Morphological studies of seeds and seedlings are important in research on the characterization of the soil seed bank, as well as for assisting in the identification of species in studies on natural regeneration of degraded areas (Araújo-Neto et al. 2002, Ferreira & Baretto 2015). Morphological study of the seeds can also have practical applications in ecological studies, and in the management and conservation of the native fauna (Bravato 1974). The size of the seed and the morphofunctional nature of the cotyledons (reserve or photosynthesizing) are the two main characteristics present at the start of the life cycle of the plants, which evolve in response to strategic changes that may reflect the ecological and evolutive history of the plants in relation to their habits (Zanne et al. 2005).
The plants show different strategies, which include the accumulation of large quantities of compounds that favor their adaptation to different environments (Buckeridge et al. 2000). According to Gonçalves et al. (2002), information on the chemical composition of tropical seeds is important for understanding their physiological process, providing more information for agroindustry. It also has ecological significance, as a source of nutrients for possible predators and pathogens. According to Hegnauer & Grayer-Barkmeijer (1993), the study of reserve carbohydrates stored in seeds has been applied as a tool for the taxonomic classification of species of Leguminosae.
In Leguminosae, both the fruits and seeds generally have particular characteristics, and are important in the taxonomy of various genera (Lima 1985, Lima 1989, Barroso et al. 1999, Oliveira 1999, Kirkbride et al. 2003, Meireles & Tozzi 2008, Leite et al. 2009, Paulino et al. 2010, Córdula et al. 2014, Pinto et al. 2014).
The genus Swartzia is subordinated to the subfamily Papilionoideae which, among the subfamilies of Leguminosae, has the highest number of taxa, i.e. around 503 genera and 14,000 species (LPWG 2017). Swartzia, which belongs to the swartzioid clade, is monophyletic. It is centered in the neotropical region, predominantly in the Amazon Rainforest, and has around 180 species, which are distributed in lowland formations from southern Mexico and the Islands of the Caribbean to the south of Brazil and Bolivia (Torke & Schaal 2008).
There are 112 species of Swartzia in Brazil, of which 63 are endemic (Flora do Brasil 2020). They are distributed across all Brazilian phytogeographic domains, but it is in the Amazon that the greatest wealth of the species is concentrated; 71 % of the estimated total for Brazil (Flora do Brasil 2020).
Swartzia is characterized by the morphology of its flowers, which in the majority of species, have entire calyx, a single white or yellow petal, and dimorphic stamens (Torke & Schaal 2008). The fruits are dehiscent, monospermic or polyspermic follicles or legumes, but indehiscent fruits are also cited for the genus (Cowan 1967), recognized as mucoid legumes (Barroso et al. 1999, Mansano & Tozzi 2004). They vary in shape, coloration and the presence of indumentum. The seeds are usually arillate, but the aril may be absent in some species (Pinto et al. 2012).
Studies on the morphology and anatomy of seeds of Swartzia species are still scarce, and represent less than 10 % of the 112 species estimated for Brazil to date (Flora do Brasil 2020). Studies have been conducted on the morphology of the seed and germination in Swartzia pinnata (Vahl) Willd. and Swartzia madagascariensis Desv. (Corner 1976), S. langsdorffii Raddi (Oliveira 1999), S. apetala Raddi var. apetala (Gonçalves et al. 2008), S. recurva Poepp. (Santos et al. 2015), S. laevicarpa Amshoff (Moreira et al. 1995), S. polyphylla DC., S. recurva Poepp., S. reticulata Ducke, S. tessmannii Harms, (Camargo et al. 2008), S. argentea Benth., S. auriculata Poepp., S. laevicarpa Amshoff, S. macrocarpa Benth., S. pendula Benth., S. polyphylla DC., S. recurva Poepp. and S. sericea Vogel (Meirelles & Souza 2015). Of these species, ten occur in the Amazon region.
The objective of this study is to characterize the morphology and anatomy, and identify the main ergastic substances of the seeds of Swartzia laevicarpa Amshoff, Swartzia macrocarpa Spruce ex Benth., Swartzia recurva Poepp. and Swartzia sericea Vogel, all of which are sympatric species restricted to the Amazon Rainforest. It should be noted that the occurrence of S. macrocarpa and S. recurva is so far restricted to forests of the Brazilian Amazon (Flora do Brasil 2020). The results obtained extend the biological knowledge of the species, through the identification of their seminal structures, and provide subsidies for understanding the germinative process. They also contribute to strengthening plans for management and conservation of the flora and fauna, through support for the identification of the species in the soil seed bank.
Materials and methods
Study area, species and matrices selected and collection of botanical material. The matrices were selected in the Sustainable Development Reserve of Tupé (RDST), located west of Manaus/AM, (03º 02' 35" S, 60º 15' 18" W). The RDST covers an area of around 12,000 hectares and is comprised of different types of vegetation, such as Campinarana, Terra Firme Forest, and Igapó Forest, with Terra Firme being the predominant one. The climate is classified as Am (Koeppen 1948): humid tropical monsoon climate with rainfall of less than 60 mm. Relative humidity of between 85 and 90 %, and mean temperatures of 26 to 27 ºC (Scudeller et al. 2005).
The species selected for this study were S. laevicarpa Amshoff, S. macrocarpa Spruce ex Benth., S. recurva Poepp. and S. sericea Vogel and the binomials are in accordance with the treatment of Swartzia in Flora do Brasil (Flora do Brasil 2020). The matrices were selected based on the availability of botanical material, among adult individuals in the flowering and fruiting phenophases.
The collections were performed during 2017 and 2018. Three individuals of each species were selected for the collection of botanical material, to be used in the preparation of exsiccates, for identification, and for obtaining fruits and seeds. The collected material was identified and deposited in the Herbarium of the Federal University of Amazonas (Table 1). Duplicates were sent to the Herbarium of the Rio de Janeiro Botanical Garden - RB (acronyms according to Thiers, continuously updated).
Table 1 Number of individuals per species, sites of occurrence and the respective vouchers.
| S. laevicarpa | S. macrocarpa | S. recurva | S. sericea | |
|---|---|---|---|---|
| Number of individuals per species | 3 | 3 | 3 | 3 |
| Type of vegetation in which it occurs | Igapó forest | Igapó forest | Terra firme forest | Igapó forest |
| Vouchers | HUAM10841 | HUAM10845 | HUAM10844 | HUAM10852 |
| HUAM10842 | HUAM10840 | HUAM10851 | HUAM10853 | |
| HUAM10848 | HUAM12042 | HUAM12041 | HUAM12043 |
Open fruits still on the tree were collected, with the seeds exposed, and fruits in which the dehiscence process was close to occurring, as established by the dimensions, change in coloration, and swollen appearance of the fruits. After collection, the fruits were kept at room temperature and transported to the laboratory for immediate selection of the mature seeds.
Morphoanatomical and histochemical analysis. For the morphological and biometric analyses, thirty mature seeds were obtained, based on the mixing and homogenization of samples collected from three individuals. The lengths, widths and thicknesses of the seeds were measured using a Mitutoyo 150 mm digital pachymeter and the weighed of fresh material was weight on a Shimadzu BL320H digital balance with precision of 0.001 g. The length was measured from the base to the apex of the seed, and the width and thickness were obtained in the mid region of the seeds. The region where the hilum is situated was considered as the base of the seed (Lima 1985). The biometry data were submitted to non-parametric analysis of variance (Kruskal-Wallis Test), using the statistical language R v4.0.2 (R Core Team 2007) and Rstudio v1.1.4. The level of significance was 0.05.
The shape and color of the seeds and surface of the testa were observed, measuring the length of the funiculus and hilum and describing their shapes. The identification of colors of the aril, seed coat and embryo was identified using the PANTONE scale (www.pantone.com/color-finder), projected according to the Munsell Color Chart (Cooper 1929).
In the embryo, the position of the hypocotyl-radicular axis in relation to the cotyledons was observed, as well as the type of plumule, according to Oliveira (1999). The seed was considered as having a rudimentary plumule when there was a small protuberance after the cotyledonary node, but without differentiation into pinnae and leaflets.
The morphological descriptions and photographic documentation were performed using a Nikon SMZ25 2x stereomicroscope with an attached Head DS-Fi2 camera, in addition to a Canon SX60 HS photographic camera.
For the anatomical studies of the seed coat and embryo, ten seeds of each species were used. The samples were fixed in FAA 70 (Johansen 1940) for 48 hours and kept in 70 % ethanol solution for subsequent anatomical procedures. Cross-sections of the seed were cut to observe the characteristics of the seed coat in the hilar region and opposite it, as well as longitudinal cuts of the embryo. For the permanent slide collection, after dehydration in ethanol series, the material was included in hydroxyethyl methacrylate (Leica) (Gerlach 1969, modified by Ruzin 1999), and then then sectioned at 8 µm in a Leica RM2145 rotary microtome, stained with 0.05 % toluidine blue, pH 4.7 (O’Brien et al. 1964) and mounted in Permount synthetic resin. The observations were performed using an Axioskop MC80 Zeiss binocular stereoscopic microscope.
Histochemical tests were performed on the cross-sections of fresh samples, obtained using a table microtome, to verify ergastic substances present in the aril, seed coat and cotyledons. The following reagents were used: Lugol to detect starch (Jensen 1962), Sudan III for total lipids (Pearse 1980), iron (III) chloride for phenolic compounds (Johansen 1940), Ponceau xylidine for proteins (O’Brien & McCully 1981), and Wagner’s for alkaloids (Furr & Mahlberg 1981). All records were obtained from photomicrographs with a Canon PC1252 digital camera coupled with a Zeiss Primo Star MicroImaging 37081 photomicroscope.
Results
The species studies have fruits of the legume type; the number of seeds per fruit varies among species, with one to three seeds in S. laevicarpa fruits, one to two seeds in S. recurva and S. sericea, and one to seven seeds in S. macrocarpa fruits.
The seeds presented variation among species in relation to weight, length, width and thickness, with 19 g, 31 × 50 × 22 mm in S. laevicarpa; 5 g, 20 × 28 × 13 mm in S. macrocarpa, 8 g, 20 × 30 × 10 mm in S. recurva and 4 g, 18 × 22 × 13 mm in S. sericea (Table 2). The only exception was the thickness measurements for S. macrocarpa and S. sericea which were similar. Among the four species, the shape of the seeds ranged from reniform (Figure 1A-C), oblong (Figure 1B) to oval (Figure 1D); the seed coloration was light brown and black (7631C, 730C, 7560C, 419C) (Table 3).
Table 2 Dimensions and fresh weight of the seeds of S. laevicarpa, S. macrocarpa, S. recurva and S. sericea (N = 30).
| Characteristics | Species | p - value2 | |||
|---|---|---|---|---|---|
|
Swartzia laevicarpa N = 301 |
Swartzia macrocarpa N = 301 |
Swartzia recurva N = 301 |
Swartzia sericea N = 301 |
||
| Weight (g) | (a)3 19 ± 5 |
(c)3 5 ± 1 |
(b)3 8 ± 1 |
(d)3 4 ± 1 |
<0.001 |
| Length (mm) | (a)3 31 ± 5 |
(b)3 20 ± 2 |
(d)3 20 ± 0 |
(c)3 18 ± 2 |
<0.001 |
| Width (mm) | (c)3 50 ± 1 |
(a)3 28 ± 3 |
(d)3 30 ± 0 |
(b)3 22 ± 2 |
<0.001 |
| Thickness (mm) | (a)3 22 ± 3 |
(b)3 13 ± 1 |
(c)3 1 ± 0 |
(b)3 13 ± 1 |
<0.001 |
1 Statistics presented: mean ± SD
2 Statistical tests performed: Kruskal-Wallis test
3 Statistical tests performed: Dunn test. Distinct letters differ at a significance level of 0.05
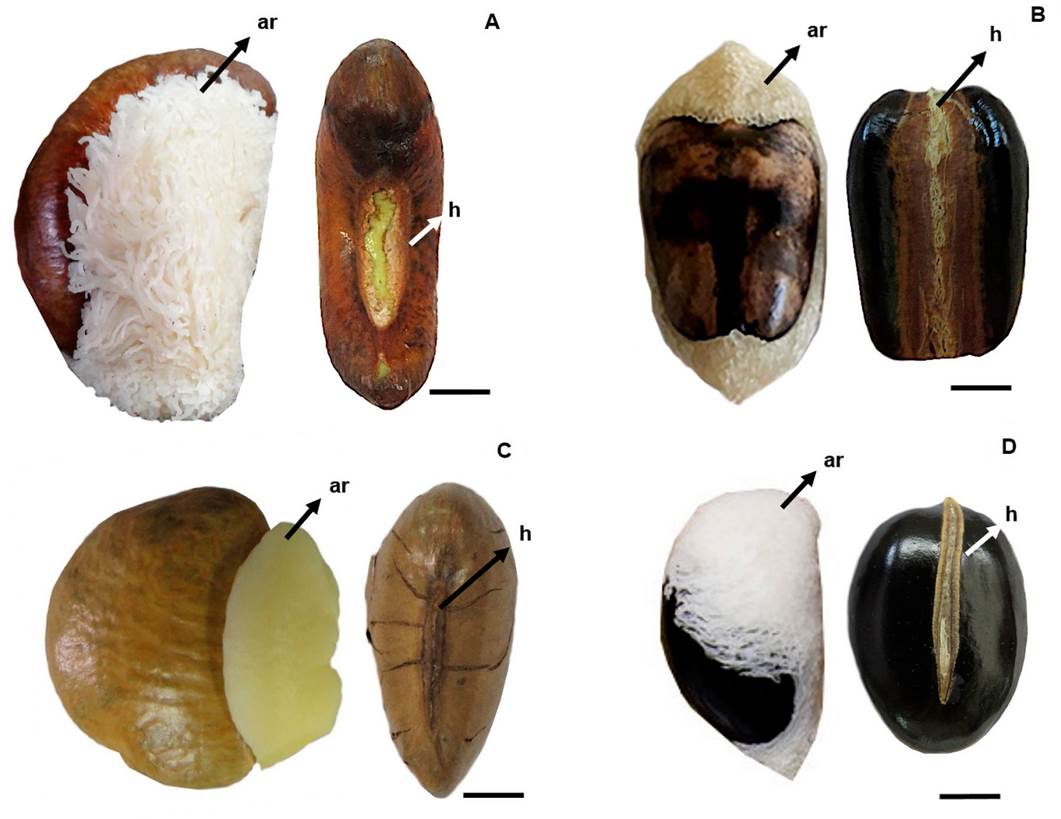
Figure 1 Seeds with aril and aril removed: A) S. laevicarpa; B) S. macrocarpa; C) S. recurva; D) S. sericea. ar, aril; h, hilum. Bars A; B; C; D 1 cm.
Table 3 Differential seeds characters of S. laevicarpa, S. macrocarpa, S. recurva and S. sericea.
| S. laevicarpa | S. macrocarpa | S. recurva | S. sericea | |
|---|---|---|---|---|
| Characteristics | ||||
| Color of the Aril | White (Cool Gray 1C) | White (Color Gray 1C) | Beige (4237C) | White (Cool Gray 1C) |
| Color of the Seed Cover | Brow (7631C) | Black (419 C) Brown (730 C) |
Light Brown (7560 C) | Black (419 C) |
| Seed Shape | Reniform | Oblong | Reniform | Oblong |
| Type of Aril | Filamentous | Filamentous | Spongy | Filamentous |
| Hilar Seed | No | Yes | No | No |
| Size of the funiculus | 3.8 cm | 0.8 cm | 3.0 cm | 1.5 cm |
| Ergastic substances Aril | Alkaloids | Protein | Lipids | Alkaloids |
| Ergastic substances Seed Coat | Phenolic Compounds Alkaloids |
Phenolic Compounds |
Phenolic Compounds |
Phenolic Compounds Alkaloids |
| Ergastic substances Cotyledons | Starch Protein | Starch Protein Lipids | Alkaloids Protein | Starch Protein |
The seeds are partially covered by a beige spongy aril (4237C) in S. recurva (Figure 1C); and a white, filamentous aril (Cool Gray 1C), in S. laevicarpa (Figure 1A), S. sericea (Figure 1D) and S. macrocarpa (Figure 1B).
The seeds of the species S. laevicarpa, S. macrocarpa, S. recurva and S. sericea present the following morphological pattern: presence of the aril, membranous seed coat; glabrous, filiform funiculus, imperceptible micropyle and linear hilum. The seeds are exotestal, characterized by a palisade layer comprised of macrosclereids, with thick, lignified cell walls, elongated radially, compactly arranged, forming the exotesta; the mesotesta is comprised of several layers of parenchymatous cells (Figure 2) and in S. recurva, groupings of tracheal elements are observed, with thick walls and spiraled thickening (Figure 2C).
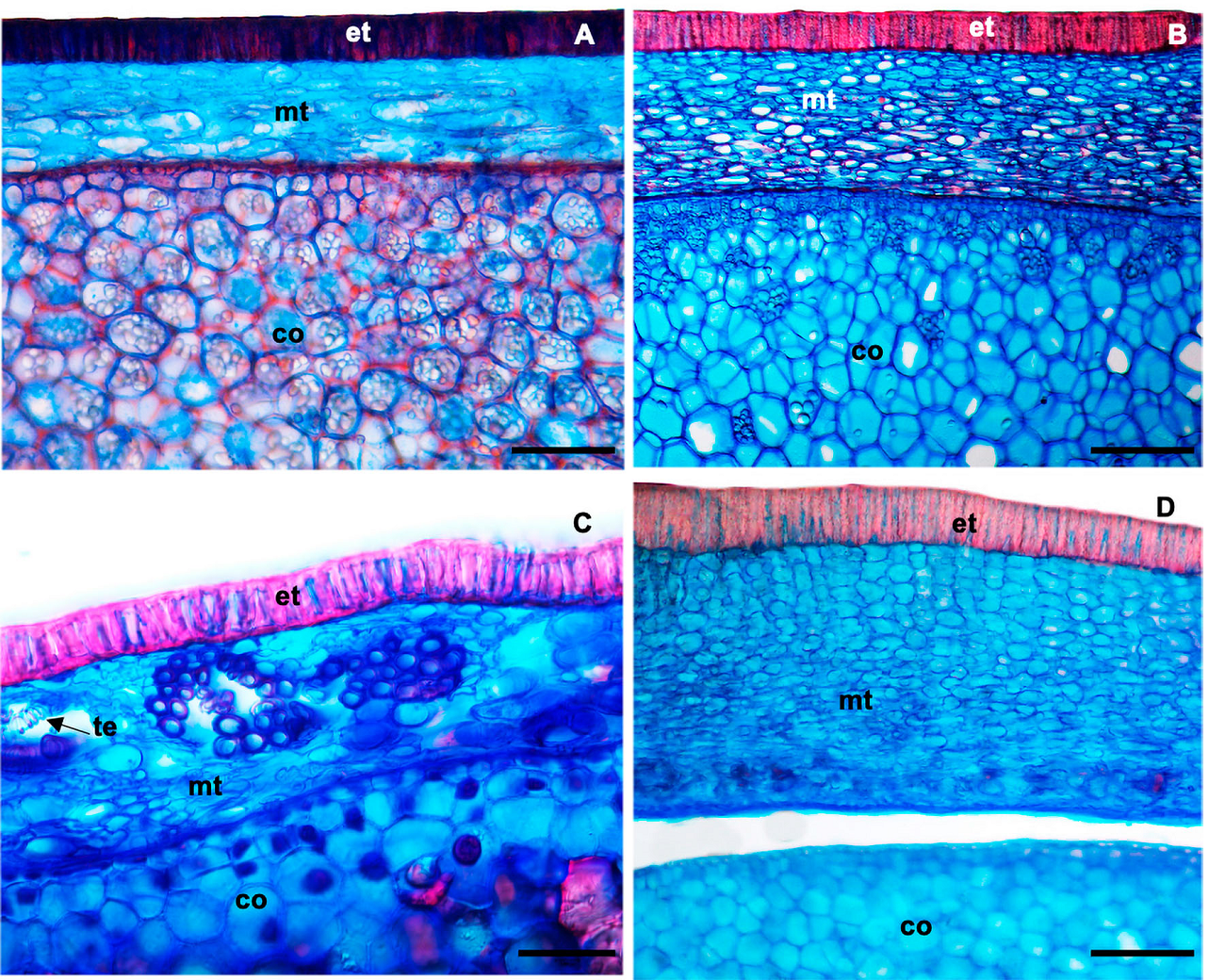
Figure 2 Cross sections of the seed coat and cotyledon of: A) S. laevicarpa; B) S. macrocarpa; C) S. recurva; D) S. sericea. co, cotyledon; et, exotesta; mt; mesotesta; te, tracheal elements. Bars A; B; D; 200 µm; C 70 µm.
In the four species studied, the absence of a palisade covering in the hilar region (Figure 3), as well as of a tracheid bar in the subhilar tissue were observed.
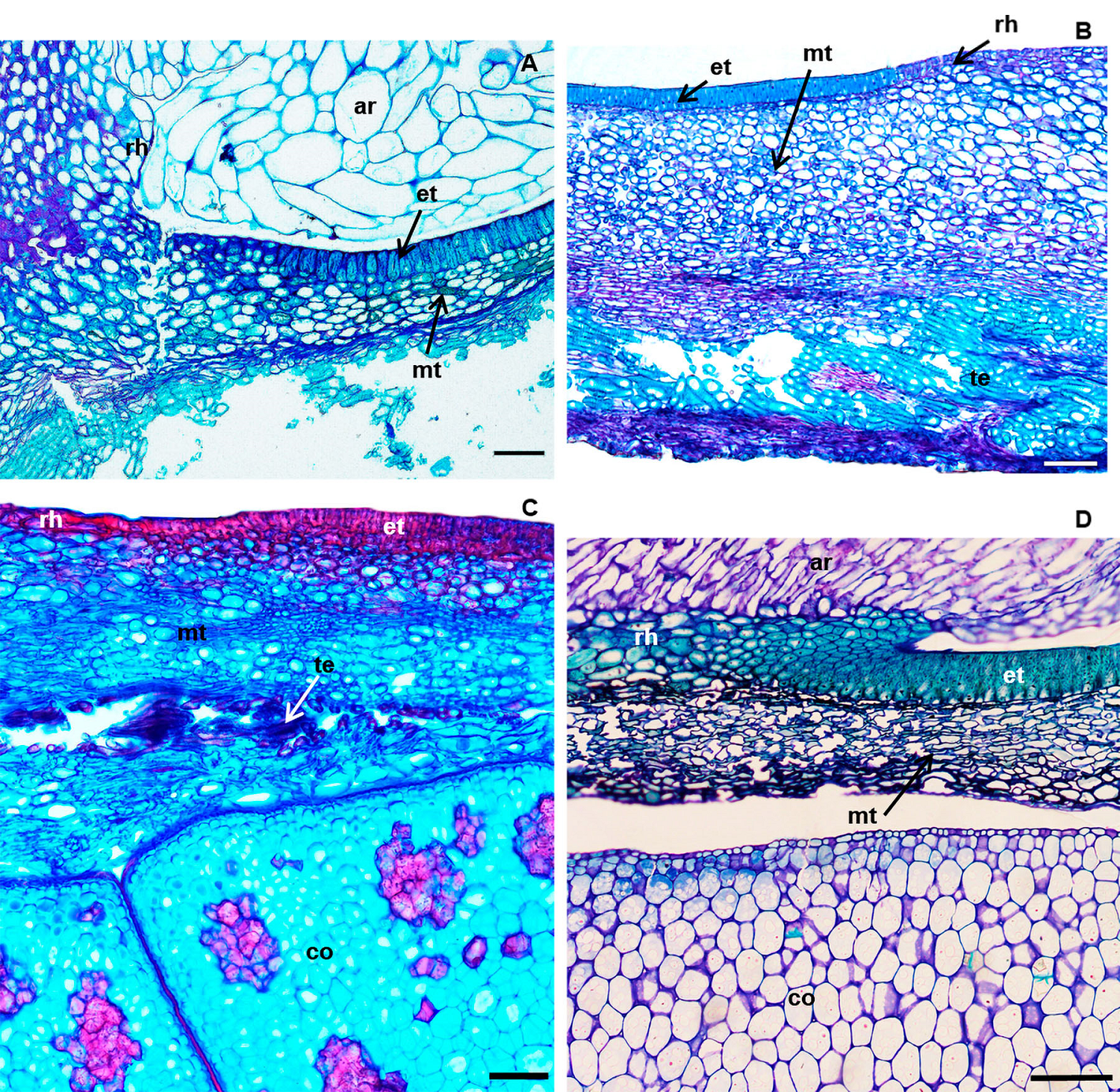
Figure 3 Cross sections of the hilar region of seeds of: A) S. laevicarpa; B) S. macrocarpa; C) S. recurva; D) S. sericea. ar, aril; co, cotyledon; et, exotesta; hr, hilar region; mt, mesotesta; te, tracheal elements. Bars A; B; C; D; 200 µm.
In all the species, the embryo is green (360 C), with fleshy cotyledons, uniseriate cotyledonary epidermis, cuboid cells, and parenchymatous, homogenous mesophyll in S. laevicarpa, S. macrocarpa and S. sericea, interrupted by groupings of sclereids in S. recurva. The embryonic axis is straight, and the meristems are visibly distinct: protoderm, fundamental meristem and procambium (Figure 4). The apical meristems of the stem and root are distinct, with initial cells with more intense color; there are no leaf primordia, characterizing the plumule as rudimentary, and no radicle in differentiation.
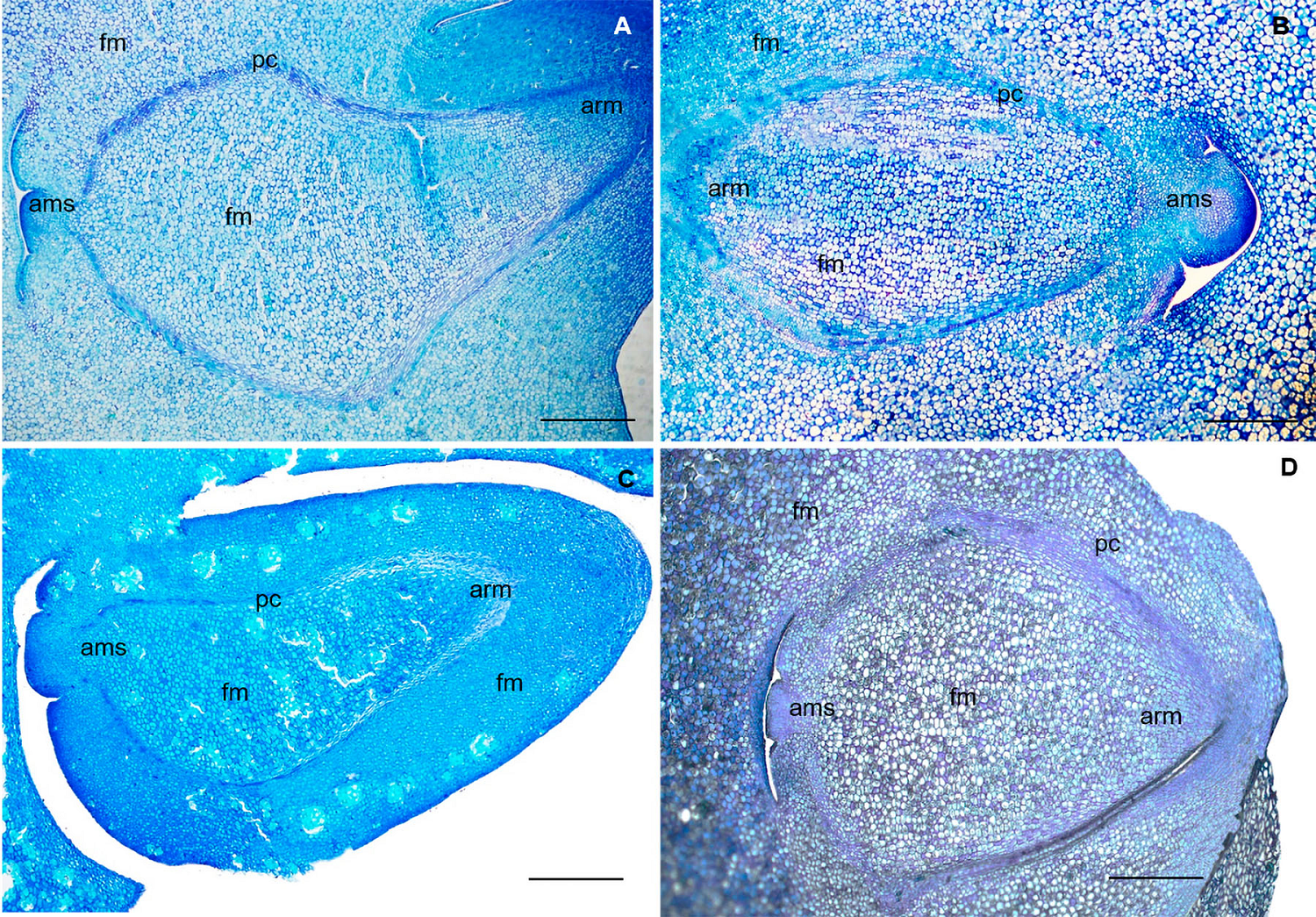
Figure 4 Longitudinal sections of the embryo. A) S. laevicarpa; B) S. macrocarpa; C) S. recurva; D) S. sericea. ams, apical meristem of the stem; arm, apical meristem of the root; fm, fundamental meristem; pc, procambium; pt, protoderm. Bars A; B; C; D; 400 µm.
The hilum in S. laevicarpa is 1.8 cm long, surrounded by a slightly prominent border of the same color as the seed coat (Figure 1A). In S. macrocarpa the hilum has an average length of 2.5 cm, extending across the entire width of the seed, characterizing the hilar seed (Figure 1B). S. recurva has a pronounced hilum, with an average length of 1.2 cm, of the same color as the seed coat (Figure 1C) and in S. sericea the hilum measures 2.1 cm long, occupying almost the entire base of the seed (Figure 1D).
Among the four species analyzed, histochemical tests showed the presence of protein (Figure 5D-J), lipids (Figure 5N) and alkaloids (Figure 5E-R in the aril, phenolic compounds (Figure 5A-G-M-P) and alkaloids (Figure 5B-O) in the seed coat, and starch (Figure 5B-H-O), protein (Figure 5C-I-L-Q) and alkaloids (Figure 5K) in the cotyledons (Table 3).
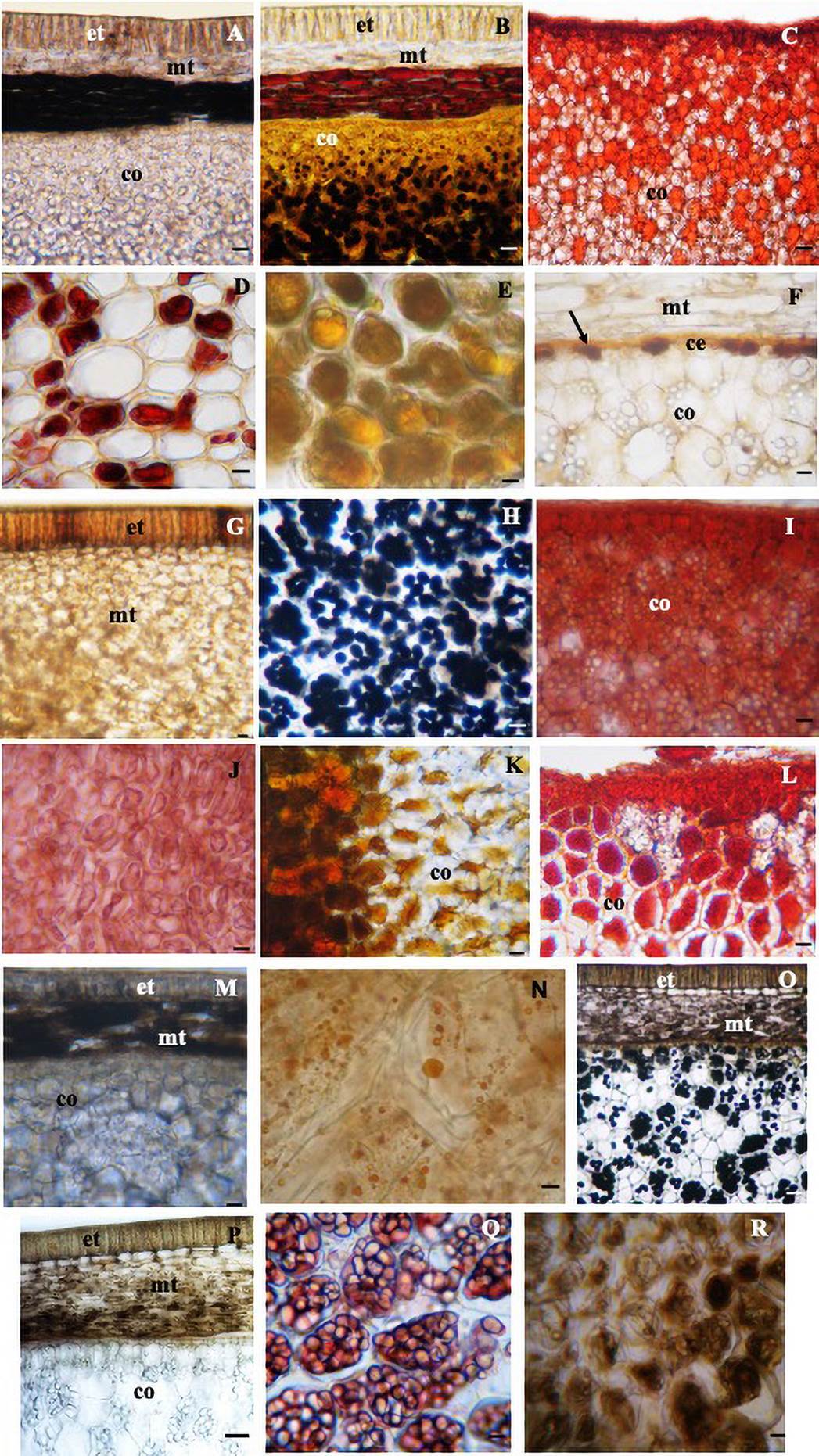
Figure 5 Cross sections of seed submitted to histochemical tests. In the seed coat: phenolic compounds in the region of the exotesta and mesotesta in G (S. macrocarpa) and P (S. sericea); only in the mesotesta in A (S. laevicarpa) and M (S. recurva), bars 150 µm. Alkaloid in the mesotesta in B (S. laevicarpa), bar 150 µm, in the mesotesta and exotesta in O (S. sericea), bar 200 µm. Lipids in the cotyledonary epidermis in F (arrow) (S. macrocarpa), bar 70 µm; starch in the cotyledon: in B (S. laevicarpa), bar 150 µm, H, (S. macrocarpa) bar 70 µm and O (S. sericea), bar 200 µm; protein in C, (S. laevicarpa) I, (S. macrocarpa) L, (S. recurva) bars 150 µm and Q, (S. sericea) bar 70 µm; alkaloids in K (S. recurva), bar 150 µm; in the aril: protein in D, (S. laevicarpa) bar 70 µm and J, (S. macrocarpa) bar 150 µm; alkaloids in E (S. laevicarpa) and R (S. sericea) and lipid in N, (S. recurva) bar 70 µm.; ce, cotyledonary epidermis; co, cotyledon; et, exotesta; mt, mesotesta.
The different characteristics between the four species are highlighted, such as size, shape, color of the seed coat, type of aril, size of the hilum, size of the funiculus and type of ergastic substances detected in the aril, seed coat and cotyledons (Table 3).
Discussion
The dimensions of the seeds varied among the four species, with S. laevicarpa having the largest length (3.1 cm), width (5.4 cm) and thickness (2.1 cm) and S. sericea having the smallest length (1.7 cm); width (2.2 cm) and thickness (1.3 cm), reinforcing the observations made by Meirelles & Souza (2015), who highlight that the dimensions of fruits and seeds are highly variable in species of Swartzia. Among seeds of the same species, a wider range of measurements for length, width, thickness and weight was found. Similar behavior was verified by Santos et al. (2015) for seeds of S. recurva.
The variation in dimensions of seeds of the same species, in the present study, can be attributed to the diversity in size of the fruits and the number of seeds per fruit, as occurred in S. macrocarpa where the number of seeds per fruit ranged from one to seven seeds, in S. recurva where fruits with two seeds were found, and in S. laevicarpa which had fruits of up to three seeds. According to Harper et al. (1970), variations in seed size may occur among individuals of the same species and in the case of polyspermic fruits, within the same fruit. Harper et al. (1970) also affirm that in elongated fruits, the distal and proximal seeds are generally smaller than the median seeds. An example of this is S. macrocarpa, which has elongated, polyspermic fruits. In tropical arboreal species, there is great variability in relation to the size of the fruits, number of seeds per fruit, and size of the seeds (Cruz & Carvalho 2003).
Another characteristic that is shared among seeds of the four species studied is the presence of the aril, which is predominant among the species of Swartzia, as the absence of this structure is mentioned as rare in the genus (Mansano & Lima 2007). The aril is white, the predominant coloration for the aril of seeds of Swartzia (Cowan 1967). The variation observed is in the texture and shape, with a spongy aril in S. recurva and a filamentous aril in S. laevicarpa, S. macrocarpa and S. sericea. The presence of the aril in seeds of Leguminosae is considered a character that supports the identification of some genera of the family (Barroso et al. 1999, Kirkbride et al. 2003) and in the present study, it contributes to the identification of the species.
According to Werker (1997), arils frequently contain oils and starch; in the present study, lipids were detected in S. recurva, protein in S. laevicarpa and S. macrocarpa and alkaloids in S. laevicarpa and S. sericea. According to Matsuura & Fett-Neto (2015), the presence of alkaloids and other secondary metabolites in the plants increases the rates of reproduction, improving the plant’s defenses against biotic and abiotic stresses or affecting pollinizers and visitation by seed/fruit dispersers. The occurrence of alkaloids in the aril of the seeds of S. laevicarpa and S. sericea analyzed in the present study is an important data in the biology of these species, as it may be related to the defense against herbivory. Vaz et al. (2018), affirm that in Swartzia langsdorffii Raddi, whose diaspore are rich in secondary compounds, the predation of seeds was not significant; when this occurred, seeds were only partially bitten but not completely consumed, suggesting that the chemical composition of the diaspore may not be adequate for consumption by vertebrates.
The presence of secondary compounds in the aril may also be related to the germinative process of the species, as verified by Vaz et al. (2016) in seeds of Swartzia langsdorffii. These authors observe that seeds sown with aril reached 33 % germination, while seeds that had the aril removed had a germination rate of 100 %.
According to Corner (1976), in Leguminosae, the seeds are exotestal, with a smooth, hard surface, epidermis with palisade cells and hypodermis of hourglass cells. This author also cite that in the seeds of Swartzia pinnata (Vahl) Willd. the seed coat is membranous, without hypoderm. The seeds here analyzed had similar characteristics to those described by Corner (1976) in that they are exotestal, with smooth surface, (as in Leguminosae), membranous and with absent hypodermis (as in S. pinnata).
In the seed coat of the four species analyzed, the presence of phenolic compounds was observed. These compounds are known to form a barrier against water loss, diffusion, and attack by pathogens (McCue et al. 2000). Phenolics have high antioxidant activity, protecting the cells against abiotic stress (Swigonska et al. 2014), as well as being important in the germinative process for lignification of the seedlings (Mng’omba et al. 2007). According to Werker (1997), these compounds are generally the main compounds responsible for the black of brown coloration of the seed coat, a coloration that was also observed in the species of this study.
In the studies of Meirelles & Souza (2015) on the germination of S. laevicarpa, S. macrocarpa, S. recurva and S. sericea, the authors suggest that there is a dormancy mechanism that is not associated with the hardness or impermeability of the seeds of these species, but to other factors, including the presence of inhibitor substances. The detection of phenolic and alkaloid compounds in the seeds of the present study may be associated with one of the dormancy mechanisms cited by the above mentioned authors.
Characteristics of the hilum related to the size, color and shape are important for identifying their respective seeds. Swartzia macrocarpa and S. sericea have hilar seeds (2.5 and 2.1cm in length, respectively) which, according to Corner (1976), are seeds in which most of the circumference is comprised of hilum, in contrast to the smaller hilum in S. laevicarpa (1.8 cm) and S. recurva (1.2 cm). In S. sericea, the color of the hilum, which is lighter than that of the seed coat, is also a characteristic that distinguishes it from the other three species studied.
The anatomy of the hilar region in the seeds studied differs from that found by other authors for species of Papilionoideae, due to the absence of a palisade layer. According to Kopooshian & Isely (1966) and Corner (1976), the hilar region in species of this sub-family presents a double palisade layer, as observed in seeds of Sophora tomentosa L. and Erythrina speciosa Andr. (Delgado et al. 2015), species of Indigofera L. (Teixeira & Corrêa 2007) and in Dipterix odorata (Aubl.) Will. (Bessa et al. 2001).
The crypto-radicular embryo, seen in the four species studied, is not a common type in Papilionoideae, which generally have a papilionaceous embryo, in which the hypocotyl-radicle axis is curved (Barroso et al. 1999). According to Kopooshian & Isely (1966), in Papilionoideae the embryo is typically curved, but some taxa of the subfamily have a straight embryo, as in Arachis where this character appears to be secondarily derived, and as in various genera of Sophoreae, where it is probably primitive. Kopooshian & Isely (1966) analyzed three undetermined species of Swartzia, and found both curved and straight embryos, considering the genus with seeds of intermediate characteristics to be between the sub-families of Caesalpinioideae and Papilionoideae.
Various authors, such as Lima (1989), Lima (1985), Oliveira (1999), Kirkbride et al. (2003), Meireles & Tozzi (2008) have emphasized the importance of characteristics of the embryo in the taxonomy of Leguminosae. Von Teichman & Van Wyk (1991) affirm that embryological characteristics are usually constant within genera, acting as indicators of taxonomic affinities.
In regard to the consistency of the cotyledons, only the fleshy type was observed in this study, which may be an adaptative characteristic to Igapó environments, where three of the four species studied S. laevicarpa, S. macrocarpa and S. sericea occur. According to Maia et al. (2005), in nutrient-poor environments, such as Igapó areas alongside blackwater rivers, the initial supply of nutrients by the mother tree, via reserve storage in the cotyledons, is crucial for survival.
The ergastic substances, starch, lipids, protein and alkaloids detected in the cotyledons of the species of this study confirm what was reported by other authors on the occurrence of reserve materials in seeds. Werker (1997) states that reserve materials in mature seeds are proteins, lipophilic substances and carbohydrates in the form of starch grains, and that these substances may be present as cell wall constituents. Paulino et al. (2010) detected the presence of alkaloids in cells of the cotyledons of three species of Indigofera L. and consider this character to have diagnostic value for this genus. According to Matsuura & Fett-Neto (2015), the accumulation of defense compounds in plants, originating from the primary or secondary metabolism (e.g. alkaloids) is closely linked to the survival strategy in the environment, guaranteeing adequate maintenance of the basic activity of the primary metabolism. The presence of phenolic and alkaloid compounds in all the seeds studies, whether in the aril, cotyledons or seed coat, indicates a defense mechanism of these species.
The results presented in this work for S. laevicarpa, S. macrocarpa, S. recurva and S. sericea highlight the form, color, consistency of the aril and size of the hilum of the seeds of these species, as important morphological characteristics for the recognition of these taxa in the field. This knowledge extends the information on the composition of the species in the soil seed bank, of the Tupé Sustainable Development Reserve in relation to species of Swartzia, which is important, particularly in a conservation area of the Amazon region.
We also highlight the adaptative relationship between the type of embryo with fleshy cotyledons and the environment occupied by the species studied.
Another important contribution is the current knowledge of the ergastic substances present in the aril, seed coat and cotyledons, which serve to support studies on seed dispersal strategies, for a better understanding of the germinative processes of the seeds and the establishment of its seedlings, considering that these substances accumulated in the seeds act as storage and defense substances.











 nueva página del texto (beta)
nueva página del texto (beta)



