Biogeographic regionalizations (kingdoms, regions, provinces, etc.) are identified based on their endemic species and represent recapitulations of the complex distribution of species within their territory (Williams 1996). Biogeographic regionalizations are traditionally represented geographically by drawing lines to delimit them (see, for example, Figure 1A). However, there are generally species characteristic of one region that penetrate a certain distance into another, an aspect of complexity around regional boundaries that is rarely discussed. These areas of overlap in species distribution from one region to another are known as transition zones (Morrone 2010, 2020) and the involved entities are biotas, which are recognized by the geographical restriction (endemism) of different plant and animal taxa to particular geographical areas (Morrone 2020).

Figure 1 Study site. A. Biogeographical provinces of central Mexico as proposed by CONABIO (1997). The pink line indicates the 1,600 m of elevation used as the boundary between the provinces in this study. BAL = Balsas Depression, SMS = Sierra Madre del Sur, VB = Volcanic Belt. B. Division of the study area into 122 grid squares of 30 minutes of latitude by 30 minutes of longitude.
Transition zones are not homogeneous in size or floristic composition. Williams (1996) argues that in some places these zones can be abrupt, with a rapid change in species from one region to the other, known as subtraction zones (Zunino & Zullini 2003, Ferro & Morrone 2014), while in other parts the change can be gradual, with wide zones with transition gradients between the species from one region to another, called addition zones. Maps delimiting biogeographic regionalizations do not indicate which boundaries more resemble a subtraction zone, an addition zone (Ferro & Morrone 2014), or something intermediate. Ferro & Morrone (2014) and Morrone (2020) review the concept of transition zones and discuss the relevance of characterizing them, both in terms of their biological content and size.
In these transition zones, not all the species are affected exactly in the same way by partial barriers or filters (Morrone 2020) and are considered areas where floristic composition changes (gradually or abruptly), generating more attention from ecologists than biogeographers. Although not specifically defined as transition zones, the ecological literature abounds with works on ecotones, hybrid zones, ecological limits, biotic transition zones, etc. (Schilthuizen 2000, Cadenasso et al. 2003, Strayer et al. 2003, Yarrow & Marín 2007), which at different scales deal with the composition, structure or replacement of species across the boundaries of communities, vegetation types or ecosystems. Ecological borders among vegetation types, biomes, ecosystems or biogeographic regionalizations constitute barriers that stop the free dispersal of many species (closed borders), although they can also serve as corridors through which biological exchange occurs (open borders). Therefore, understanding how richness and endemism distribute from one region to another is essential to explaining how their floras have been assembling and evolving (Hernández-Bermejo & Sainz-Ollero 1984).
For some authors, transition zones should be compared with areas of endemism and should be considered in conservation strategies (Smith et al. 2001). They could constitute additional zones where parapatric speciation processes are most likely occurring (Schilthuizen 2000), and if transition zones contain species that evolved in these zones and are adapted and known only from such hybrid regions, they could also constitute areas of endemism.
Considering the arguments laid out above, we ask the following questions: Is it possible to identify transition zones between the biogeographic provinces of Mexico? Can transition zones also be considered areas of endemism? To answer them we propose a series of objectives. The first was to document the Asteraceae species considered strictly endemic to three biogeographic provinces of central Mexico-the Balsas Depression (BAL), the Volcanic Belt (VB), and the Sierra Madre del Sur (SMS) and map their distribution. Our second objective was to outline the breadth of distribution of each of these species, documenting their biogeographic affinity based on the elevation intervals recorded by their collection locations, in order to assign each species as characteristic to one of the three biogeographic provinces evaluated or as a species typical of the transition zones among provinces. Using that information, a potential geographic extension of the transition zones is presented in order to better understand the characteristics of the borders among provinces, beyond the simple linear representation observed in biogeographic regionalization maps. Finally, using the floristic similarity among units of the same area (grid squares of 30 minutes latitude and longitude), we compared the floristic regions obtained in this study with previously proposed biogeographic regionalizations of the study area.
Materials and methods
Study area. The study area includes the biogeographic provinces located in central Mexico, according to the biogeographic regionalization proposal of Arriaga et al. (1997) and CONABIO (1997). This zone includes three provinces—BAL, VB and SMS. Figure 1 shows the geographical position and extension of these three biogeographic provinces as defined in the CONABIO (1997) proposal. We selected this scheme as it was the result of a workshop where 20 specialists evaluated previous regionalization proposals and developed a consensus among them (Arriaga et al. 1997).
Asteraceae endemic to the biogeographic provinces of central Mexico. The information analyzed in this work was obtained from a database of records of Asteraceae from Mexico (the database is available on request to the corresponding author), derived from the review of herbarium specimens deposited in both national and foreign institutions. Much of the material reviewed is already available in the SNIB-REMIB online databases of the National Commission for the Knowledge and Use of Biodiversity (CONABIO), as well as in the digital repository of the National Herbarium of Mexico (MEXU-UNIBIO) of the Institute of Biology, National Autonomous University of Mexico (UNAM). Species were selected by consulting the literature on endemism in the provinces analyzed (for example Rodríguez-Jiménez et al. 2005, Alcántara & Paniagua 2007, Villaseñor & Ortiz 2007, Rzedowski 2020) and on unpublished data of one of the authors (JLV).
Using the geographic coordinates of the collection sites, the records of endemic species were filtered to locate their correct position within their biogeographic limits, and duplicates and records with incomplete or dubious information were eliminated. Considering the possible uncertainty of the limit drawn on the map of biogeographic provinces (CONABIO 1997), records located within 5 km outside the province borders were included in the analyses, considered as the possible spatial error in some coordinates (Graham et al. 2008). Once the records had been selected, the elevation of each record was standardized using the geographic coordinates of the collecting sites and a digital elevation model downloaded from the GTOPO30 page (http://eros.usgs.gov/"\l"/Find_Data/Products_and_Data_Available/gtopo30_info), as well as the biome (Villaseñor & Ortiz 2014) where they were found.
Due to the uncertainty of the completeness of the collecting effort in the study area, we considered important to generate a map of the distribution of the known and estimated richness of endemic species in the study area by using the Universal Kriging geostatistical method following the strategy applied by Cruz-Cárdenas et al. (2013). This will allow obtaining a more uniform scenario on the distribution of richness and endemism (Hortal & Lobo 2011). Thus, the spatial prediction of species richness was based on the known richness in the grid squares into which the study area was divided (Figure 1B).
Elevation limit among biogeographic provinces. The VB and SMS provinces are mainly composed of humid mountain forest and temperate forests (fir, oak, pine, pine-oak forests, as well as typical vegetation of high uplands, Villaseñor & Ortiz 2014). In contrast, the BAL province is mainly seasonally dry tropical forest and its derived subtropical scrub. The mean upper elevation limit of the seasonally dry tropical forest in BAL to be approximately 1,600 m (Flores-Tolentino et al. in preparation). This elevation corresponds relatively well with the limit for this biome in Mexico as discussed by Rzedowski (2006), who stated that seasonally dry tropical forest can be found from sea level to 1,900 m of elevation, but is most frequently below 1,500 m. We therefore considered 1,600 m asl (Figure 1A) the limit of BAL in relation to VB and SMS. Thus, all the endemic species records in the province polygon above this elevation were considered to belong to the VB or SMS, while records below this elevation were ascribed to the BAL. Species with both records above and below 1,600 m asl were assigned to both provinces (i.e., BAL-VB or BAL-SMS).
Size of transition zones. Transition zones among the biogeographic provinces were defined based on the distances from the collection sites of the endemic Asteraceae species to the 1,600 m elevation line. Only the endemic species shared among them were evaluated in terms of the number of records they contain in one or another province (Table S1).
The width of the transition zones was calculated as the average of the distances of the records of the species shared among provinces at the limit level. In this way, a BAL → VB and BAL → SMS value was obtained for the species shared between those provinces but with a higher frequency of records in BAL, while the VB → BAL and SMS → BAL values corresponded to the species shared between them but with higher frequency of records in high areas. Once the distance averages were obtained, the results were projected onto the map of the biogeographic provinces to illustrate the width of the transition zones. Finally, based on the width of the zones, the characteristic species of each of the evaluated regions (provinces and transition zones) were defined. In addition, with the purpose of evaluating the coincidence of these species with the identified transition zones, a biogeographic track (Morrone 2004) was drawn. All the spatial analyses were carried out using the ArcMap 10.1 program (ESRI 2013), using the ‘Raster Calculator’ (to calculate the elevation of the collection sites) and ‘Euclidean Distance’ tool (to calculate the distances from the collection sites to the 1,600 m elevation line).
Floristic similarities and areas of endemism. The study area was divided into grid squares of 30 × 30 minutes of latitude and longitude, and we estimated the floristic similarities among all pairs of grid squares using the Sorensen-Dice coefficient of similarity (SD = 2c / (2c + a + b)), where for A and B represent two grid squares, a are the taxa exclusive to A, b are the taxa exclusive to B and c are the shared taxa between A and B. The similarity values were used to generate a dendrogram of floristic similarities using the UPGMA grouping method (unweighted grouping using arithmetic means). Once the groups with similar floristic composition (floristic regions sensuBirks 1976) were defined in terms of their floristic composition, the species restricted to their territories were identified, thus recognizing floristic elements that have similar geographic distribution (Birks 1976). Grid squares that shared at least two restricted species were considered areas of endemism (Morrone 1994, Noguera-Urbano 2016). Data were analyzed using the computer program NTSYS, version 2.21v (Rohlf 2007).
Hernández-Bermejo & Sainz-Ollero (1984) propose a strategy using the floristic similarity along province borders to determine whether these borders act as open corridors or closed barriers in the dispersal of species among provinces. Their proposal consists of evaluating the shared species with respect to the total species in each flora. In essence, their calculations correspond to the Simpson (S = c / min (a, b)) and Braun-Blanquet (BB = c / max (a, b)) indexes of similarity, where c is the number of taxa shared by the two floras (or grid squares in this case), a and b are the number of taxa in the compared floras, in S the least rich and in BB the richest. The data obtained with these two indices also correspond to the q and q' values estimated by Hernández-Bermejo & Sainz-Ollero (1984). This comparison essentially helps quantify the degree of migration or dispersal between two areas (S) or species impoverishment due to possible barriers that limit migration or dispersal between them (BB, Hengeveld 1990).
Results
From the literature and database review, we compiled a total of 2,061 distribution records from 315 endemic Asteraceae taxa-301 species and 15 infra-specific categories (varieties or subspecies, hereafter referred to as species)-in the three biogeographic provinces (Table 1). Species’ distribution by province is indicated in Figure 2 and Table 1.

Figure 2 Distribution of the collection sites of endemic Asteraceae species from three biogeographic provinces of central Mexico. The green squares represent the records in VB, blue dots in BAL and red triangles in SMS.
Table 1 Endemic species richness of Asteraceae in the biogeographic provinces of central Mexico (CONABIO 1997) and their distribution in the main biomes of Mexico (Villaseñor & Ortiz 2014). In parenthesis the number of species restricted to the province is indicated. BAL = Balsas Depression, VB = Volcanic Belt, SMS = Sierra Madre del Sur. BHM = humid mountain forest, BTEM = temperate forest, BTES = seasonally dry tropical forest, BTHU = humid tropical forest, MXE = xerophilous scrub.
| Biogeographic Province | Species | BHM | BTEM | BTHU | BTES | MXE |
|---|---|---|---|---|---|---|
| VB | 164 (125) | 15 | 102 | 6 | 1 | |
| SMS | 150 (105) | 24 | 83 | 2 | 4 | 4 |
| BAL | 71 (29) | 10 | 1 | 15 | ||
| VB-BAL | 11 | 1 | 9 | 5 | ||
| SMS-BAL | 15 | 4 | 15 | 3 | 6 | |
| VB-SMS | 16 | 6 | 15 | 1 | ||
| VB-SMS-BAL | 14 | 5 | 13 | 7 | ||
| Total | 315 | 55 | 247 | 6 | 44 | 5 |
The VB province had the highest number of species (164), followed by SMS (150) and BAL (71). A significant number of species’ distributions (259) were restricted to only one of the three biogeographic provinces; VB had 125 exclusive species, followed also by SMS with 105 and BAL with only 29. The other 56 species were distributed in at least two provinces (Table 1). Table S1 contains a list of the species analyzed and indicates the province(s) in which they are distributed.
Throughout the three biogeographic provinces analyzed, the five main types of biomes discussed by Villaseñor & Ortiz (2014) are recorded. Endemic species are documented in all of them, with the highest richness in those dominating the provinces’ landscapes (Table 1). The temperate forest (BTEM) had the highest richness, with 247 out of the 315 endemic species, followed by the humid mountain forest (BHM) with 55, and the seasonally dry tropical forest (BTES) characteristic of the BAL lowlands with 44 species (Table 1). Finally, six endemic species in humid tropical forest (BTHU) small areas and five additional species in two patches of xerophytic scrub (MXE) were recorded.
Kriging interpolation, used to smooth species distribution data, revealed that the highest concentration of species is focused on the center of the three provinces, where the variable richness is predicted to range from 8 to 23 species (Figure 3). Most of the provinces are expected to have a richness of at least four species, with lower richness values along the eastern and western edges of the study region.
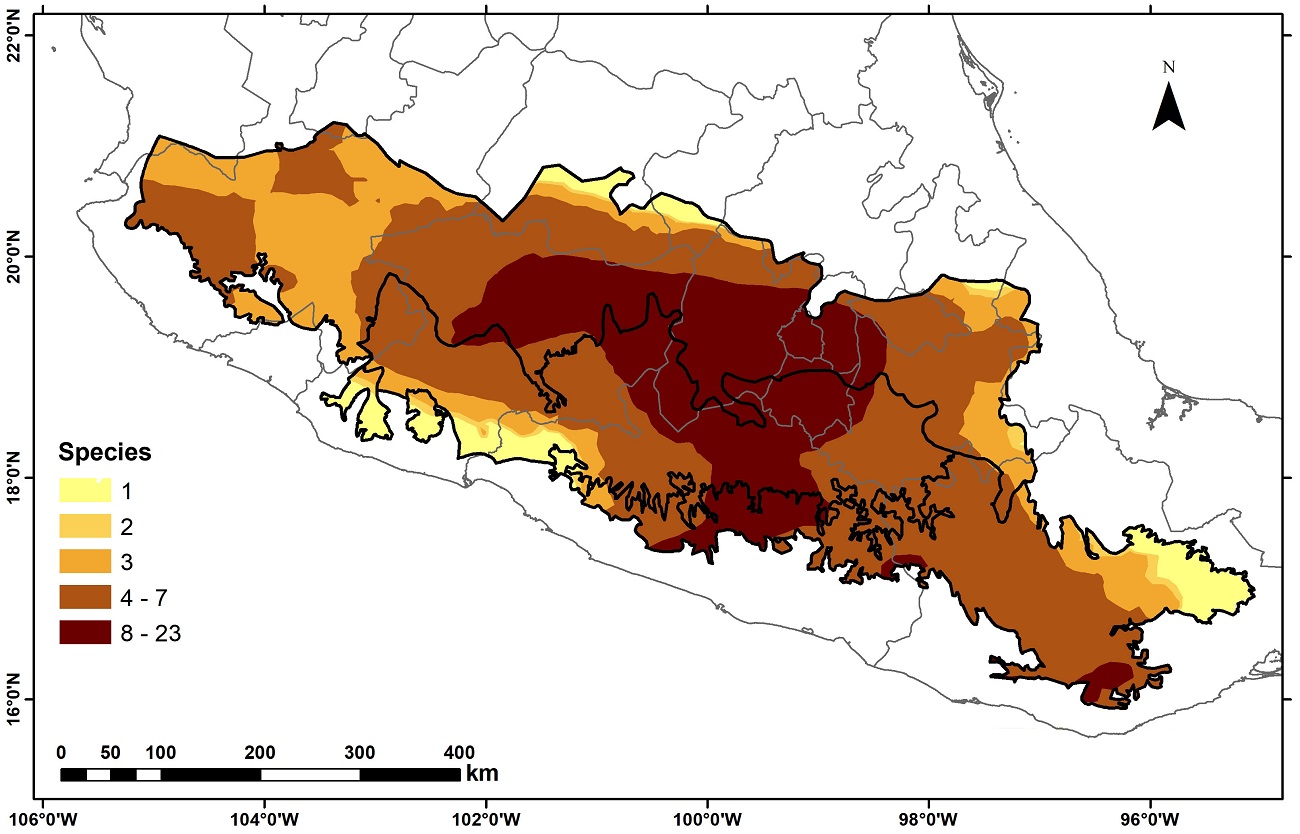
Figure 3 Map of potential species richness of endemic Asteraceae species from three biogeographic provinces of central Mexico.
Breadth of distribution of endemic species. The study area was divided in 122 grid squares (Figure 1B), from these 81 grid squares included at least one record and 41 lack records of endemic species. Only four of these 20 grid squares were completely included within provinces (squares labeled as 21 and 72 in VB, 48 in BAL and 85 in the eastern limits of VB and SMS). The other 37 empty squares occupy portions of the provinces located in their periphery (1, 2, 3, 4, 5, 10, 11, 12, 13, 14, 15, 21, 23, 24, 26, 28, 29, 42, 45, 48, 57, 60, 72, 74, 75, 76, 77, 85, 86, 88, 95, 96, 97, 98, 99, 108, 110, 115, 116, 117 and 121). In summary, 81 grid squares contained at least one record of the endemic species, and 55 squares had exclusive species-that is, endemics recorded only within the grid square surface.
Most species (148) constitute micro-endemisms in the study region, that is, species known from only one grid square (Table S1). Only 10 species were identified as having wide distribution (recorded in more than 10 grid squares): Senecio iodanthus in 18 squares, Cirsium velatum in 17, Verbesina klattii in 16, Roldana robinsoniana in 15, Perymenium globosum var. globosum and Tridax trilobata in 14, Pseudognaphalium oxyphyllum var. nataliae in 13 and Lagascea heteropappus, Psacalium holwayanum and Trixis alata in 11. The 148 species with restricted distribution are recorded in a total of 55 grid squares; if eight further grid squares are added to them (selected for containing the largest number of endemic species, greater alpha diversity), the total richness of endemism (315 species) is fully recorded. Floristic similarity analyses were carried out using only these 63 grid squares, and species’ distribution within them is indicated in Table S1.
Breadth of transition zones among biogeographic provinces.Table 2 shows the results of the average distance of penetration by species considered elements of one province into the neighboring province. The BAL elements penetrated the VB zone an average of 4.7 km, while the VB elements penetrated the BAL on average 8.3 km. In contrast, the BAL elements only penetrated an average of 2.6 km into the SMS province, while the SMS elements penetrated the BAL an average of 9.7 km (slightly more than into the VB). In summary, it is concluded that the northern transition zone between BAL and VB covers a width of about 13 km, and the southern transition zone between BAL and SMS covers a width of about 12.3 km along their territory borders. Figure 4 shows the extension of these transition zones as well as the distribution of the species that characterize them by joining their collecting sites in a biogeographic track. The biogeographic tracks that portray the distribution of the characteristic species of each biogeographic province are also shown in Figure 4.
Table 2 Average distance of the collection sites of endemic Asteraceae species in the biogeographic provinces of central Mexico. The line demarcating an elevation of 1,600 m asl, considered to be the boundary between the provinces. Arrows indicate the direction of the incursion into the neighboring province, e.g., BAL → VB = penetration of species typical of the BAL into VB territory. Min elev = Minimum elevation, Max elev = Maximum elevation, s.d. = standard deviation.
| North transition zone | Distance (km) | Min elev | Max elev | Average ± s.d. |
|---|---|---|---|---|
| BAL → VB | 4.7 | 180 | 1,600 | 1,173.3 ± 363.9 |
| VB → BAL | 8.3 | 1,601 | 3,150 | 2,111.1 ± 344.9 |
| South transition zone | ||||
| BAL → SMS | 2.6 | 360 | 1,600 | 1,205.0 ± 301.9 |
| SMS → BAL | 9.7 | 1,601 | 3,000 | 2,137.7 ± 361.9 |

Figure 4 Biogeographic tracks of the species distributed exclusively in the biogeographic provinces of central Mexico and the north and south transition zones. BAL = Balsas Depression, SMS = Sierra Madre del Sur, VB = Volcanic Belt.
Within the two transition zones (BAL-VB in the north and BAL-SMS in the south), 45 species were identified. Twenty-two of these species were restricted to the BAL-VB (northern) transition zone: Acourtia cuernavacana, A. lepidopoda, A. platyptera, A. pringlei, Ageratina leiocarpa, Aldama morelensis, A. subcanescens, Bidens pringlei, Cosmos nitidus, Chaptalia hintonii, Galinsoga triradiata, Pectis exilis, P. holochaeta var. holochaeta, Perymenium rogmacvaughii, Piqueria glandulosa, Porophyllum warnockii, Psacalium matudae, P. mollifolium, Roldana hederifolia, Stevia hypomalaca, Steviopsis amblyolepis and Verbesina pterocaula. Thirteen were restricted to the BAL-SMS (southern) transition zone: Alloispermum guerreroanum, Dahlia cordifolia, Erigeron tephropodus, Microspermum tenue, Pentacalia guerrerensis, Psacaliopsis paneroi var. paneroi, Psacalium guerreroanum, Roldana langlassei, Stevia calzadana, S. filodecaballoana, S. zephyrantha, Symphyotrichum hintonii and Tagetes arenicola. Five additional species were recorded in all three provinces and the transition zones (Ageratina macvaughii, Lagascea heteropappus, Perymenium globosum var. globosum, Verbesina stenophylla and Wedelia hintoniorum). Finally, we recorded four species with disjunct distributions in the VB and SMS provinces, recorded only at elevations higher than 1,600 m (Ageratina amblyolepis, Dahlia atropurpurea, Davilanthus huahuapanus and Stevia seemannioides).
Importance of boundaries among floristic provinces as possible barriers to dispersal.Table 3 presents the results to determine how efficient the boundaries among provinces can be in preventing or allowing the flow of individuals of species from one province to another. High values of the two indices between provinces suggest that no factor limits the dispersion between them, while low values suggest there are barriers of some kind that are limiting such dispersion. The results obtained indicate that the borders among the provinces limit species dispersion to some extent; Simpson’s values (q from Hernández-Bermejo & Sainz-Ollero 1984) were above 50 % similarity for only four of the 14 adjacent squares, and none of the borders had values above this threshold using the Braun-Blanquet Index (q').
Table 3 Species per grid-square belonging to the biogeographic province and similarity values between them using the Simpson and Braun-Blanquet indices. Only the grid squares in Figure 1 that show borders between the biogeographic provinces studied are included. NA = Does not apply because the grid squares did not contain the province.
| Grid-square | BAL | VB | SMS | Species shared | BAL → VB (q) |
VB → BAL (q’) |
BAL → SMS (q) |
SMS → BAL (q’) |
|---|---|---|---|---|---|---|---|---|
| 47 | 2 | 3 | NA | 0 | 0 | 0 | NA | NA |
| 49 | 6 | 14 | NA | 4 | 0.67 | 0.29 | NA | NA |
| 50 | 4 | 14 | NA | 4 | 1.0 | 0.29 | NA | NA |
| 53 | 3 | 25 | NA | 1 | 0.33 | 0.04 | NA | NA |
| 65 | 2 | 0 | NA | 0 | 0 | 0 | NA | NA |
| 67 | 5 | 8 | NA | 4 | 0.8 | 0.5 | NA | NA |
| 68 | 3 | 18 | NA | 3 | 1.0 | 0.17 | NA | NA |
| 69 | 8 | 15 | NA | 3 | 0.38 | 0.20 | NA | NA |
| 90 | 5 | NA | 20 | 3 | NA | NA | 0.60 | 0.15 |
| 91 | 5 | NA | 12 | 1 | NA | NA | 0.20 | 0.10 |
| 92 | 2 | NA | 5 | 2 | NA | NA | 1.0 | 0.40 |
| 93 | 2 | NA | 1 | 0 | NA | NA | 0 | 0 |
| 94 | 2 | NA | 2 | 0 | NA | NA | 0 | 0 |
| 104 | 0 | NA | 18 | 0 | NA | NA | 0 | 0 |
Floristic similarities among the biogeographic provinces of central Mexico. The analysis of floristic similarities among the grid squares evaluated is shown in Figure 5. The results indicate that throughout these three biogeographic provinces, five floristic groups were recognized, which constitute areas of endemism, because they fully contain the distributions of more than two endemic species. One of these areas is located at the western end of VB (Area 1: “Western VB”) and includes the northwestern portion of the SMS in the state of Michoacán. It comprises the 15 westernmost grid squares (Figure 5), characterized mainly by temperate forests (pine and pine-oak forests) and a few patches of seasonally dry tropical forest. The main characteristic of these temperate forests is that they are located at low elevation (less than 1,600 m), where seasonally dry tropical forest would be expected to dominate. Sixty-five species were recorded in this territory, 45 of which are restricted to it (Table 4).

Figure 5 Floristic similarities among the biogeographic provinces of central Mexico. A. Dendrogram obtained of UPGMA using the Sorensen-Dice coefficient of similarity. B. Floristic similarities between 49 grid squares distributed in the three biogeographic provinces. The numbers correspond to the numbering of the grids in Figure 1B.
Table 4 Floristic regions defined in the three biogeographic provinces of central Mexico based on the analysis of floristic similarities (see Figure 5).
| Biogeographical area (Floristic group) |
Grid squares included | Biogeographic province | Biomes included | Number of exclusive endemic species |
|---|---|---|---|---|
| 1 | 1, 2, 3. 4, 5, 6, 7, 8, 9, 10, 16, 17, 18, 19, 20, 29, 30, 31, 32, 33, 45, 46, 47, 61, 62 | VB and NW edge of SMS | BTEM, BTES | 45 |
| 2 | 36, 37, 38, 39, 40, 41, 42, 43, 44, 49, 50, 53, 54, 55, 56, 57, 58, 59, 60, 67, 68, 72, 73 | VB and adjacent portions of BAL | BHM, BTEM, BTES | 86 |
| 3 | 69, 81, 82 | BAL and part of VB | BTES and adjacent portions of BTEM | 7 |
| 4 | 64, 78, 79, 90, 91, 92, 100, 101, 102 | BAL and SMS | BHM, BTEM | 47 |
| 5 | 104, 105, 111, 112, 117, 118, 119, 122 | SMS | BHM, BTEM, BTES | 56 |
| Floristic elements (exclusive endemic species of Group 1) “Western VB” | Ageratina barriei, A. iltisii, A. jocotepecana, A. manantlana, Ageratum platypodum, Bidens melchertii, Brickellia secundiflora var. monticola, Cosmos deficiens, C. intercedens, C. jaliscensis, C. landii var. achalconensis, C. longipetiolatus, C. sessilis var. sessilis, C. sessilis var. stellata, Cymophora hintonii, Digitacalia hintoniorum, Eremosis pugana, Grindelia sublanuginosa, Hymenostephium kingii, Jaegeria sterilis, Lasianthaea machucana, Lundellianthus jaliscensis, Melampodium mayfieldii, Microspermum gonzalezii, M. gracillimum, Perymenium alticola, P. cualense, Philactis fayi, Pittocaulon hintonii, Psacalium perezii, Roldana kerberi, Stevia macvaughii, S. mascotensis, S. talpensis, Verbesina cuautlensis, V. culminicola, V. curatella, V. fusiformis, V. linearis, V. machucana, V. paneroi, Viguiera ayutlana, V. iltisii, Wedelia cordiformis, W. simsioides | |||
| Floristic elements (exclusive endemic species of Group 2) “Eastern VB” | Acourtia pringlei, Ageratina enixa, A. lasia, A. moorei, A. neohintoniorum, Ageratina perezii, A. photina, A. spooneri, Aldama subcanescens, Archibaccharis simplex, A. veracruzana, Astranthium laetificum, A. reichei, Baccharis erosoricola, B. macrocephala, Bidens gracillima, B. purpusorum, Brickellia leonis, B. squarrosa, Cirsium jorullense subsp. lanosum, C. liebmannii, C. lomatolepis, C. nivale, C. pascuarense, C. tolucanum, Coreocarpus ixtapanus, Cosmos diversifolius var. dahlioides, C. nitidus, C. schaffneri, Chaptalia hintonii, Chionolaena lavandulifolia, Dahlia barkeriae, D. brevis, D. parvibracteata, Dendroviguiera splendens, Eremosis solorzanoana, Erigeron annuactis, Galinsoga triradiata, Guardiola thompsonii, Gutierrezia dunalii, Heliopsis brachactis, Hymenostephium woronowii, Hymenothrix greenmanii, Liabellum hintoniorum, Mexerion sarmentosum, Microspermum debile var. arsenei, M. flaccidum, M. michoacanum, Nelsonianthus tapianus, Oritrophium orizabense. Pectis exilis, P. holochaeta var. holochaeta, Perymenium garciaruizii, P. ibarrarum, P. paneroi, P. reticulatum, P. rogmacvaughii, Piqueria glandulosa, Porophyllum warnockii, Psacalium hintonii, P. matudae, P. mollifolium, Roldana hederifolia, R. hintonii, Sabazia leavenworthii, Selloa plantaginea, Senecio jacalensis, S. mairetianus, S. mulgediifolius, S. orizabensis, S. procumbens, S. roseus, Stevia hintonii, S. hypomalaca, S. totalcoana, S. vernicosa, Tridax trilobata, Trigonospermum alexandri, Verbesina pterocarpha, V. pterocaula, V. seatonii, V. xicoana, Viguiera moreliana, V. sultepecana, Villanova achillaeoides, Wedelia keilii | |||
| Floristic elements (exclusive endemic species of Group 3) “Core BAL” | Bidens gypsophila, Dendroviguiera mirandae, Erigeron morelensis, Pseudognaphalium altamiranum, Sinclairia moorei, Steviopsis amblyolepis, Viguiera tepoxtlensis | |||
| Floristic elements (exclusive endemic species of Group 4) “Guerrero SMS” | Achyrocline guerreroana, Ageratina josepaneroi, A. pelotropha, A. yaharana, Aldama torresii, Alloispermum guerreroanum, Archibaccharis almedana, Arnicastrum guerrerense, Bidens balsana, B. cronquistii, B. hintonii, B. minensis, B. rosemaniana, Brickellia jimenezii, Carminatia papagayana, Carphochaete macrocephala, Cosmos mattfeldii, Critonia paneroi, Dahlia cordifolia, Dendroviguiera guerrerana, Koanophyllon revealii, Microspermum hintonii, M. tenue, Neurolaena balsana, Otopappus mexicanus, Pentacalia guerrerensis, Perymenium episcopale, Psacalium nanum, P. napellifolium, P. sharpii, P. villosum, Roldana tlacotepecana, Rumfordia revealii, Sabazia lapsensis, Simsia spooneri, Stevia chilapensis, S. filodecaballoana, S. neurophylla, S. triangularis, S. velutinella, S. zephyrantha, Symphyotrichum hintonii, Tagetes arenícola, Trigonospermum auriculatum, Verbesina alcabrerae, V. chilapana, V. elgalloana | |||
| Floristic elements (exclusive endemic species of Group 5) “Oaxaca SMS” | Ageratina juxtlahuacensis, A. macdonaldii, A. miahuatlana, A. ozolotepecana, A. pauciflora, A. pendula, A. peracuminata, A. serboana, A. solana, A. textitlana, Alepidocline macdonaldana, Aphanactis macdonaldii, Archibaccharis macdonaldii, A. nephocephala, Bartlettina juxtlahuaca, B. solavegana, B. textitlana, B. yaharana, Bidens oaxacana, Cosmos juxtlahuacensis, Chionolaena macdonaldii, Dahlia hintonii, Decachaeta serboana, Desmanthodium hintoniorum, Erigeron quiexobrensis, Eupatoriastrum chlorostylum, Heliomeris serboana, Hieracium macdonaldii var. quiexobranum, Oteiza scandens, Perymenium oaxacanum, Psacaliopsis macdonaldii, Psacalium hintoniorum, P. schillingii, Roldana calzadana, R. mixtecana, R. uxordecora, Simsia benziorum, Sinclairia manriquei, Sinclairiopsis ismaelis, Smallanthus putlanus, Stevia calzadana, S. oaxacana, S. quiexobra, S. schiblii, S. serboana, Tagetes oaxacana, Tridax paneroi, T. serboana, Verbesina calzadae, V. juxtlahuacensis, V. macdonaldii, V. miahuatlana, V. textitlana, V. villasenorii, Vernonia confusa, V. occulta | |||
The second floristic group is located adjacent to this first area of endemism and includes the squares easterly located within the VB (“Eastern VB”), some of them bordering BAL (Table 4); 123 species were recorded in this area, 86 of which are restricted to it (Table 4), found mainly in humid mountain forests (BHM) or temperate forests (BTEM), with some spreading to patches of seasonally dry tropical forests (BTES).
The third biogeographic unit identified contains areas located within the BAL and east of the VB (“Core BAL”), circumscribed by three grid squares (69, 81, and 84) that contained 19 species, where seven are exclusive endemics. Although in the dendrogram (Figure 5) this area appears to be composed of topologically disjunct units, these units are actually relatively close to each other, although not clearly nested like the other areas of endemism.
The last two floristic groups are located in portions of the BAL and SMS. One is located in the states of Guerrero, Michoacán and Oaxaca (Area 4 in Table 4: “Guerrero SMS”), is delimited by nine grid squares, and contains 78 species, 47 of which are restricted to its territory. The other is in the SMS in the state of Oaxaca (Area 5 in Table 4: “Oaxaca SMS”). This area of endemism contains 73 species, 56 of which are restricted to it.
Figure 5 summarizes the distribution of the five biogeographic units identified within the three provinces and considered areas of endemism, and Table 4 summarizes for each area of endemism the grid squares contained, the province where it is located, the main biomes found, and the species that qualify them as areas of endemism. Grid squares unassigned to floristic units (not used in the biogeographic analysis) were placed in its proper group according to their geographical position and their floristic composition (Table 4).
Discussion
This work documents for the first time, the endemic component of most species-rich and endemic-rich plant family in Mexico in the three main biogeographic provinces of central Mexico. However, several previous important efforts were fundamental to the integration of this number of endemic species, each one considering a geographic circumscription different to the provinces proposed by CONABIO (1997) used in this paper. Such differences may influence the number of species analyzed in each study; for example, Rodríguez-Jiménez et al. (2005) reported 48 species of Asteraceae as endemic to BAL, a figure that increases to 71 here, although only 29 cited by them are recognized as strictly endemic to the province. Rzedowski (2020) reported 120 endemic species to the VB, a figure that here increases to 165. There is no previous source of comparison for SMS, since previous studies in the province have only considered portions of the endemic richness (for example, Santiago-Alvarado et al. 2016). In summary, the total richness evaluated amounts to 315 taxa (301 species and 14 subspecific taxa), from 99 genera. Considering the total number of endemic Asteraceae species in Mexico (1,988) reported by Villaseñor (2018), 15.9 % of the family’s total endemisms in Mexico are present in the three biogeographic provinces included in this study.
From 122 total grid squares into which the study area was divided (Figure 1B), only 41 had no endemic species recorded. Most of these squares were located on the periphery of the study area, and so only partially overlapped with the study provinces, so there was little area included from the provinces studied. Only four grid squares (48 in BAL, 21, and 72 in VB, and 85 in the eastern limits of VB and SMS, Figure 1B) that were fully contained within the study area had a complete lack of recorded endemics, and future exploration in those locations will likely show that the absence of records is due to insufficient collecting effort rather than a true lack of endemic species. In fact, it is obvious that the distribution of important elements of the Mexican flora, such as those analyzed here, is still far from being well known. Of the 122 grid squares, 11 of them have records for a single species and 13 have only two species, while the grid-square with the highest richness reports 100 endemic species (grid-square number 100). Comparing the three provinces, a median of five species per grid-square was obtained, a figure that can hardly be considered satisfactory, and surely further exploration will demand that results of this work be re-evaluated soon.
The transition between biogeographic provinces, for example BAL-VB or BAL-SMS also represents ecotones or environmental gradients between different habitats or biomes (Table 1). Species adapted to different biomes surely contain special attributes that have allowed them to adapt to the gradual or abrupt changes in the transition zones where they thrive (Smith et al. 2001). For example, their tolerance to both tropical and temperate environments may provide adaptive advantages over endemics specialized to a single biome.
Cadenasso et al. (2003) consider that biogeographic boundaries or transition zones show a particular biotic composition, with dynamics and ecosystem functions different from adjacent regions. It is worth recapitulating, as Yarrow & Marín (2007) emphasize, whether the boundary between two provinces is really a biogeographic barrier (a limit between them, that is, a closed border) or rather a transition zone (an area of change from one floristic composition to another). Here, we identified some species endemic to the transition zones between adjacent biogeographic provinces, which make up a unique floristic composition. These 22 exclusive species of BAL-VB and 13 of BAL-SMS determine such transition zones as areas of endemism, within larger areas of endemism defined as biogeographic provinces. They may be additional examples of the “Circum-Balsas river basin subhumid mountain pattern” identified by Espinosa-Organista et al. (2008) and Morrone (2020). The results support the arguments of Schilthuizen (2000) to consider them areas of endemism, where diversification processes are occurring that lead to very particular micro-endemisms. Future work will be necessary to determine the processes that could be generating these endemisms in hybrid areas due to their floristic composition.
The Balsas Depression (BAL) has been largely shaped by the formation of the Volcanic Belt (VB) and the Sierra Madre del Sur (SMS). For more than 15 million years, the orographic shadow effect that prevents the flow of cold and humid winds from the north and the Pacific Coast, have led to the formation of seasonally dry tropical forest biome that is so characteristic of the BAL. The approximate age of seasonally dry tropical forest formations in BAL is determined by Becerra (2005) from studies in Bursera to be between 7.5 to 17.5 million years in the western portion of the region and a maximum of 7.4 million years in the east. The western BAL tropical dry forest is determined to be older than the eastern part because it mirrors the evolution of Late Miocene mafic episode of the VB (Gómez-Tuena et al. 2005), which initiated with tectonic activity in the west and progressed eastward.
Rodríguez-Jiménez et al. (2005) in their study of endemism in BAL report 337 plant species, 48 of which (14.2 %) belong to the Asteraceae family; in this work, we recognize only 22 strict endemics to the BAL, representing only 6.5 % of the endemisms recognized by those authors. Much of the difference can be explained by the different criteria used to define the provinces; Rodríguez-Jiménez et al. (2005) included species typical of temperate forests, which constitute characteristic elements of the upper parts of the basin that drain into the Balsas River, but which in this work were considered part of VB or SMS. Examples of these species include Stevia hintonii and Microspermum flaccidum, assigned here as exclusive to the VB, or Microspermum hintonii, here considered exclusive to the SMS. Other species they cited have since been detected beyond the recognized boundaries for BAL; examples include Calea pringlei B.L. Rob., now synonymous with Calea ternifolia Kunth, which is widely distributed in the country, or Lasianthaea crocea (A. Gray) K.M. Becker, whose distribution now reaches the southern Mexican Highlands, the Pacific Coast and the Tehuacán-Cuicatlán Valley.
The VB is a volcanic arc located along the central portion of Mexico in a west-east direction (Figure 1A). Ferrusquía-Villafranca (2007) describes it as beginning in Cabo Corrientes, Nayarit and ending in the Sierra de Chiconquiaco in Veracruz (Figure 1A). The VB was formed in stages; first emerging in the west during the Miocene (15-17 million years ago), with the eastern part forming more recently, during the Pliocene and Pleistocene (about 7.4 million years ago) (Becerra 2005, Ferrari et al. 2012, Mastretta-Yanes et al. 2015, Moran-Zenteno 1994).
Alcántara & Paniagua (2007) evaluate 63 endemic VB species to determine patterns of richness and rarity, identifying priority areas for conservation based on either richness or endemism. Some of the areas they identify correspond to floristic regions identified in this work; for example, their richest area (the Sierra Nevada) is included in this work within Floristic Group 2 (Table 4), followed by the Sierra de Manantlán (Jalisco-Colima), included here within the Floristic Group 1 (Table 4). These authors considered 12 endemic Asteraceae species, but four of those were not included in this work because more recent data have shown that three of those species’ distributions extend beyond the limits of the three provinces, and the fourth species is a recently introduced exotic species (Senecio inaequidens DC.).
The floristic groups identified correspond with the geological divisions discussed by Ferrari et al. (2012, Figure 2). For example, the western portion of VB, where Floristic Group 1 is identified (“Western VB”, Table 4), corresponds in part with their western division of the VB, which would represent one of the oldest areas of the study region. The central and eastern portions of the VB correspond to our Floristic Group 2 (“Eastern VB”) and makes it evident that in these geologically differentiated portions, equally important speciation processes have occurred in the family. Similarly, two other important areas of endemism identified are in the Guerrero Tectonostratigraphic Terrain (Floristic Group 4 in Table 4: “Guerrero SMS”) and in the Mixteco and Oaxaca terranes (Floristic Group 5 in Table 4: “Oaxaca SMS”), both corresponding to different portions of the SMS (Ferrari et al. 2012).
The SMS is perhaps the most difficult biogeographic province to characterize of the three studied. Some authors consider that it extends to Jalisco, precisely where the western end of the VB is also located (Cabo Corrientes, Jalisco and southern Nayarit), then runs parallel to the Pacific Coast to the Isthmus of Tehuantepec, where it bifurcates toward the north, encompassing the mountains of northern Oaxaca (Luna-Vega et al. 2016). However, as is the case with BAL and VB, taxa that strongly support the full SMS as a biogeographic unit are not recorded. The areas of endemism identified inside each biogeographic province position part of their extension as geographical portions within a geological, morphotectonic or paleontological surface that appears to be homogeneous with particular floristic elements. They undoubtedly reflect different cenocrons (groups of taxa with a common biogeographic history and constitute an identifiable subset within a biota, sensuMorrone 2010, 2020). As indicated above, in this work we identified two important areas of endemism within the SMS: one forming part of the Guerrero Tectonostratigraphic Terrain (Floristic Group 4 in Table 4: “Guerrero SMS”) and another in the Mixteco and Oaxaca terranes (Floristic Group 5 in Table 4: “Oaxaca SMS”). Both areas of endemism correspond to the Eastern Subprovince, one of the three subprovinces recognized by Morrone (2017) in the SMS; the first (Area 4) is identified with its Guerreran District and the second (Area 5) with the Highlands of Oaxaca District. The results found here do not support Morrone’s (2017) proposal to include the portion of the SMS in Michoacán and Jalisco (Western Subprovince) as part of this biogeographic province, since the sites that comprise it are more floristically associated with the VB (Floristic Group 1, Figure 5, Table 4). It is interesting to note the contradiction between the paleontological information locating these parts of Colima, Michoacán, Jalisco, and Nayarit as part of SMS and the floristic information, which suggests that their endemic species have a closer affiliation with the VB. Only an analysis of the complete floristic richness of this region, which is complex in both its tectonics and its biological evolution, will provide more robust conclusions since partial analyses with some elements of its biodiversity do not seem to allow us to reach conclusive results. The results found here suggest that, floristically, the northwestern limit of the SMS is the mouth of the Balsas River, and that the mixture of species found in the mountains north of the Balsas River in Michoacán, Colima, Jalisco and Nayarit is dominated by elements that are more characteristic of the VB.
In conclusion, the polygons used in this work (Arriaga et al. 1997, CONABIO 1997) locate the northwestern boundary of the SMS in Michoacán, south of the mouth of the Balsas River on the Pacific Coast, in the municipalities of Aguililla and Coalcomán, and the southeastern border of the province is in the districts of Juchitán and Miahuatlán in the state of Oaxaca, where the mountains give way to the characteristic lowlands of the Isthmus of Tehuantepec. This polygon excludes the mountains of Colima and Jalisco (such as the Sierra de Manantlán) and Nayarit (such as the Sierra de San Juan), considering them to be part of the VB. As here delimited, the provinces agree better with the results of recent stratigraphic studies, reported by geologists and paleontologists (for example Ferrari et al. 2012).
Santiago-Alvarado et al. (2016) recognized areas of endemism in the SMS and recovered two areas (considered sub-provinces): one in Guerrero extending to Michoacán and Jalisco and another in Oaxaca. In their analyses, they only used two species of Asteraceae (Axiniphyllum corymbosusm Benth., Psacalium guerreroanum B.L. Turner), neither of which were considered SMS endemics in this study (A. corymbosum because its lowland distribution reaches the Pacific Coast, which corresponds to another biogeographic province, and P. guerreroanum because it is characteristic to the transition zone between the BAL-SMS rather than to the SMS; Table S1). Despite the differences in species selection criteria, the important point is that results both of Santiago-Alvarado et al. (2016) and this work coincide in identifying the same areas of endemism, which reinforces the idea that their endemisms constitute cenocrons that will help understand the evolutionary history of these biogeographic units.
Some authors may consider that important species have been left off the list of species included in Table S1. Many of the species that could be omitted were considered and assessed to be characteristic of the transition zones with other biogeographic provinces. For example, the VB is bordered on the north by the Altiplano-Sur province (Zacatecano-Potosino); there, shared species have been detected, such as Bidens aequisquama (Fernald) Sherff, Grindelia oxylepis Greene or Stevia ovalis (B.L. Rob.) B.L. Rob. Similarly, the VB is bounded on the west by temperate forests (pine and pine-oak) with the lowest recorded elevation (500-600 m). It is not straightforward whether such plant communities belong to VB or to the Pacific Coast province (CPA); however, according to the criteria used in this work, species with records at elevations lower than 1,600 m should be classified as belonging to the BAL, but since the BAL province does not include this part of the country in its territory, communities at such elevations were considered to belong to the CPA. It will be important to determine the limits between the CPA and VB, especially in this region that undoubtedly represents a biogeographic complex where characteristic elements of CPA, VB and the Sierra Madre Occidental are mixed. Examples of species that characterize this complex zone and were therefore not included here include Ageratina jaliscensis (B.L. Rob.) Gage ex B.L. Turner, Trixis jaliscana B.L. Turner or Verbesina glaucophylla S.F. Blake. Finally, similar scenarios must be considered between the CPA and SMS, where there are species that are surely part of their transition zones, such as Ageratina pochutlana B.L. Turner, Axiniphyllum corymbosum or Verbesina macvaughii B.L. Turner.
The strategy followed in this work was to evaluate the specimens collected over time, that are physically housed in both national and foreign herbaria, which represent a valuable information source of biodiversity (James et al. 2018), but more importantly, which are recorded and maintained in digitized databases. It is possible that results obtained with the information analyzed (2,061 records and 315 taxa) change when we have full inventories of the provinces studied. But to reach this utopic goal is virtually impossible or will still take many decades of inventory effort. Consequently, the accumulation of floristic information over time, contained in online databases (such as REMIB-SNIB of CONABIO and MEXU-UNIBIO of the Institute of Biology, UNAM at the national level or GBIF at the international level) seems to be the best offer for inventory and biodiversity studies in complex regions, in both physiography and climate, as the three biogeographic provinces studied here.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2768











 text new page (beta)
text new page (beta)



