Temporal and spatial environmental heterogeneity is common in terrestrial ecosystems and, sessile organisms such as plants must cope with this heterogeneity (Bazzaz 1996, Valladares et al. 2007). Phenotypic plasticity (PP), defined as the ability of a single genotype to express multiple phenotypes in response to environment changes (Bradshaw 1965, Schlichting & Pigliucci 1998, Sultan 2000), is recognized as one important way in which plants can deal with environmental heterogeneity (Bradshaw 1965, Bradshaw & Hardwick 1989). At present, it is widely accepted that PP is genetically inherited and, natural selection may therefore optimize PP in environmentally variable habitats (Via et al. 1995).
Humans are an important driver of plant evolution, an influence that is particularly notable in domesticated plants (Ladizinsky 1998). As a result of domestication, a suite of phenotypic traits favorable to humans is exhibited in a number of phylogenetically unrelated crops (i.e., domestication syndrome; Harlan 1975, Hammer 1984). Artificial and natural selection have different targets and may lead to phenotypic divergence between crops and their wild relatives, and the presence of the novel phenotypes of domesticated plants may have some ecological consequences that have only recently begun to be documented (Whitehead et al. 2017, Egan et al. 2018, Chang et al. 2021). One aspect that is poorly understood is how domestication has affected PP in cultivated plants (Piperno et al. 2015, Matesanz & Milla 2018). Although crops with greater plasticity can take advantage of the beneficial environmental conditions typically found in agroecosystems (i.e., greater water and nutrient availability) and have a greater ability to buffer the negative effects of environmental variation (Nicotra et al. 2010, Des Marais et al. 2013), high PP is also often considered an undesirable trait in many breeding programs aimed at increasing yield uniformity (Peltonen-Sainio et al. 2011, Des Marais et al. 2013). Since PP is often inadvertent from the human perspective (Valladares et al. 2007), it has been unconsciously selected (Zohary 1994). Assessment of the effect of artificial selection on PP in crops therefore requires experimental approaches in which the reaction norms (i.e., the array of phenotypes generated by a specific genotype in response to different environments; Schmalhausen 1949) of domesticated plants are compared to those of their wild relatives (Matesanz & Milla 2018). However, studies employing such an approach are scarce in the literature and the crop species studied to date (chard [Beta vulgaris L], cabbage [Brassica oleracea L], sunflower [Helianthus annuus L], tomato [Solanum lycopersicum Medik.], durum wheat [Triticum durum Desf.], maize [Zea mays L.] and pea [Pisum sativum L.]) are sexually propagated (Matesanz & Milla 2018).
To the knowledge of the author, only one study (Matesanz & Milla 2018) has compared the reaction norms of domesticated plants with their wild relatives. The authors found non-parallel reaction norms in domesticated and wild accessions for a set of morphological and physiological traits in most of the crops they studied; i.e., the PP tended to be greater in domesticated plants, which were able to capitalize on greater water availability and outperform their wild relatives in terms of growth rates and biomass gain, while their performance does not differ when water availability is low. Paradoxically, the same crops exhibited mainly parallel reaction norms for most traits under different nutrient levels. Furthermore, using a different approach, greater PP in response to light was found in the number of branches in wild compared to domesticated accessions of maize (Zea mays) (Doebley & Stec 1991, Doebley et al. 1997) and millet (Setaria viridis (L.) Thell. and S. italica (L.) P. Beauv.) (Doust & Kellogg 2006). The effect of domestication on PP may therefore be contingent upon the environmental variable and crop species in question. Another aspect that may preclude generalization is that all the previously studied crops reproduce through seeds. It has been suggested that PP could have played a more important role in the evolution under domestication of clonally propagated crops than in those that reproduce sexually (McKey et al. 2010, Denham et al. 2020). For example, Denham et al. (2020) suggested that morphological differences exhibited between domesticated plants and their wild relatives are often due to plastic responses to environmental differences that prevail in their respective habitats (i.e., agroecosystems for domesticated plants and natural habitats for wild plants). Moreover, Ménard et al. (2013) observed that wild and cultivated manioc (Manihot esculenta Crantz) exhibited different growth habits in open savannah (shrub) and forested areas (liana). Based on this, these authors inferred that PP in growth form has been unaffected by domestication in this clonally propagated crop (Ménard et al. 2013). However, the currently available evidence comes from observational studies in which the genotype of plants was not controlled and therefore the apparently negligible effect of domestication on PP sensu stricto remains to be confirmed in clonally propagated crops.
In this study, the PP of traits related to yield, growth rate and resource allocation, as a response to light availability, was assessed in chaya (Cnidoscolus aconitifolius (Mill.) I.M. Johnst), a clonally propagated crop with edible leaves (Ross-Ibarra & Molina-Cruz 2002, Munguía-Rosas et al. 2019). Chaya offers an excellent study model with which to address the effect of domestication on PP given that, in contrast to many other clonally propagated crops (Denham et al. 2020), its domestication history, artificial selection targets and wild ancestor are all known (Ross-Ibarra 2003, Munguía-Rosas et al. 2019, Solís-Montero et al. 2020). Chaya was domesticated by the Maya, who selected for plants with a greater quantity of larger and softer leaves (Munguia-Rosas et al. 2019). Domesticated and wild plants coexist; however, domesticated plants only grow in home gardens while the wild plants grow in a variety of habitats ranging from forest gaps to heavily disturbed vegetation (Munguía-Rosas et al. 2019). Light availability was selected as the independent variable because chaya is a light demanding plant and the phenotypic response of these plants to shade is well known (i.e., the shade avoidance syndrome), featuring allocation of resources to height at the expense of leaves, branches and stems (Franklin 2008). The specific goal of this study was to assess the effect of domestication on PP in leaf traits, growth rates and allocation patterns to light variation by comparing reaction norms between wild and domesticated chaya. If domestication has affected PP, the incidence of non-parallel reaction norms in wild and domesticated plants can be predicted, for at least some of the traits measured.
Materials and methods
Study system.Cnidoscolus aconitifolius (Mill.) I.M. Johnst (Euphorbiaceae) is a shrub of up to 5 m in height, distributed mainly in tropical America (Ross-Ibarra & Molina-Cruz 2002, Maya-Lastra & Steinmann 2019). C. aconitifolius is a monoecious plant species (i.e. staminate and pistillate flowers are produced by the same plant), and its short-lived (24 h) flowers are insect-pollinated (Arceo-Gómez et al. 2009). The fruits are dry capsules containing up to three seeds that are dispersed ballistically (Standley & Steyermark 1949). The reproductive season lasts from March to November with a flowering peak in summer (Munguía-Rosas & Jácome-Flores 2020). The leaves are deciduous and have highly urticant trichomes, which are also present on the stems and fruit (Parra-Tabla et al. 2004, Abdala-Roberts & Parra-Tabla 2005). Trichome density and resource allocation is influenced by some environmental variables such as herbivory (Abdala-Roberts & Parra-Tabla 2005, Arceo-Gómez et al. 2009). C. aconitifolius grows in forest gaps and heavily anthropized habitats (Parra-Tabla et al. 2004).
The cultivated form of C. aconitifolius, known as chaya in Yucatan, belongs to the same species as its wild relative (Maya-Lastra & Steinmann 2019). Chaya was domesticated relatively recently by the Maya in the Yucatan Peninsula, where it is grown in home gardens and co-occurs with its wild relatives up to the present (Ross-Ibarra 2003, Munguía-Rosas et al. 2019). Despite their co-occurrence, there is complete reproductive isolation between the wild and domesticated forms, mainly due to poor pollen production and fertility of the latter (Munguía-Rosas & Jácome-Flores 2020). The syndrome of domestication includes greater production of bigger leaves with significantly fewer trichomes (Munguía-Rosas et al. 2019, Solís-Montero et al. 2020). Domesticated plants are clonally propagated by stem cuttings, which are more succulent than those of their wild relatives (Munguía-Rosas et al. 2019).
Experimental design and data collection. Stem cuttings were collected from 160 accessions (69 cultivated and 91 wild plants) in 40 sites across the Yucatan Peninsula. Cuttings were planted in an experimental plot located in the municipality of Merida in central Yucatan (21° 01’ 18” N; 89° 39” W; 11 m asl) in the year 2017. By spring 2019, when this study begun, the plantation had 67 adult plants (33 wild and 34 domesticated). This plantation was used as a source of cuttings in a plasticity experiment to reduce potential environmental and mother effects. Eight stems were cut from secondary branches of 20 different (10 wild and 10 cultivated) mother plants (hereafter referred as genotypes: n = 160 cuttings). These mother plants were selected under the following criteria: they (i) had at least 10 secondary branches, (ii) were apparently healthy (i.e., showed no sign of pathogens and/or herbivory) and (iii) were similar in height (1.5-2 m). The selected stems were cut diagonally at 1-2 cm from the nearest node into segments of 30 cm in length. Stem cuttings were planted in 6 L pots with a 7:3 mix of local soil and gravel, placed in a plant nursery with full exposure to sunlight and watered as required. Within the first month after planting, stems with signs of wilting or rotting were replaced. Despite this replacement procedure, one year later, only 97 plants (64 domesticated and 33 wild) from the 20 genotypes had survived. The difference in sample size was due to greater mortality rate in wild clones. Approximately half of the clones available per genotype were allocated randomly to one of two different light treatments: full exposure to sunlight (n = 48 plants; 14 wild and 34 domesticated) or grown beneath a nylon shade cloth that reduced solar radiation by ≈ 70 % (n = 49 plants; 19 wild and 30 domesticated). Genotypes with a single clone (one genotype for domesticated and two genotypes for wild plants) were also randomly allocated into the light treatments. Photosynthetic active radiation (PAR) was measured at six different points beneath the cloth (hereafter referred to as the “shadow” treatment) and near clones fully exposed to sunlight (hereafter referred to as the “light” treatment) at 1,200 h on a clear, sunny day with a LI-500 light sensor logger (LI-COR, Lincoln, NE, USA). The PAR available in the shaded environment (156 ± 53 µmol· m2· s-1) was only 22 % of that recorded in the open spaces (696 ± 36 µmol·m2·s-1).
Data collection began one month after allocating the clones to their respective light treatments (July 2020). At that time, all of the plants were aged 12-13 months. Once a month, and for a period of six months, plant height and the diameter at the base of the main stem were measured. Every month, the number of leaves was counted, and one fully expanded leaf, with no visible damage, was collected from each plant. These leaves were then taken to the laboratory where the area and perimeter were measured with a leaf area meter (CID Biosciences Inc. CI-202, Camas, WA, USA). The number of trichomes on the leaf border were also counted because there is a larger density of trichomes and also because the trichomes on other parts of the leaves were scarce or absent in the domesticated plants and, therefore, not comparable (Solís-Montero et al. 2020). Leaves collected during the last month of sampling were also weighed fresh and then oven-dried at 75 °C for 36 h.
Data processing and statistical analyses. Since the longevity of fully expanded leaves is shorter than the duration of the inter-sampling periods, monthly leaf count was used as a proxy of leaf production per plant during the experiment. Leaf trichomes were expressed as number of trichomes per cm in length to control for leaf size. Stem elongation and growth in stem diameter were also calculated as: x2- x1 · (t2 - t1)-1 where x1 and x2 are plant height or stem diameter during the first (t1) and last (t2) weeks of sampling. Furthermore, a slenderness index was calculated as the plant height divided by the diameter of the stem at the end of the experiment (Barros et al. 2011). Leaf specific area (LSA) of the leaves collected during the last week of sampling was calculated by dividing the area of each leaf by its dry mass.
The overall trend of phenotypic variation of wild and domesticated plants in the two light treatments was first assessed using a principal component analysis (PCA) with the eight phenotypic variables. Then, generalized linear mixed models were used to assess the main effect of domestication (two levels: wild vs. domesticated), light treatment (light vs. shadow) and their interaction, on the individual phenotypic traits: (i) leaf size (leaf area in cm2), (ii) leaf perimeter in cm, (iii) LSA in cm2·g-1, (iv) leaf production in number of leaves produced in a period of six months, (v) number of trichomes·cm-1, (vi) stem elongation (mm·week-1), (vii) growth in stem diameter (mm·week-1) and (viii) the slenderness index (mm·cm-1). Leaf variables i, ii, iv and v are known selection targets in chaya, with variable iv also representing the yield (Munguía-Rosas et al. 2019), while variables iii and vi-viii are related to changes exhibited by plants under limited light conditions (Griffith & Sultan 2005, Barros et al. 2011). A Gaussian error distribution was assumed for all of these variables, except leaf production, for which a Poisson error distribution was chosen. In all models, genotype was included as a random grouping factor. Since several leaves from the same plant were measured, in the specific case of models for leaf size, leaf perimeter and trichomes, the leaves nested in the plant were also included in the random part of the models to account for correlated data. Owing to variation in the structure of the random part of the model, as well the incidence of missing data (i.e. some leaves did not met selection criteria), the denominator degrees of freedom varied among models. Differential phenotypic plasticity between wild and domesticated plants was inferred when the interaction domestication x light treatment was significant. In that case, one-way analyses within a single level of the second factor were then performed in order to elucidate the nature of the interaction.
The direction and intensity of reaction norms among wild and domesticated plants was expressed as the slope between mean values and between light environments for each domestication level and phenotypic trait (Morrissey & Liefting 2016). Although the slope of the reaction norms is suitable to show the phenotypic change produced by the environment, it offers little opportunity for comparing PP among different traits, given the differences in units. A plasticity index based on relative distance (RDPI) was therefore also calculated (Valladares et al. 2006). The RDPI is defined as the difference between minimum and maximum trait values divided by the maximum trait value, and ranges from 0 (no plasticity) to 1 (maximum plasticity). The index was calculated per genotype, when more than one clone survived per genotype and per environment, using the mean values of the phenotypic traits (Valladares et al. 2006). Only one value per trait was shown, which was the sum of phenotypic distances for each genotype divided by the number of genotypes (Valladares et al. 2006). Genotypes with only one clone were not used to calculate RDPI. To assess inter-genotype variation, the coefficient of variation (CV) of RDPI at genotype level was calculated for each trait for wild and domesticated plants. All of the analyses were run in R v4.0.3, packages: lme4, nlme and stats (R Core Team 2020). All data are available as online supplementary material.
Results
Environmental and domestication effects. All traits were significantly affected by the light treatment, except stem elongation (Table 1). Similarly, most of the traits measured (leaf area, leaf perimeter, trichome density, stem elongation and growth in stem diameter) were affected by domestication (Table 1). Interaction between light treatment and domestication was significant for leaf production and the slenderness index (Table 1); however, the effect of these factors was additive for the rest of the traits measured (Table 1, Figure 1). Examination of the significant interactions indicated that, while domesticated plants did not produce a significantly different number of leaves in the two light treatments (χ2 1 = 2.76, P = 0.09), the wild plants (χ21 = 16.36, P << 0.01) produced significantly less leaves in the shadow than in the light treatment (Figure 1a). Wild plants also exhibited a significantly higher slenderness index than domesticated plants in the shadow treatment (F1,43 = 3.33, P = 0.04); however, the wild and domesticated plants did not differ in this regard in the light treatment (F1,43 = 0.28, P = 0.60) (Figure 1h).
Table 1 Effects of light treatment (light vs. shadow) and domestication (wild vs. domesticated) and their interaction on eight vegetative traits (Trait) in Cnidoscolus aconitifolius: Leaf area, leaf perimeter, leaf specific area (LSA), leaf production, trichome density (Trichomes), stem elongation (Elongation) and growth in stem diameter (Stem diameter), as well as a slenderness index (Slenderness).
| Trait | Source of variation | Statistics |
|---|---|---|
| Leaf area | Light | F1,66 =11.37** |
| Domestication | F1,17 = 25.71** | |
| Light × Domestication | F 1,66 = 1.02 n.s. | |
| Leaf perimeter | Light | F1,66 =4.65* |
| Domestication | F1,17 = 5.03* | |
| Light × Domestication | F1,66 = 1.14 n.s. | |
| LSA | Light | F1,59 =15.19** |
| Domestication | F1,17 = 0.46 n.s. | |
| Light × Domestication | F1,59 = 0.16 n.s. | |
| Leaf production | Light | Χ21= 13.41 ** |
| Domestication | Χ21= 0.23 n.s. | |
| Light × Domestication | Χ21= 5.75* | |
| Trichomes | Light | F1,66 =15.24** |
| Domestication | F1,17 = 44.99** | |
| Light × Domestication | F1,66 = 0.14 n.s. | |
| Elongation | Light | F1,68= 5.19 * |
| Domestication | F1,68=10.63** | |
| Light × Domestication | F1,68=0.35 n.s. | |
| Stem diameter | Light | F1,68=0.09 n.s. |
| Domestication | F1,68=3.14 * | |
| Light × Domestication | F1,68= 0.37 n.s. | |
| Slenderness | Light | F1,68 = 19.71** |
| Domestication | F1,68= 0.72 n.s. | |
| Light × Domestication | F1,68 =3.73* |
n.s. = No significant effect, *P < 0.05, **P < 0.01.
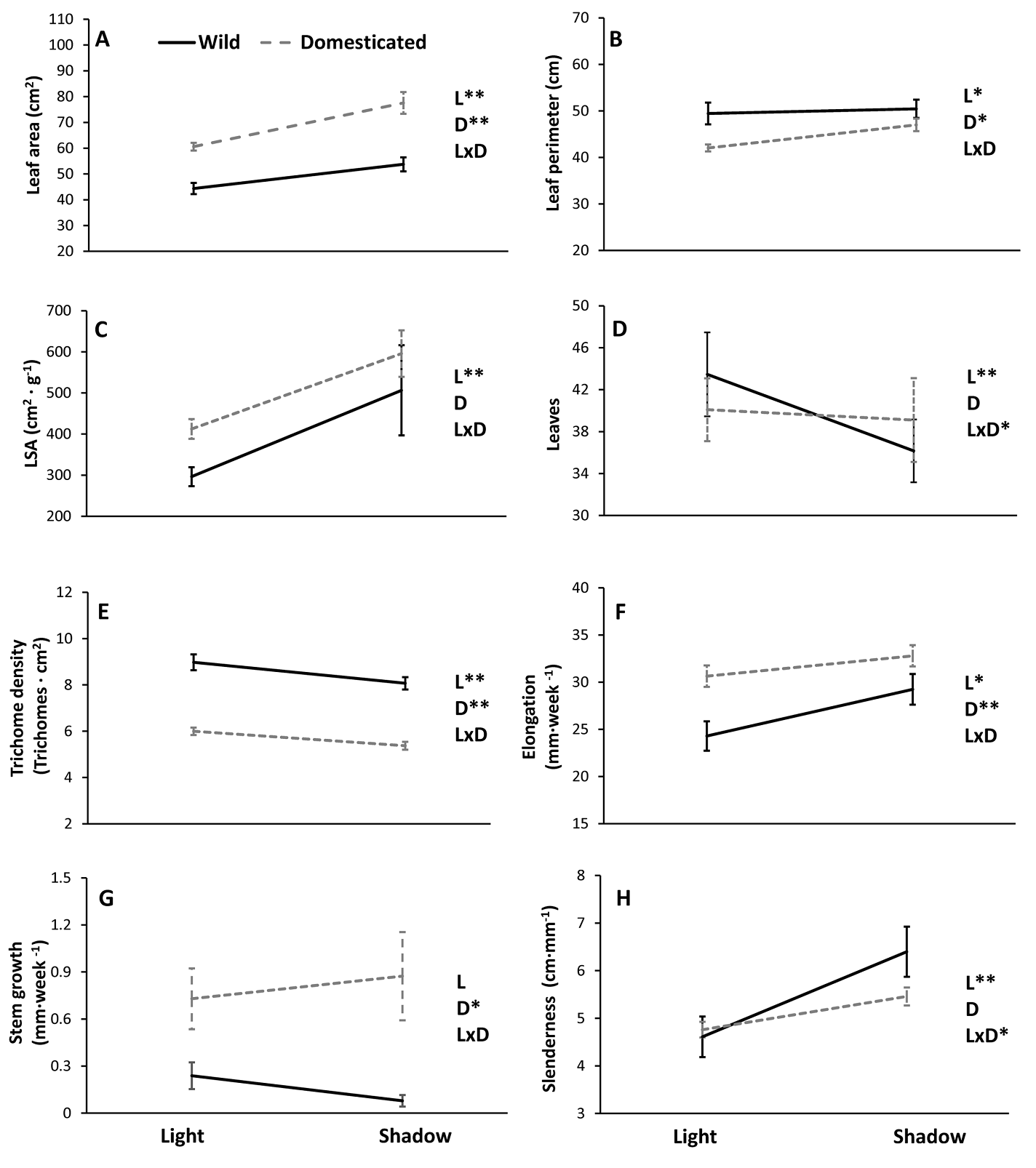
Figure 1 Reaction norms observed in eight vegetative phenotypic traits in wild and domesticated Cnidoscolus aconitifolius under two contrasting light treatments: light vs. shadow. Data are mean values ± 1 standard error. Phenotypic traits measured were: (A) leaf area, (B) leaf perimeter, (C) leaf specific area (LSA), (D) leaf production, (E) trichome density, (F) stem elongation, (G) growth in stem diameter and (H) a slenderness index. The letters L (light treatment), D (domestication) and L x D (light x domestication), as well as the asterisks (* = P <0.05, ** = P < 0.01) indicates sources of variation tested and their statistical significance.
Plants in the light treatment produced 1.08 times more leaves and 1.11 times more trichomes than those in the shadow treatment. However, the leaves of the plants in the full light were 1.25 times smaller and presented perimeter and LSA values that were 1.09 and 1.48 times lower, respectively, than those of the plants in the shadow treatment (Table 2). Regarding plant growth, plants in the shadow treatment presented faster elongation and greater slenderness than plants in the light treatment (Table2). Regardless of light treatment, the domesticated plants produced leaves with 1.39 times greater leaf area and presented a more rapid elongation (1.18 times) and growth in stem diameter (3.85 times) than the wild plants. However, the domesticated plants also produced leaves with a 1.13 times smaller perimeter and 1.49 times fewer trichomes than the wild plants (Table 2).
Table 2 Mean values (± 1 SE) of eight vegetative phenotypic traits (Trait) of wild and domesticated (Domestication) Cnidoscolus aconitifolius under two contrasting light treatments: light vs. shadow. Traits measured were: Leaf area, leaf perimeter, leaf specific area (LSA), leaf production, trichome density (Trichomes), stem elongation (Elongation) and growth in stem diameter (Stem diameter) as well as a slenderness index (Slenderness). Different superscript letters indicate statistically significant differences (P < 0.05) between factor levels: light vs. shadow or wild vs. domesticated.
| Light treatment | Domestication | |||
|---|---|---|---|---|
| Trait | Light | Shadow | Wild | Domesticated |
| Leaf area (cm2) | 55.81 ± 1.36a | 70.01 ± 3.15b | 48.95 ± 1.79a | 68.47 ± 2.19b |
| Leaf perimeter (cm) | 44.24 ± 0.91a | 48.11 ± 1.11b | 49.94 ± 1.52a | 44.35 ± 0.76b |
| LSA (cm2·g-1) | 379.74 ± 20.22a | 560.78 ± 54.58b | 420.74 ± 67.89a | 498.76 ± 31.89a |
| Leaf production (n) | 41.02 ± 2.62a | 37.96 ± 2.77b | 39.12 ± 2.91a | 39.62 ± 2.48a |
| Trichomes (Trichomes·cm-1) | 6.87 ± 0.19a | 6.22 ± 0.18b | 8.53 ± 0.22a | 5.71 ± 0.12b |
| Elongation (mm·week-1) | 28.91 ± 0.95a | 31.69 ± 1.02b | 27.02 ± 1.02a | 31.91 ± 0.84b |
| Stem diameter (mm·week-1) | 0.59 ± 0.15a | 0.63 ± 0.21a | 0.21 ± 0.55a | 0.81 ± 0.18b |
| Slenderness (cm·mm-1) | 4.72 ± 0.16a | 5.75 ± 0.21b | 5.52 ± 0.34a | 5.11 ± 0.14a |
Intensity and variation of phenotypic plasticity. The first two components of the PCA explained 52 % of phenotypic variation in the study plants. Leaf perimeter (0.51) and area (0.48), as well as stem elongation (0.43) and slenderness (0.37), had the biggest loadings in PC1, while trichome density (0.54), elongation (-0.45) and leaf perimeter (37) presented the biggest loadings in PC2. When plotting PCI and PC2 values for wild and domesticated plants under the two light treatments, a variable degree of separation among groups was observed. Wild plants in both the light and shadow treatments presented greater separation, while the distribution of their domesticated counterparts under different light treatments overlapped extensively. The smallest overlap was detected between wild plants in the light treatment and domesticated plants in the shadow treatment (Figure 2).

Figure 2 Scatter plot showing the distribution of wild and domesticated plants of Cnidoscolus aconitifolius under two light treatments (light vs. shadow) along two principal components (PC1 & PC2) obtained from eight morphological vegetative traits (leaf area, leaf perimeter, leaf specific area, leaf production, trichome density, stem elongation, growth in stem diameter and slenderness). Confidence ellipses (90 %) for each group: wild plants exposed to full light (W-Light), wild plants beneath a shade cloth (W-Shadow), domesticated plants in full light (D-Light) and domesticated plants beneath shade (D-Shadow) are also shown. Symbols are defined in the legend.
The reaction norms of wild plants exhibited steeper slopes than domesticated plants in most of the traits measured, although to a variable extent (Table 3). Specifically, leaf production was 7.38 times, slenderness 2.59 times, stem elongation 2.31 times, trichome density 1.47 times, LSA 1.15 times and growth in stem diameter 1.14 times greater in wild than in domesticated plants (Table 3). In contrast, the domesticated plants presented reaction norms with steeper slopes only for leaf perimeter (4.86 times) and leaf area (1.74 times) (Table 3). In general, the slopes of the reaction norms of wild and domesticated plants had the same sign, except for growth in stem diameter, which was positive in domesticated but negative in wild plants (Table 3, Figure 1). The RDPI for phenotypic traits in wild genotypes ranged from 0.11 (trichome density) to 0.64 (growth in stem diameter) and averaged 0.29 ± 0.05. The same index for domesticated plants ranged from 0.09 (stem elongation) to 0.71 (growth in stem diameter) and averaged 0.24 ± 0.07 (Table 3). In general, the RDPI was greater in wild than in domesticated plants for most traits (63 %) and, when the opposite was found, the differences were relatively low (Δ = 0.04 - 0.07) (Table 3).
Table 3 Slope of reaction norms (Slope), phenotypic plasticity index (RPDI) and coefficient of variation of RPDI at genotype level (CV) for wild and domesticated plants of Cnidoscolus aconitifolius growing under two contrasting light treatments. Traits measured were: Leaf area, leaf perimeter, leaf specific area (LSA), leaf production, trichome density (Trichomes) stem elongation (Elongation) and growth in stem diameter (Stem diameter) as well as a slenderness index (Slenderness).
| Slope | RPDI | CV (%) | ||||
|---|---|---|---|---|---|---|
| Trait | Wild | Domesticated | Wild | Domesticated | Wild | Domesticated |
| Leaf area | 9.48 | 16.49 | 0.21 | 0.24 | 51.42 | 58.51 |
| Leaf perimeter | 1.01 | 4.91 | 0.23 | 0.12 | 77.63 | 71.19 |
| LSA | 210 | 183 | 0.31 | 0.29 | 67.61 | 53.13 |
| Leaf production | -7.31 | -0.99 | 0.31 | 0.21 | 54.94 | 120.01 |
| Trichomes | -0.91 | -0.62 | 0.11 | 0.15 | 76.98 | 75.52 |
| Elongation | 4.95 | 2.15 | 0.21 | 0.09 | 87.12 | 82.73 |
| Stem diameter | -0.16 | 0.14 | 0.64 | 0.71 | 60.28 | 42.49 |
| Slenderness | 1.79 | 0.69 | 0.31 | 0.15 | 50.33 | 53.43 |
The coefficient of variation for RDPI at genotype level ranged from 50.33 to 87.12 % (mean: 65.78 ± 1.71 %) for wild genotypes and from 42.49 to 120.01 % (mean: 69.62 ± 3.04 %) for domesticated genotypes (Table 3). Differences in the CV between wild and domesticated genotypes tended to be low (mean Δ = 14.00 ± 3.00 %), except for leaf production (Δ = 65 %), which was evidently greater than the mean (Table 3). In all traits measured, one or two genotypes showed the opposite pattern relative to the rest (Figure 3).

Figure 3 Reaction norms at genotype level of wild and domesticated Cnidoscolus aconitifolius under two contrasting light treatments: light vs. shadow. Data are mean values ± 1 SE. Phenotypic traits measured were: (A) leaf area, (B) leaf perimeter, (C) leaf specific area (LSA), (D) leaf production, (E) trichome density, (F) stem elongation, (G) growth in stem diameter and (H) a slenderness index.
Discussion
The results of this study suggest that domestication and light availability influence the phenotype of C. aconitifolius. A non-additive effect of domestication and light on phenotypic plasticity was found in leaf production and slenderness (Figure 1, Table 1). Reduced plasticity in these traits was observed in domesticated plants compared to their wild relatives. Wild plants exhibited greater PP than domesticated plants in other traits (leaf perimeter, LSA and stem elongation), albeit to a lesser extent (non-intersecting reaction norms). Although wild and domesticated plants exhibited some degree of plasticity in response to the light in all measured traits, the morphological differences between wild and domesticated plants were mostly consistent in the two light treatments (Figure 1, Table 2). These results suggest that artificial selection has not only changed the phenotype of chaya but has also reduced PP in some traits of this clonally propagated crop.
The results of the PCA clearly showed that domestication and light treatment induced an overall variation in the phenotype of C. aconitifilous. Environmentally induced variation was more evident in the wild than in the domesticated plants (Figure 2). Considering the loading and the orientation of confidence ellipses (Figure 1), the wild plants under full light exposure appear to exhibit less stem elongation and produce bigger leaves with more trichomes than is the case for wild plants in the shadow treatment. Wild and domesticated plants in both light treatments presented incomplete separation in the multivariate morphological space because these plants differed in some traits but not in others. Plasticity patterns are therefore clearer when analysing the phenotypic traits individually. The non-additive effect of the domestication x light treatment interaction on the number of leaves is compatible with the notion that artificial selection may lead to relative yield stabilization (Peltonen-Sainio et al. 2011, Des Marais et al. 2013). In contrast to the wild plants, which produced fewer leaves in the shadow than in the light treatment, leaf production in domesticated plants exhibited no significant variation between light treatments. Chaya is a vegetable crop; the leaves form the edible part of this plant and leaf production is one of traits that have been deliberately selected by the growers in the Yucatan Peninsula (Munguía-Rosas et al. 2019). Therefore, artificial selection has probably limited the PP in leaf production in domesticated chaya (i.e. yield stability). Similar results have been reported previously in some cultivated accessions of maize (Doebley & Stec 1991, Doebley et al. 1997) and millet (Doust & Kellogg 2006), in which limited plasticity to light/density was observed in axillary branches relative to that of the wild relatives. In contrast, Matesanz & Milla (2018) found greater PP in domesticated accessions than in wild relatives of chard, cabbage, sunflower, tomato, durum wheat, maize and pea. Moreover, Gallardo et al. (1996) found greater PP for root development in domesticated lettuce. Since the previous studies measured PP with environmental variables (water and nutrient availability) that were not evaluated in this research and, in contrast to this study, all of the previously studied crops were sexually reproduced, short-lived herbaceous plants, it was not possible to identify the cause(s) of the differences between those studies and the present study. However, this result clearly suggests that yield stability as a result of domestication is not exclusive to sexually propagated crops where the seed is both the edible and the reproductive part of the plant (e.g., maize and millet). This pattern can be also present in clonally propagated, vegetable crops such as chaya.
The domestication × light treatment interaction was also significant for the slenderness index. While slenderness was undistinguishable between wild and domesticated plants in the light treatment, wild plants exhibited greater slenderness than domesticated plants in the shaded environment. The plastic response in both slenderness and leaf production of the wild plants is in accordance with the shade avoidance syndrome, in which plants under light limitation reduce resource investment in leaf production and stem thickening in order to reallocate resources to growth in height (Griffith & Sultan 2005, Franklin 2008). Although this interaction was not significant for stem elongation, the steeper slope and greater PP index observed in wild plants for this trait support the notion that the shade avoidance syndrome is more evident in wild than in domesticated chaya. Artificial selection could have constrained the ability of domesticated plants to reallocate resources when facing light limitation.
Although the reaction norms of wild and domesticated plants did not intersect in most traits (i.e., non-significant domestication × light treatment interaction), those of the wild plants presented steeper slopes and/or greater PP indexes than the cultivated plants in most of these traits (Figure 1, Table 3); i.e., despite the plasticity exhibited, wild and cultivated chaya differed morphologically in the two light treatments in terms of leaf area, trichome density and stem elongation and growth in stem diameter. This suggests that the morphological difference observed between wild and domesticated plants in their respective habitats (i.e. home gardens for domesticated plants and secondary forest/anthropized habitat for wild plants) is not due to PP, as has been suggested for other clonally propagated crops (Ménard et al. 2013, Denham et al. 2020). Domesticated chaya did not regress to “the wild phenotype” when growing in a common garden resembling the habitat of wild chaya; indeed, it exhibited a set of traits compatible with a domestication syndrome i.e. domesticated chaya had more, bigger, and softer leaves as well as more succulent stems (Munguía-Rosas et al. 2019). As is the case in sexually reproduced crops, these traits are related to the size and palatability of the edible part, as well as ease of cultivation (McKey et al. 2010, Meyer et al. 2012). As with other crops, wild and domesticated C. aconitifolius probably simultaneously exhibit phenotypic differences and important PP despite artificial selection, since the trait mean and variance (i.e., PP) are genetically decoupled (Des Marais et al. 2013).
Regarding the magnitude of plasticity, the RDPI averaged 0.29 ± 0.05 (range: 0.11-0.64) in wild and 0.24 ± 0.07 (range: 0.12-0.71) in domesticated chaya for pooled traits. The direction (wild > domesticated) and magnitude (1.3-fold) of this difference concurs with the review of Des Marais et al. (2013), who reported greater (1.8-fold) plasticity in natural than in cultivated crop species, a difference that the authors attributed to historical artificial selection for environmentally stable crops. However, these results must be interpreted with caution because these authors reviewed studies that adopted a wide variety of approaches and phylogenetically unrelated species (See supplementary materials in Des Marais et al. 2013). To make a sensible comparison with previous studies using a similar approach, the RDPI was calculated using mean phenotypic values in the different environments provided by the authors. This exercise was restricted to the traits that were also measured in this study. For the seven crops studied by Matesanz & Milla (2018), the RDPI for leaf area had an average value of 0.24 for wild and 0.26 for domesticated plants in environments under contrasting nutrient availability. These values are very similar to those reported here for chaya (wild = 0.21, domesticated = 0.24). However, the RDPI values for the same trait, but under contrasting water environments (wild = 0.28, domesticated = 0.45), were substantially higher than that observed in chaya, particularly for domesticated plants. The RDPI for stem elongation was also similar between the wild plants (wild chaya = 0.21, wild relatives of previously studied crops = 0.21- 0.29) but substantially different between domesticated plants (chaya = 0.09, other crops = 0.44-0.56). Again, because there are several differences between the crops studied by Matesanz & Milla (2018) and chaya, it was not possible to identify the factor underlying the differences between studies. Not only did this and previous studies manipulate different environmental variables, but also the plants present several differences in life history traits and cultivation modes.
Variation in PP among genotypes was important for wild (CV range: 50-87 %) as well as domesticated (CV range: 42.49-120 %) plants. This variation was highly influenced by 1-2 genotypes of wild and domesticated plants that exhibited either the opposite trend compared to the rest or an exacerbated plasticity in the same direction as that of the rest of genotypes (Figure 3). This variation among genotypes in terms of PP may also explain the overlap of the ellipses of wild and domesticated plants in different light treatments in the multivariate phenotypic space (Figure 2). The presence of genotypes with atypical reaction norms may be due to spatial variation in the strength, duration and/or direction of artificial selection. The genotypes used in this study came from different populations across all over the Yucatan Peninsula; growers may therefore have selected different traits in some populations. Indeed, a previous study identified four different varieties of domesticated chaya based on leaf shape in Mexico and Guatemala (Ross-Ibarra 2003), supporting the notion that growers have selected different traits across the distribution rage of chaya. Other authors have suggested that chaya was recently domesticated and artificial selection has simply had insufficient time to filter undesired phenotypes (Munguía-Rosas et al. 2019).
To summarize, domestication in chaya affected phenotypic plasticity in response to the light exposure. Changes in PP are particularly relevant to leaf production and slenderness. Domesticated chaya also exhibits an attenuated shade avoidance syndrome compared to that of its wild progenitors. Despite this plasticity, differences between wild and domesticated plants are consistent throughout the contrasting light availability levels in most of the traits measured. This is the first study to assess the effect of domestication on phenotypic plasticity in a clonally propagated crop.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2879











 nueva página del texto (beta)
nueva página del texto (beta)



