca. Together, grid cells AA located in the Mixteca region, AE in the limits of the Sierra Norte and Valles Centrales regions, AW in the Istmo region, BO and BZ in the Costa region, N in the Papaloapan region, and U in the Sierra Norte, harbor nearly 80 % of Oaxaca’s fern and lycophyte species, so they are relevant in terms of biological conservation.
In relation to other Mexican states, Oaxaca has few Protected Natural Areas (ANPs) designated by the federal government (Briones-Salas et al. 2016, Ramírez-Toro et al. 2017). In this state, only eight ANPs are recorded (Martin et al. 2011, Briones-Salas et al. 2016), in contrast with the neighboring state of Chiapas, where 19 ANPs are present (CONANP 2016) and which is also considered a megadiverse Mexican state (Luna-Vega et al. 2013). Fortunately, in Oaxaca there are alternative conservation areas called Areas Voluntarily Designated for Conservation or ADVCs (‘Áreas Destinadas Voluntariamente para la Conservación’), which are protected by local communities (Briones-Salas et al. 2016). If we compare these areas with ANPs, these ADVCs are more efficient in conservation terms (Martin et al. 2011, Briones-Salas et al. 2016, Ramírez-Toro et al. 2017). Some of these ADVCs are in the Sierra Norte region (CONANP 2014), where the highest diversity of ferns and lycophyte species are recorded and conservation in situ could be carried out in a more efficient way, as these ADVCs include different ecosystems (Ramírez-Toro et al. 2017). Grid cells M and N are located in the Papaloapan and Cañada regions and are scored in the first places regarding richness. Both grid cells also include a higher number of ADVCs, so we propose that regional studies for conservation purposes are necessary in these grid cells, because areas with the highest beta diversity must have a higher number of protected areas (Rodríguez et al. 2003) and therefore these ADVCs should be considered part of the state's natural protected areas. In addition, migration to the USA has played a positive role in forest conservation, because in some municipalities of Oaxaca, local stakeholders have decided to live from economic resources derived from remittances from migrants and preserved about 90 % of their almost pristine forests for conservation and ecotourism activities (Velázquez et al. 2003, Martin et al. 2011). Unfortunately, the main problems for conservation of natural ecosystems in Oaxaca are those associated with social disputes over land ownership (Peterson et al. 2003, Ramírez-Toro et al. 2017) and deforestation (Velázquez et al. 2003).
From the 78 grid cells with just one record, 32 of them show a low richness of less than 10 species (Aguilar-Aguilar & Salgado-Maldonado 2006). This low richness could be associated with unexplored areas and insufficient sampling, as occurs in many zones of Oaxaca (García-Mendoza & Meave 2011, Mendoza-Ruiz et al. 2017, Ramírez-Toro et al. 2017). Grid cells P, X, and CB are examples of this, but also reflect the type of environment, as occurs with grid-cells D and K associated with arid scrub in the Tehuacán-Cuicatlán Valley. Some grid cells cover much less of Oaxaca’s area, such as A, D, and AM, whereas others, such as BW, BX, CB, and CC have more than 50 % of the grid-cell surface in the ocean (Figure 3). However, most of these grid cells have several fern records, such as E with 51 species; the exceptions are BB, BE, and BS grid cells, where no records were obtained (Figure 5A). Future explorations in these areas will very likely show that absence of records is due to insufficient collecting effort rather than a true lack of ferns and lycophyte species. The grid cells with low richness could partially explain the high value for beta diversity, because these represent 40 % of all grid cells with just one record. The value obtained for beta diversity must be taken with caution, because it is necessary to explore each grid cell with care to record the presence of all fern and lycophyte species, a task that involves many years of extreme effort in order to have values closer to reality. Future collection efforts in poorly sampled areas are necessary in order to increase the fern and lycophyte records (Almeida & Salino 2016), which highlights the importance of fieldwork, an activity that is reflected in the comparison of observed alpha and estimated alpha values of the pteridoflora of Oaxaca.
Bolivia and Costa Rica are countries with similar number of species (nearly 1,200) of ferns and lycophytes to Mexico (Almeida & Salino 2016), where 10 % of its species are included in some risk category; in contrast, Mexico has only 3 % (Tejero-Díez et al. 2016). Our study included 620 species of ferns and lycophytes. Of these, only 23 are listed under some category of risk in NOM-059 (SEMARNAT 2010). Considering the diversity of ferns and lycophytes in Oaxaca as a whole (683 species), Tejero-Díez & Mickel (2011) established that only 3.3 % of the species are included in NOM-059 (SEMARNAT 2010). This low number of species implies that other fern species inhabiting Oaxaca must be included. This task has been proposed for other areas of the country, such as Sierra Madre Oriental (Sanginés-Franco et al. 2011) and the Sierra Madre del Sur (Tejero-Díez et al. 2016). Unfortunately, there are no species endemic to Oaxaca included in NOM-059 (SEMARNAT 2010), as occurs with endemic species of flowering plants (Espejo-Serna 2012).
The tuber sword fern Nephrolepis cordifolia (L.) C. Presl, unfortunately included in NOM-059 (SEMARNAT 2010) in the category in danger of extinction, is probably not native in Mexico, but is introduced and naturalized in many localities (Mickel & Smith 2004) due to this species being used as an ornamental plant (Muñiz et al. 2007). N. cordifolia is one example, among others, such as Schizaea fluminensis Miers ex J. Sturm, known as Caribbean curly-grass fern, that show the mistakes included in NOM-059 (SEMARNAT 2010), because several plants that have been introduced into Mexico or do not inhabit the country are included in some category of risk. Thus, this red list produced by the Mexican government needs an urgent, serious and critical revision by specialists in different biological groups.
The lycophytes and ferns that inhabit the state of Oaxaca are mainly distributed in humid and temperate forests. We noted that in the case of the grid cells M, N, U, V, AD, AE and AF that include areas of the Cañada, Papaloapan, Sierra Norte and Valles Centrales regions, all these with high richness and CWE values, an elevation gradient associated with different vegetation types and high moisture are located here and let the development of many species of ferns and lycophytes. An example is observed between Valle Nacional (65 m) and Cerro Pelón (3,000 m), two locations apart on horizontal plane only by 30 km (Meave et al. 2017). In contrast, some species belonging to the genera Cheilanthes, Gaga, Myriopteris and Notholaena, inhabit in vegetation types with less rainfall (Tejero-Díez et al. 2016), such as xerophytic scrubland and tropical deciduous forest and are mainly located in the Mixteca and Costa regions. Notwithstanding, the distributional patterns obtained in the present study are mainly defined by mountainous and humid landscapes and reflect Mexico’s wonderful biodiversity.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2844.











 nueva página del texto (beta)
nueva página del texto (beta)