As a rare and endangered tree species endemic to China, Zelkova schneideriana Hand.-Mazz. is a versatile material (Wu & Raven 2003, Yin 2013). Firstly, it can be used as a precious high-density wood timber (Jin et al. 2009). Secondly, its bark contains 46 % fiber, so it can be used to make artificial cotton, cord, and paper. Thirdly, it is an ornamental tree species, with a graceful crown, straight trunk, and red leaves in autumn. In addition, its leaves and bark are of great importance in Chinese Traditional Medicine. Unfortunately, over the past several decades, the distribution of its natural populations has significantly decreased due to deforestation and overharvest (Ma & Zhang 2009). Since 1999, Z. schneideriana has been listed as a national second-grade protected endangered plant species in China (Yin 2013).
According to the Flora of China Vol. 5, Z. schneideriana is distributed in 16 provinces in China (Wu & Raven 2003), with it primarily being distributed in subtropical regions of China (Ma & Zhang 2009). More recently, according to the results of the second key protected wild plant resources survey conducted in China, the mountainous area of southern Jiangsu is an area with a concentrated distribution of Z. schneideriana (Jiangsu Forestry Bureau 2017). Previous studies have focused on leaf architecture (Wang et al. 2001), phylogeny (Denk & Grimm 2015), genetic diversity (Liu et al. 2016), and seedling ecology (Yu et al. 2015); however, little is known about its niche and interspecific association.
A niche is the position of one species in a community in relation to other species. In other words, it refers to the spatial and temporal position and functional relationship of each species in the community (Grinnell 1917, Bai & Zhang 2017, Lu et al. 2020). Niche breadth can be used to reflect the utilization of resources, the distribution and quantity of species; niche overlap can be measured to determine the degree of similarity between species in their ability to exploit resources or adapt to the environment (Colwell & Futuyma 1971). Thus, a niche may reflect the position, function, and importance of a species in a community. Interspecific association refers to the interrelation of different species in spatial distribution, reflecting relationship formed by the mutual influence and interaction of various species in different habitats in the community (Gleason 1926, Feroz et al. 2014, Chai et al. 2016). Accordingly, it can reflect the species’ spatial distribution relationship and their functional dependency on the environment. Niche and interspecific association, to a great extent, altogether explain the spatial distribution of species and their utilization of environmental resources in the community (Jiang et al. 2019). Therefore, the joint research on niche and interspecific association of the community will help to better understand the ecological habits of the dominant species on resource utilization and their interrelationships, which will provide a basis for biodiversity conservation and forest management of endangered plant species.
This study examined the niche and interspecific association of dominant tree populations of Zelkova schneideriana communities in southern Jiangsu, eastern China. The aim of this study was to provide a theoretical basis for the protection and effective management of this endangered species. More specifically, the following four questions were addressed: (1) What is the status of Z. schneideriana populations in the communities? (2) How is Z. schneideriana’s ability to exploit environmental resources, compared with other dominant tree species in the communities? (3) What is the interspecific relationship between this species and associated tree species in Z. schneideriana communities? (4) What are the implications for the conservation of Z. schneideriana based on the results of niche indices and interspecific relationships?
Materials and methods
Study area. The study was in the mountainous area of Liyang and Jintan in Changzhou City (31º 09′ - 32º 04′ N, 119º 08′ - 120º 12′ E), which is located in southern Jiangsu Province, eastern China (Figure 1). Changzhou has a northern subtropical monsoon climate, with mild, humid weather and an average annual temperature of 16.3 °C, total annual precipitation of 1,068.9 mm, and an annual frost-free period of 237 d (Yang et al. 2013). The soil is predominantly yellow-brown soil. The vegetation type is mainly deciduous broadleaved forests with few evergreen broadleaved tree species; the dominant species include Z. schneideriana, Celtis sinensis Pers., Dalbergia hupeana Hance, and Aphananthe aspera (Thunb.) Planch. In addition, there are some coniferous and broadleaved mixed forests, bamboo forests, and shrublands in the hilly land of Changzhou.
Sampling design. Based on our comprehensive field survey in 2019, three sites were selected for study in a mountain area in southern Jiangsu Province where there was a large distribution area of Z. schneideriana forests (Table 1). At each of the three sites, we designated seven 20 × 20 m sampling plots. Thus, a total of 21 plots with a total area of 8,400 m2 at three sites were sampled. The forest was divided into three layers: tree layer, shrub layer, and herb layer. For the convenience of field investigation, each plot (or quadrat) was divided into four 10 × 10 m subplots. The tree layer (DBH ≥ 5 cm) was investigated for each species, and species name, tree height, and diameter at breast height (DBH at 1.3 m) were recorded (Li & Zhang 2015). Topographic elements including longitude, latitude, and elevation were also recorded (Table 1).
Table 1 The basic information of Z. schneideriana communities sampled at three sites in southern Jiangsu, eastern China.
| Sites | Area (m2) | Latitude | Longitude | Elevation (m) | Tree density (no./hm2) |
Average DBH of trees (cm/hm2) |
|---|---|---|---|---|---|---|
| Huijiacun, Liyang | 7 (20 × 20) | 31° 12′ 50″ N |
119° 27′ 44″ E | 122 - 140 | 871.43 | 7289.14 |
| Longtan, Liyang | 7 (20 × 20) | 31° 16′ 13″ N |
119° 29′ 02″ E | 148 - 160 | 942.86 | 7756.93 |
| Shijiashan, Jintan | 7 (20 × 20) | 31° 43′ 16″ N |
119° 19′ 15″ E | 108 - 130 | 960.71 | 5338.42 |
Importance value. The importance value (IV) can reflect the position and function of species in the community, which was calculated with the following formula (Curtis & McIntosh 1950, Zhang 2011, Li et al. 2020):
where IV is the importance value, RA is the relative abundance, RD is the relative dominance, and RF is the relative frequency. a i is the number of individuals of population i, d i is the basal area at the height of 1.3 m of population i, f i is the number of quadrats in which the population i appeared, and S is the total number of species.
Niche breadth. The Levins index and Shannon-Wiener index were used to calculate the niche breadth of the dominant species (the top 10 species in terms of IV) in Z. schneideriana communities (Shannon et al. 1949, Levins 1968, Tian et al. 2018, Lu et al. 2020).
(1) Levins index:
(2) Shannon-Wiener index:
where n
ij
is the importance value of species i in resource j, N
i
is the sum of the importance value of species i in all resources
Niche overlap. The niche overlap of the dominant populations in Z. schneideriana communities was calculated using the niche similarity index and niche overlap index (Pianka 1973, Schoener 1974, Lu et al. 2020).
(1) Niche similarity index:
(2) Niche overlap index:
where C ik and O ik are the niche similarity index and niche overlap index between species i and k respectively; P ij and P kj are the proportion of resources j used by species i or k, respectively.
It is generally regarded that the importance value of each species in a plant community, reflects its utilization of various resources and the spatial relationship relative to other plant species (Zhang et al. 2003). In this study, we took the investigated quadrat as the resource status, the quadrat number as the number of resource gradient. Accordingly, we applied the importance value of a species as quantitative index to measure its niche breadth and overlap.
Overall interspecific association. The variance ratio (VR) was used to determine the overall interspecific relationships, and the significance was further tested using the W value. The formula is as follows (Schluter 1984, Wang et al. 2019):
Where n
i
is the number of quadrats containing species i, N is the total number of quadrats,
If VR = 1, species have no association because they are assumed independent. Species show a positive association when VR > 1; VR < 1 indicates that species have a negative association. The W value was used to test whether the VR value deviated significantly from 1. If
Chi-square test. Chi-square (χ 2) was conducted based on a 2 × 2 contingency table for the qualitative study of interspecific association. Since this study was a discontinuous sample, χ 2 was corrected by Yates' continuous correction formula (Greig-Smith 1983, Dai et al. 2020). When the frequency of occurrence of a certain species is 100 %, the b and d values are weighted to 1 in order to avoid a noncomputable situation when the denominator is 0 (Jiang et al. 2019). The aim is to obtain more objective results. This was calculated as follows:
Where N is the total quadrat number, a is the number of quadrats containing species i and k, b is the number of quadrats containing species i but not k, c is the number of quadrats containing species k but not i, and d is the number of quadrats in which neither i nor k is found.
When χ2 < 3.841, there is no interspecific association (P > 0.05); when 3.841 ≤ χ 2 < 6.635, the interspecific association was significant (0.01 < P ≤ 0.05). When χ 2 ≥ 6.635, the interspecific association was highly significant (P ≤ 0.01). In addition, if ad > bc, there is a positive association and the species pairs tend to appear at the same time. Conversely, if ad < bc, there is a negative association and the species pair tends to be independent.
Spearman’s correlation coefficient. Spearman’s correlation coefficient of dominant species in Z. schneideriana communities was calculated using the following formula (Spearman 1904, Xu et al. 2016, Huang et al. 2017):
Where r
s
,(i,k) is Spearman’s correlation coefficient; N is the total number of quadrats; x
ij
and x
ik
is the importance value of species i and k, respectively, in quadrat j;
R 4.0.3 (R Development Core Team 2020) and Excel 2016 (Microsoft Corporation, USA) were used for all statistical analyses. The "spaa" package was used to analyze niche and interspecific association (Zhang 2016). In addition, ArcGIS 10.6 (Environmental Systems Research Institute, USA) was used to generate the maps of Z. schneideriana sampling plots.
Results
Importance values of dominant species. In our study, the three sites are not far away from each other (Table 1). Most tree species occurred at several plots, rather than at only one plot. Therefore, we used the IV to determine the number of dominant species in the tree layer of the communities. Among the 21 sample plots, there were 39 species belonging to 25 families and 32 genera in the tree layer. The sum of importance value of the 10 most dominant species accounted for 76.64 % (DBH ≥ 5 cm); therefore, these ten species were selected as dominant species for niche and interspecific association analyses (Table 2). In the tree layer, the importance value of Z. schneideriana was the highest (37.96), followed by C. sinensis (15.02); Phyllostachys edulis (Carriere) J. Houzeau had the lowest importance value (1.65). Therefore, there was a striking difference in the IV of these species.
Table 2 Importance value and niche breadth of dominant tree species in Z. schneideriana communities in southern Jiangsu, eastern China.
| No. | Dominant tree species | IV/% | Levins index | Shannon-Wiener index | ||
|---|---|---|---|---|---|---|
| BL | Ordination | BS | Ordination | |||
| T1 | Zelkova schneideriana | 37.96 | 19.84 | 1 | 3.02 | 1 |
| T2 | Celtis sinensis | 15.02 | 14.80 | 2 | 2.77 | 2 |
| T3 | Dalbergia hupeana | 4.77 | 7.33 | 3 | 2.09 | 3 |
| T4 | Aphananthe aspera | 4.49 | 5.76 | 4 | 1.88 | 4 |
| T5 | Firmiana simplex | 2.92 | 3.57 | 8 | 1.51 | 8 |
| T6 | Liquidambar formosana | 2.83 | 5.20 | 6 | 1.78 | 5 |
| T7 | Platycarya strobilacea | 2.70 | 4.17 | 7 | 1.51 | 7 |
| T8 | Ligustrum lucidum | 2.32 | 5.24 | 5 | 1.72 | 6 |
| T9 | Phoebe sheareri | 1.98 | 3.48 | 9 | 1.30 | 9 |
| T10 | Phyllostachys edulis | 1.65 | 1.75 | 10 | 0.77 | 10 |
Note: IV, importance value; B L , Levins index; B S , Shannon-Wiener index. T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 correspond to Zelkova schneideriana, Celtis sinensis, Dalbergia hupeana, Aphananthe aspera, Firmiana simplex, Liquidambar formosana, Platycarya strobilacea, Ligustrum lucidum, Phoebe sheareri, and Phyllostachys edulis respectively. The same below.
Niche breadth of dominant populations. There was a large difference in the niche breadth of the 10 most dominant species in the tree layer of Z. schneideriana communities. The Levins index (B L) ranged from 1.75 to 19.84, while the Shannon-Wiener index (BS) ranged from 0 to 3.04. In terms of BL and BS , Z. schneideriana had the highest niche breadth (BL : 19.84, BS : 3.02), followed by C. sinensis (BL : 14.80, BS : 2.77), and Ph. edulis had the lowest niche breadth (BL : 1.75, BS : 0.77) (Table 2). For these dominant species, the ranking result of BL was largely consistent with BS , except for Liquidambar formosana Hance and Ligustrum lucidum Ait (Table 2).
Niche overlap of dominant populations. Of the 45 species pairs consisting of 10 dominant species, the niche similarity indices (Cik) ranged from 0.00 to 0.71 and the average was 0.21. In the tree layer, only one pair with Cik > 0.5 was found, accounting for 2.22 % of all species pairs. The other 44 pairs had Cik ≤ 0.5, accounting for 97.78 %. These findings suggested that there was a considerable difference in the requirements of those species on environmental resources (Figure 2A). The niche overlap index (Oik) ranged from 0.00 to 0.82, and the average was 0.27. Seven pairs with Oik > 0.5 were found, accounting for 15.56 %; the other 38 pairs had Oik ≤ 0.5, accounting for 84.44 % (Figure 2B). Generally, if the niche overlap value was greater than 0.5, it was considered as relatively high; if less than 0.5, it was considered as relatively low (Tian et al. 2018). Similarly, the niche overlap indices (O ik) of most species pairs were also relatively low (Ōik = 0.27), suggesting there was little or no competition among most species in the tree layer of Z. schneideriana communities (Figure 2B). In addition, the average C ik and O ik between Z. schneideriana and other associated species were 0.32 and 0.47, respectively, and the greatest overlaps were found between Z. schneideriana and C. sinensis (C ik : 0.71, O ik : 0.82).
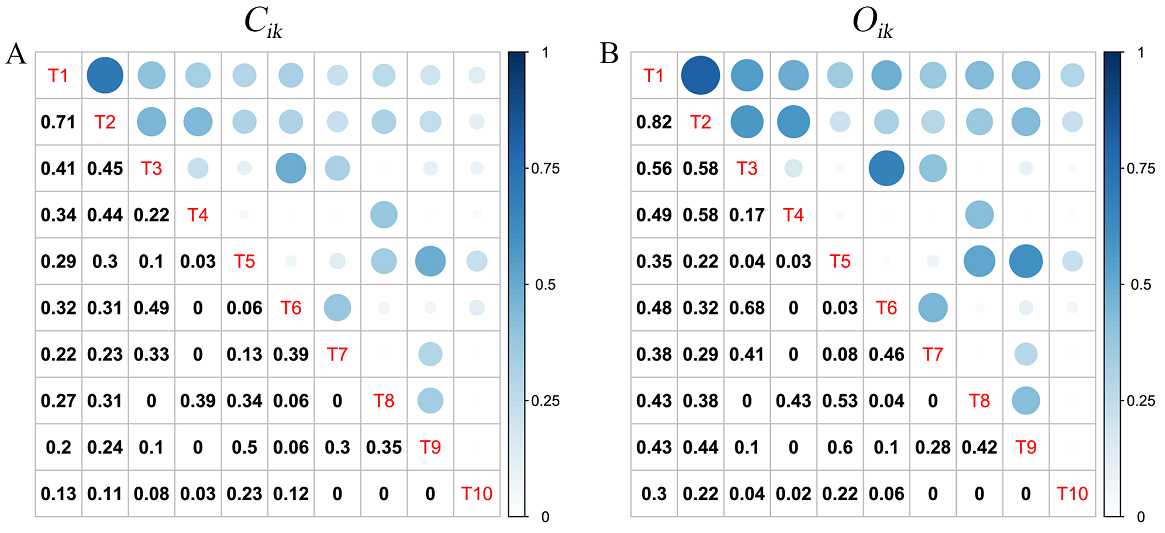
Figure 2 Niche overlap of dominant tree species in Z. schneideriana communities in southern Jiangsu, eastern China. (A) Niche similarity index (C ik ); (B) niche overlap index (O ik ). Tree species code (T1~T10) according to Table 2. The size and color of the circles (above the diagonal) show the value of niche overlap (below the diagonal). Color intensity and the size of the circles are proportional to the absolute value of niche overlap (C ik or O ik ).
Overall interspecific association. The variance ratio (VR) of the overall interspecific associations among the dominant tree species was 0.80 (VR < 1) in the tree layer of Z. schneideriana communities, indicating a negative interspecific association. Furthermore, the W value was 16.89
Association between major species pairs. In the χ 2 test, 15 species pairs showed positive associations in the tree layer, among which there was a highly significant difference between one pair and no significant difference between 14 pairs. Firmiana simplex (T5) and Phoebe sheareri (T9) showed highly significant positive correlation in the χ2 test. Twenty-eight pairs showed negative associations, among which there were significant differences between two pairs and no significant difference between 26 pairs. The two species pairs namely Dalbergia hupeana (T3) and Ligustrum lucidum (T8), Aphananthe aspera (T4) and Liquidambar formosana (T6), presented significantly negative correlation in the χ 2 test. In addition, the remaining two pairs showed no association (Figure 3). The semi-matrix diagram of Spearman’s correlation coefficient further revealed that 18 pairs showed positive associations in the tree layer, among which there were highly significant differences in two pairs and no significant difference in 16 pairs. The two species pairs namely Firmiana simplex (T5) and Phoebe sheareri (T9), Dalbergia hupeana (T3) and Liquidambar formosana (T6), presented highly significant positive correlation in light of Spearman’s correlation coefficient. Twenty-seven pairs showed negative associations, among which there were significant differences between two pairs and no significant difference in 25 pairs (Figure 4). The two species pairs namely Dalbergia hupeana (T3) and Ligustrum lucidum (T8), Aphananthe aspera (T4) and Liquidambar formosana (T6), presented significantly negative correlation in light of Spearman’s correlation coefficient.
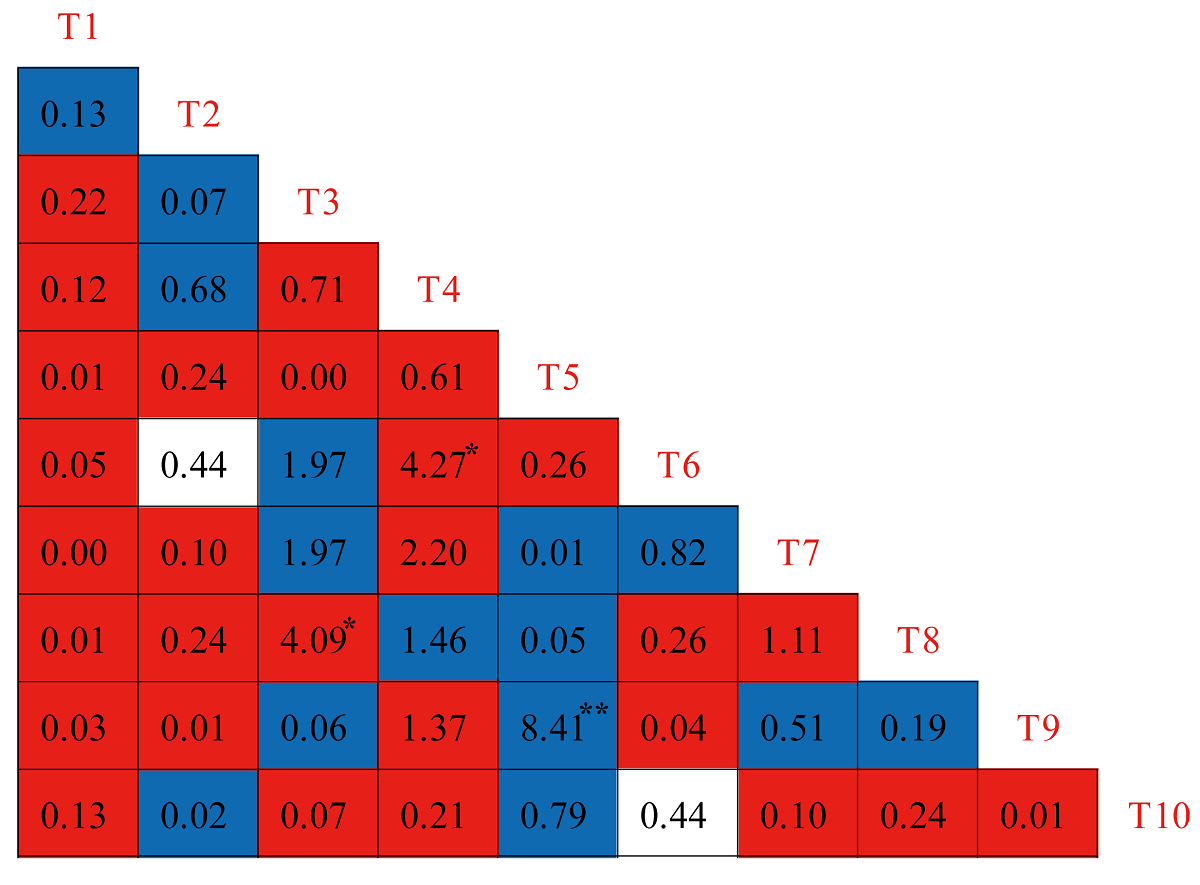
Figure 3 χ 2 test of dominant tree species in Z. schneideriana communities in southern Jiangsu, eastern China. Blue, red, and white color represents positive association (VR > 0, 15 pairs), negative association (VR < 0, 28 pairs) and no association (VR = 0, 2 pairs), respectively. Species codes in the diagonal are the same as in Table 2. *: P < 0.05, ** : P < 0.01.
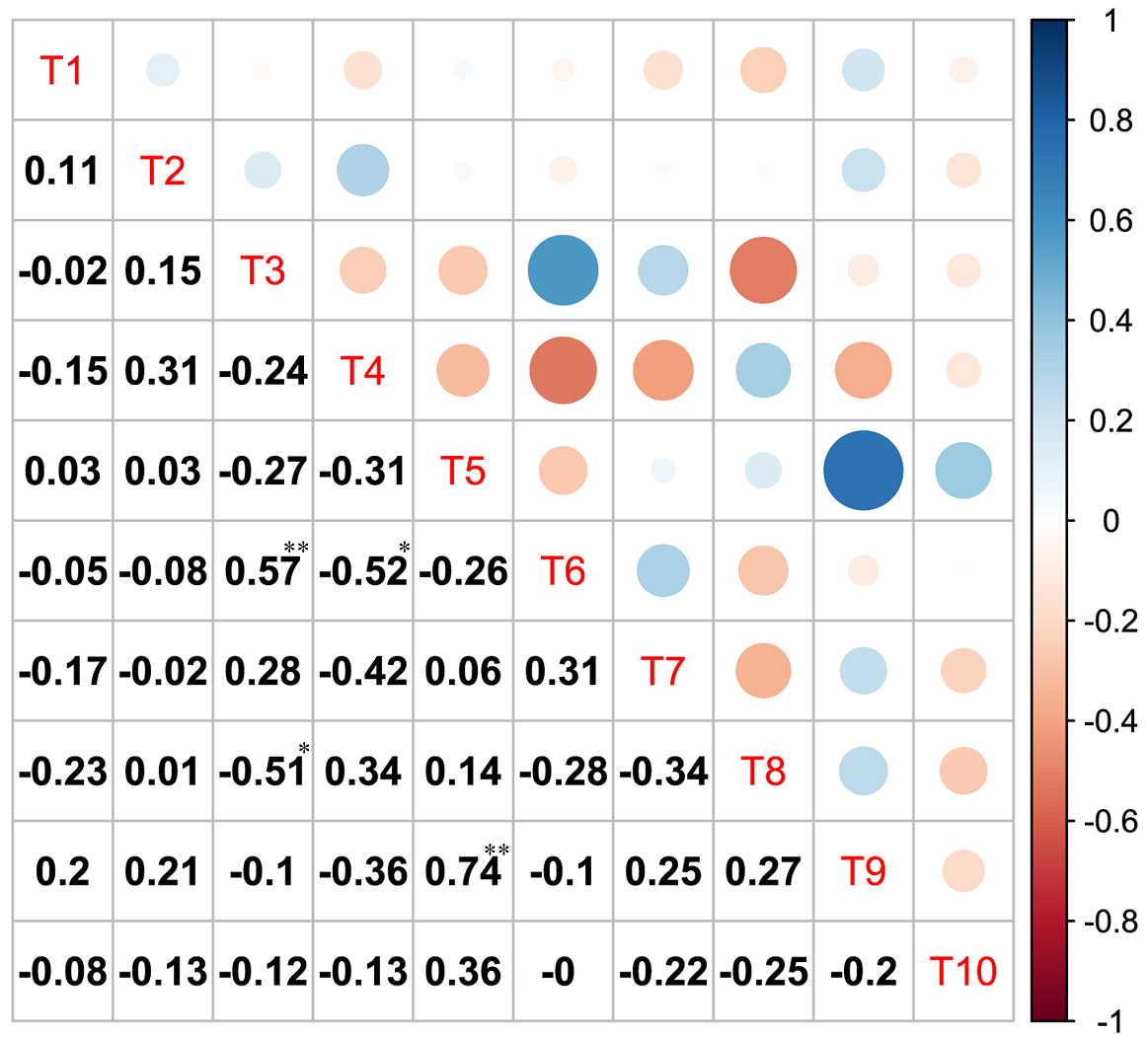
Figure 4 Spearman’s correlation coefficient of dominant tree species in Z. schneideriana communities in southern Jiangsu, eastern China. Tree species code (T1~T10) according to Table 2. The size and color of the circles (above the diagonal) show the value of Spearman’s correlation coefficient (below the diagonal). Blue and red color represents positive association (18 pairs), negative association (27 pairs) respectively. Color intensity and the size of the circles are proportional to the absolute value of Spearman’s correlation coefficient.*: P < 0.05, ** : P < 0.01.
In the tree layer of Z. schneideriana communities, the results of the χ 2 test and Spearman’s correlation coefficient showed that the species pairs were more negatively associated than positively associated, which was consistent with the results of the overall interspecific association analysis. The results showed that most associations between pairs of species were not statistically significant.
Discussion
Importance values and niche breadth. Importance value and niche breadth are comprehensive indices that can be used to measure the role and status of species in a community, but the two indices have different ecological significance. The importance value reflects the status, advantage, and function of species in the community, whereas niche breadth evaluates the ability of a species to utilize environmental resources (Liu et al. 2018, Tian et al. 2018). Our results showed that Z. schneideriana had the highest importance value and niche breadth, indicating that Z. schneideriana was the most important dominant species. In general, the dominant species in dominant layer is commonly known as the instructive species in a forest community (Zhou 2010). Our investigation shows that Z. schneideriana communities can be divided into three layers: tree layer, shrub layer and herb layer. Z. schneideriana in the tree layer had the IV of 37.97 (Table 2), taking almost half of the total IV of the first ten tree species. This indicates that Z. schneideriana was an instructive species or edificator tree. Z. schneideriana made full use of environmental resources and had strong environmental adaptability, thus, playing a significant role in the stability and composition of the communities in the sampled locations. In contrast, the importance values and niche breadth of other species were relatively low, indicating that their adaptability and ability to utilize local resources were poor. Consequently, their distributions were relatively narrow. For example, Phoebe sheareri (Hemsl.) Gamble, an evergreen tree endemic to China (Ding et al. 2018), was typically found in valleys in the study area.
Niche overlap and interspecific association. Interspecific association and niche overlap are closely correlated. Many studies have shown that a positive association indicates the use of similar resources and a niche overlap, whereas a negative association indicates that species have different habitat and resource requirements (Su et al. 2015, Gu et al. 2017, Li et al. 2017). In this study, both Z. schneideriana and C. sinensis had a positive association, with a large overlap in habitat and niche, which may be related to the fact that both species are sun-loving deciduous trees. In contrast, D. hupeana and L. lucidum are deciduous and evergreen trees, respectively, but L. lucidum is more salt-tolerant than D. hupeana (Su et al. 2011, Lai et al. 2020). As a result, D. hupeana and L. lucidum had a negative association and had a minimal shared niche and habitat.
Niche overlap and interspecific association reflect the degree of interspecific competition and community stability (Moloney & Levin 1996, Wu et al. 2013). In the tree layer of Z. schneideriana communities, the niche similarity and niche overlap indices of most species were relatively low, which indicated that the competition among these species was not intense due to their different demands for environmental resources. At the same time, the overall interspecific association showed negative association that was not significantly different demonstrating that most tree species were relatively independent and less likely to be affected by other co-occurring species. Based on the χ2 test and Spearman’s correlation coefficient, most species pairs exhibited no significant correlation and the number of negatively associated species pairs was greater than positively associated species pairs, which was consistent with the overall interspecific results. As such, we hypothesize that dominant species of tree layer is relatively independent during the early stage of forest community succession in subtropical China. However, with succession proceeding, the communities may gradually become stable and more species pairs may exhibit positive associations (Zhang et al. 2016, Gao et al. 2017, Liu et al. 2017).
Besides, the niche overlap between Z. schneideriana and C. sinensis is the greatest in tems of C ik or O ik (Figure 2) among the 45 species pairs. This indicates that both species may have relatively consistent requirements for environmental resources. When environmental resources are insufficient, intense competition may occur between these two species.
Implications for conservation. Our results indicate that most of the dominant tree species in the Z. schneideriana communities exhibited a distinctive demand for resources, with little competition, and the species were only slightly associated or even independent. At present, except for the Huijiacun population, the Longtan population is protected due to its location in Tianmuhu National Forest Park, Jiangsu Province, and the Shijiashan population was protected because it was located in a small nature reserve for endangered plant species. According to the genetic analysis, most variation consistently originated from within Z. schneideriana population, whereas its genetic differentiation among population was not significant (Liu et al. 2005, Liu et al. 2016). Therefore, we suggest establishing a small nature reserve to protect the wild population in Huijiacun.
Our results also indicate that most of the interspecific associations between Z. schneideriana and its co-occurring tree species were primarily negative. Recent research has found that Z. schneideriana had long seed dormancy, a high empty-nut ratio, and low maximum net photosynthetic rate (Shen et al. 2011, Zhang et al. 2013, Tian et al. 2014). Although Z. schneideriana currently occupies a dominant position in the stand, the relationship between its population and other associated tree species may change with the continued ecological succession. In other words, the competition between species may intensify in the future. Thus, we recommended conducting dynamic monitoring in these dominant populations of Z. schneideriana communities.
In summary, our study is the first to show the niche characteristics and interspecific associations of the dominant species in Z. schneideriana communities. Based on the importance value and niche breadth, Z. schneideriana was the constructive species or edificator tree in the investigated communities. We also found that dissimilarity in environmental requirements existed among most dominant tree species, thus, decreasing interspecific competition. This is likely due to the instability of the Z. schneideriana community because it is in the early stages of succession. In addition, we suggest strengthening the population conservation of Z. schneideriana by establishing a small nature reserve and by conducting dynamic monitoring of these dominant populations in the investigated communities.











 nueva página del texto (beta)
nueva página del texto (beta)