Orchids represent one of the most awesome and intriguing examples of complex interactions with pollinators (Roberts 2003, Scopece et al. 2015). About 60 % of all orchid species interact with a single pollinator species (Tremblay 1992), suggesting an important adaptive process in the family (Cozzolino & Widmer 2005). Although this high specificity represents important advantages to plants, it also entails substantial drawbacks such as the possibility of pollination failure. In turn, pollination failure may be due to one or a combination of these factors: (1) pollinator limitation, (2) inefficient pollen removal or pollen loss during transport, and (3) poor pollen quality or quantity received by the plant (Wilcock & Neiland 2002).
A distinctive feature among orchids is their pollen aggregated in structures known as pollinia. Cross-pollination requires the removal of pollinia by an animal, normally in a single all-or-nothing event (Tremblay 1992). Actually, pollen removal does not guarantee the occurrence of pollination, as pollen must be also adequately placed on the animal’s body, and then the pollinator must successfully transport the pollen and deposit it on another flower’s stigma. Overall, pollen deposition is less successful in orchids than pollinia removal (Nilsson 1992); for example, after examination of a sample of 100 flowers of Oncidium sphacelatum, Damon & Cruz-López (2006) only found three events of pollinia deposition and 31 of pollinia removal. Apparently, many orchids have faced such pollination limitation over their evolutionary history (Tremblay et al. 2005), particularly those having a pollination deceptive strategy, and consequently have a low fruit set (Sabat & Ackerman 1996, Sonkoly et al. 2016, Phillips et al. 2020).
Plants attract pollinators through a large suite of floral traits, including nectar, aromas and floral displays, which are capable of driving pollinator behavior, ideally increasing floral visitation rates (Stpiczyńska et al. 2004, Flach et al. 2004). Paradoxically, deception mechanisms in which flowers do not offer rewards are typical among orchids; an estimated one third of all species in this family possess such mechanisms (van der Pijl & Dodson 1966, Dressler 1981, Ackerman 1984, Dafni 1984, Schiestl 2005, Jersáková et al. 2006). Similarly, floral display (i.e., number of flowers, inflorescence length, flower density and spatial pattern) plays a key role in the pollination process (Willmer 2011), given its influence on pollinator visits and the plant’s reproductive success from the perspectives of both the male and the female functions of flowers (Castillo et al. 2002).
Orchids are a good system to investigate factors affecting pollination success in plants, as the arrangement of their pollen in pollinia facilitates the assessment of pollen removal and deposition rates (Nilsson et al. 1992, Coombs et al. 2009). Nonetheless, cross-pollination in orchids has been seldom observed under natural conditions, given the low frequency of pollinator visits (Neiland & Wilcock 1998, Widmer et al. 2000, Tremblay et al. 2005). This is probably why studies on tropical orchid pollination are so scant, especially for those species from seasonally dry regions in the Neotropics. This is regrettable, as tropical regions host the largest orchid diversity (Dressler 1981), and the study of their pollination may offer new insights on the mechanisms involved in their diversification (Bawa 1990, Roberts 2003).
Our goal was to examine pollination biology in three orchid species (Barkeria whartoniana (C. Schweinf.) Soto Arenas, Clowesia dodsoniana E. Aguirre, and Cyrtopodium macrobulbon (La Llave & Lex.) G.A. Romero & Carnevali.) from a seasonally dry tropical region of southern Mexico, through the assessment of pollination success, both regarding the male and female functions, and its relation to floral display, as well as the frequency and diversity of floral visitors. These analyses were supplemented with an examination of floral micromorphology as a way to gain insight into the existence of structures potentially producing rewards to attract pollinators in these species. We hypothesized that floral display (assessed through the number of open flowers, and number and length of inflorescences) has a positive effect on pollinia removal and deposition rates.
Materials and methods
Study site and species. We conducted this study in the surroundings of Nizanda, a small village located in the southern portion of the Isthmus of Tehuantepec, Oaxaca state, Mexico (16° 39’ N, 95° 00’ W; Figure 1). Regional climate type is Aw (Equatorial savannah with dry winter; Kottek et al. 2006), with summer rains concentrated between June and September. Mean annual temperature is 27.6 ºC and mean annual precipitation is 902.6 mm (CLICOM Project, National Meteorological Service, CICESE, http://clicom-mex.cicese.mx). The regional vegetation is a complex mosaic encompassing various communities (Pérez-García et al. 2010), among which the most widely spread one is tropical dry forest. In this forest type, epiphytic and lithophytic orchids are rather scarce due to the relatively low humidity in most part of the year. Yet, in our study region these plants are relatively abundant in small forest patches growing on limestone outcrops. The study was conducted in these rocky environments, at elevations ranging from 150 to 250 m asl.
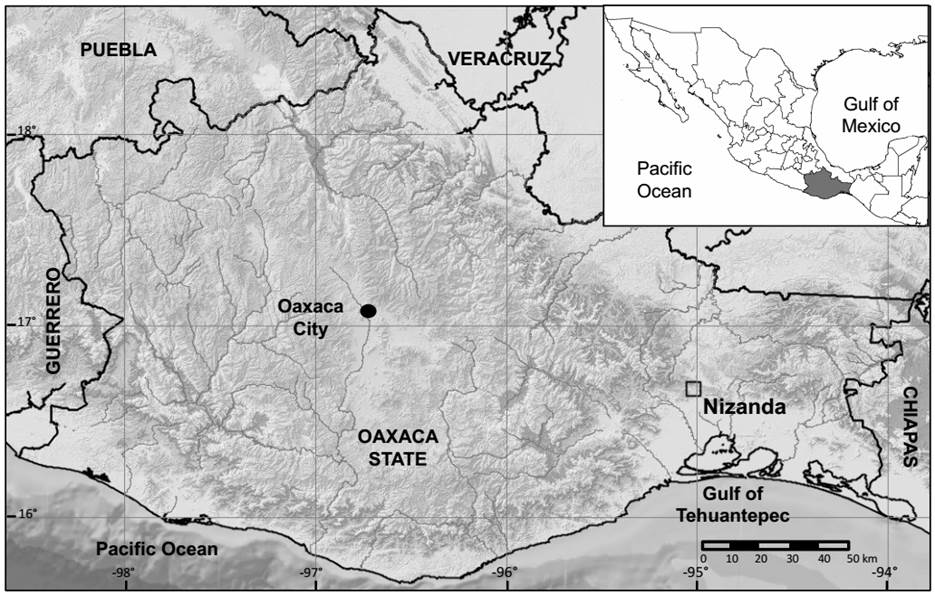
Figure 1 Location of the study site around the village of Nizanda, Isthmus of Tehuantepec, Oaxaca State, Mexico.
We selected three orchid species for the study, all of them apparently being pollinated by diurnal organisms, most likely bees: Barkeria whartoniana (Figure 2A), Clowesia dodsoniana (Figure 3A), and Cyrtopodium macrobulbon (Figure 4A). These are relatively abundant species, thus easy to find in the field, while their distinctive morphology ensures their correct identification; moreover, they are relatively synchronous in their flowering, and their floral sizes are similar (Table 1).
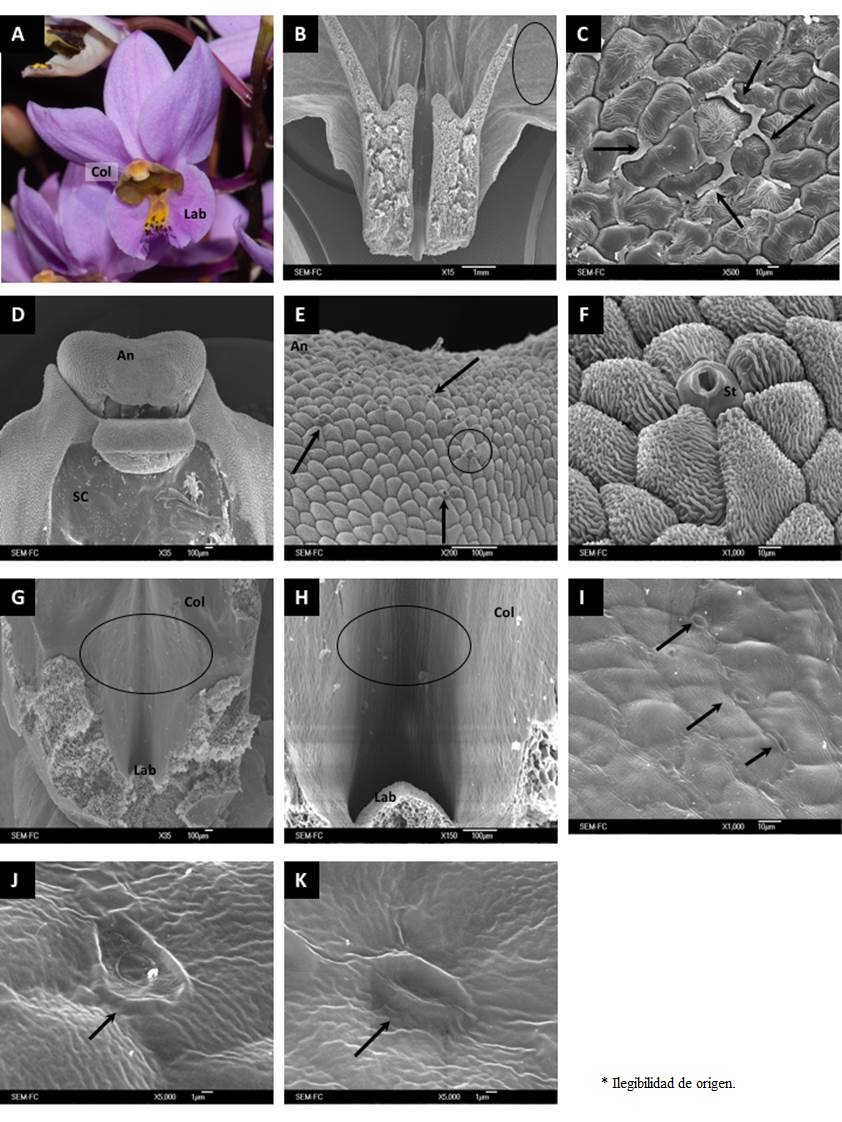
Figure 2 General aspect and micromorphology of Barkeria whartoniana flowers. (A) Flower of B. whartoniana in anthesis. (B) Longitudinal cut of the labellum showing a probable secretion zone. (C) Secretion zone towards the intercellular space. (D) View of the column, showing the anther and the stigmatic cavity. (E) Upper view of the anther where the presence of open stomata is indicated. (F) Stoma located on the anther surface. (G) View of the column-labellum intersection, pointing out the zone where secreting structures were found. (H) Column-labellum intersection at a larger zoom. (I) Stomata along the column. (J)-(K) Grooves on the column. An, Anther; Col, Column; Lab, Labellum; SC, Stigmatic cavity; St, Stoma.
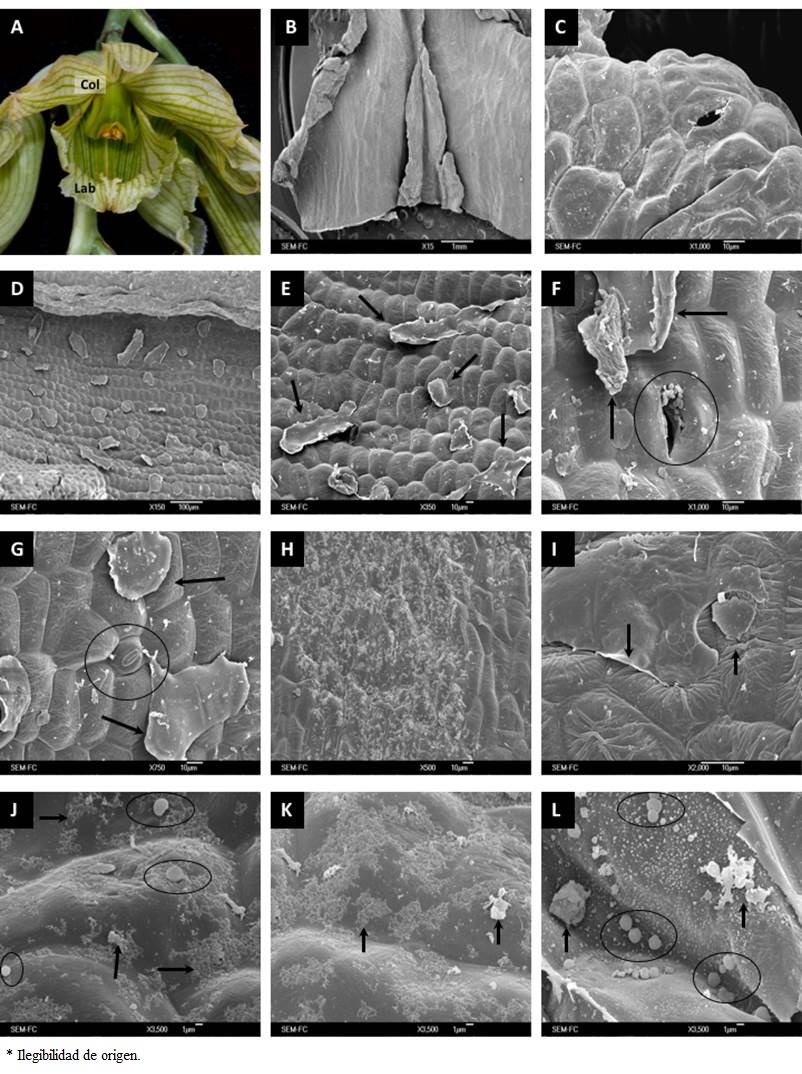
Figure 3 General aspect and micromorphology of Clowesia dodsoniana flowers. (A) Flower of C. dodsoniana in anthesis. (B) Longitudinal cut of the labellum. (C) Stomata on the epidermal surface in labellum’s apical zone. (D) Epicuticular wax detachments in labellum’s apical zone. (E) Epicuticular wax detachments in labellum’s middle portion. (F) Stomata in labellum’s middle part with secretions around it; arrows point to secretions at the periphery. (G) Stomata in the labellum surface. (H) Secretions in labellum’s middle zone, near the base. (I) Epicuticular secretions near the labellum’s base. (J) Elements on the epidermal surface of labellum’s basal zone; the arrows and circles point to the observed substances. (K) Substances observed on the labellum’s basal surface. (L) Various types of secretions at the labellum’s base; the arrows point to the observed substances. Col, Column; Lab, Labellum.
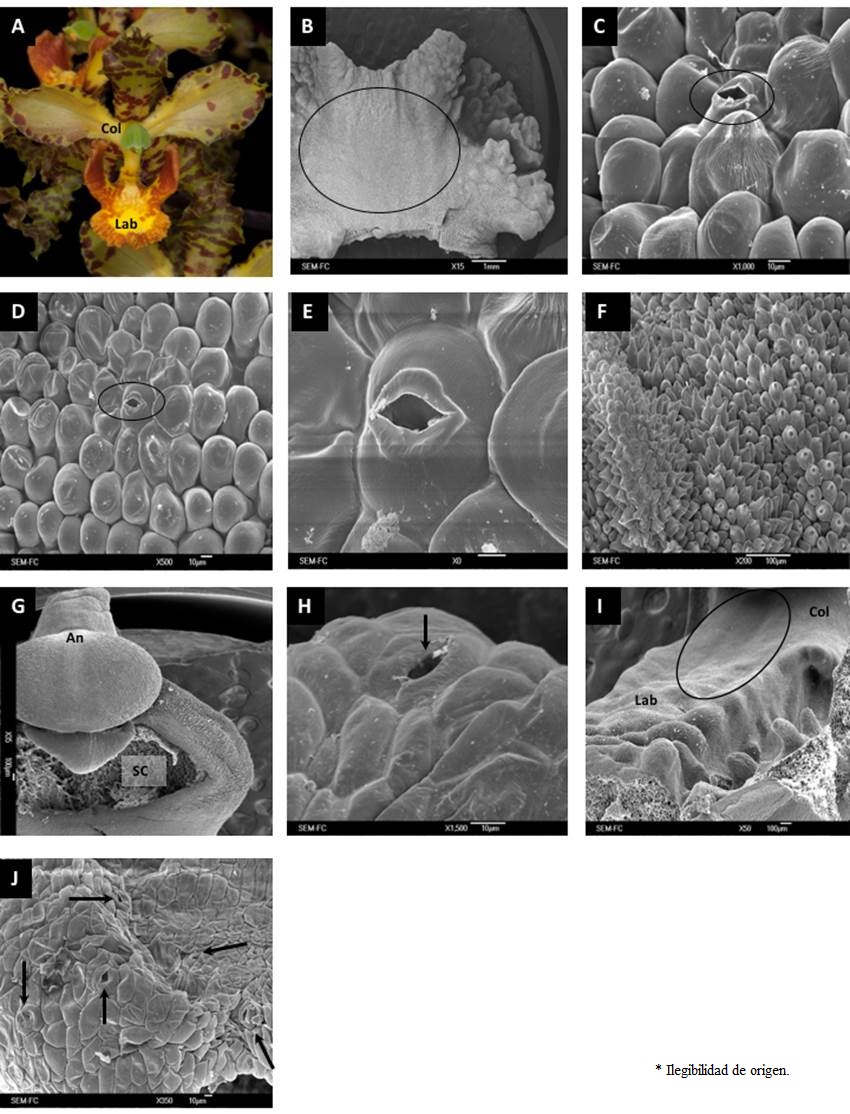
Figure 4 General aspect and micromorphology of Cyrtopodium macrobulbon flowers. (A) Flower of C. macrobulbon in anthesis. (B) Longitudinal cut of the labellum showing the area where papillae and stomata were observed. (C) Globose cells spread over the labellum. (D) Papilla cells on the middle part of the labellum. (E) Papilla with an opening stoma on its external periclinal wall. (F) Trichomes and papillae in labellum’s basal zone. (G) Column showing the stigmatic cavity and the anther. (H) Stoma located on column’s epidermal surface. (I) Labellum-column intersection zone, showing secreting structures. (J) A set of stomata. An, Anther; Col, Column; Lab, Labellum; SC, Stigmatic cavity.
Table 1 A comparison of key biological and ecological attributes in three species included in the study of orchid pollination in the region of Nizanda, Oaxaca, Mexico.
| Trait | Barkeria whartoniana | Clowesia dodsoniana | Cyrtopodium macrobulbon |
|---|---|---|---|
| Flowering type | Successive | Synchronous | Successive |
| Flowering season | November to January | May to mid-June | April to May |
| Inflorescences | Consisting in a succession of between one to five racemes, each with 2-15 flowers. | One or two hanging racemes by pseudobulb, each with as many as 20 flowers. | One or two panicles by pseudobulb, with as many as 50 flowers each. |
| Flowers | Lacking perceptible aroma, ca. 2.5 cm in diameter, often with a whitish color just after anthesis, turning lilac when mature. Both petals and sepals with a bright appearance. Self-compatible. | With intense aroma, ca. 4 cm in diameter. Light green color, with dark green longitudinal lines. Self-incompatible. | Lacking perceptible aroma, ca. 2.5 cm in diameter, yellow with reddish speckles. Colorful petaloid bracts. |
| Growth habit | Epiphytic, rarely rupicolous, growing mostly on the stems of Beaucarnea recurvata Lem., Comocladia engleriana Loes., Neobuxbaumia scoparia (Poselg.) Backeb. and Plumeria rubra L. | Epiphytic, usually growing on dead branches and stems of various tree species. | Lithophytic, very common on limestone outcrops. |
| Elevational range | 200-300 m asl | 200-400 m asl | 200-950 (-2,200) m asl |
| Geographic distribution | Micro-endemic of the Isthmus of Tehuantepec, Oaxaca. | Mexican Pacific watershed, from Sinaloa to Oaxaca. | Broadly distributed in the Pacific and Atlantic watersheds of Middle America, from Mexico to Panama. |
Data collection. Field work was conducted during the flowering periods of the studied species. To maximize sample size, observations were done in two yearly periods (2013 and 2014), except for B. whartoniana, for which observations were done twice in a single flowering season (2013), at the onset of flowering and a month later, at the end of this phenological event. A different site was selected for each species; these sites were located as close as possible to each other to reduce site effects on pollinator abundance and diversity.
To evaluate pollination success, we marked each flowering individual and labelled each flower in anthesis. For all labelled flowers we made sure that they had pollinia at the column apex but had no pollinia deposited in the stigmatic cavity. For each individual, we recorded number of open flowers, number of inflorescences, and the length of each inflorescence (to the nearest cm), and for each flower, we logged the removal or deposition of pollinia by observation period, as respective measures of the male and female reproductive success (Nilsson 1992). We restricted the sampling to sunny days (i.e., not overcast) with little wind, as this region is characterized by constant strong winds (Brennan et al. 2010) that seem to affect insect activity. This resulted in four to seven effective observation days by flowering period. Observations were conducted between 0700 and 1500 h (summer daylight saving time, from the third week of April to the third week of October, with solar time adjusted in the winter). In each day there were three 2-h periods of direct observation alternated with resting 1-h periods; the latter was used to trap potential pollinators with entomological nets to build a reference collection. Trapped insects were sacrificed in a lethal chamber with ethyl acetate, mounted, labelled and deposited at the Alfonso L. Herrera Zoology Museum at the Faculty of Sciences, Universidad Nacional Autónoma de México (UNAM).
Given the low number of visits recorded in the first two observation cycles (2013), we incorporated the use of two video recording cameras (Canon R400 Full HD, Canon Inc., Tokyo, Japan) in a continuous schedule from 0800 to 1300 h. Through the cameras we acquired a digital visual record of the entire sampling period for B. whartoniana (2013), and of the second year for C. dodsoniana and C. macrobulbon (2014; Table 2). Every time an animal made direct contact with the flower was tallied as a visit to a flower, no matter whether the insect made contact with the same flower repeatedly or with different flowers on the same plant.
Table 2 Total observation period (hours) of floral visitors by species in each flowering season.
| Season | Video recording | Direct observations | Total |
|---|---|---|---|
| Barkeria wharthoniana | |||
| 2013-1 | 27:28:47 | 12:00:00 | 39:28:47 |
| 2013-2 | 43:07:51 | 88:30:00 | 131:37:51 |
| Clowesia dodsoniana | |||
| 2013 | 0:00:00 | 80:00:00 | 80:00:00 |
| 2014 | 19:35:27 | 36:00:00 | 55:35:27 |
| Cyrtopodium macrobulbon | |||
| 2013 | 0:00:00 | 48:00:00 | 48:00:00 |
| 2014 | 29:17:05 | 64:30:00 | 93:47:05 |
To compare plants having different numbers of flowers and sampled in different time periods, we calculated a Flower Visitation Rate by observation period, as follows:
where NFV is the number of visits received by a flower in the period, NF is the number of flowers recorded, and NH is the number of hours in the period.
Characterization of floral micromorphology. We collected five flowers in anthesis and fixed them in FAA (formaldehyde-acetic acid-alcohol-water) solution for one week. Next, we dissected the flowers in three areas, namely the labellum, the column and the labellum-column point of intersection. The samples were washed with distilled water and dehydrated in a gradual ethanol series (50, 70, 85, 96 and 100 %; 2 h in the first solution, one day in each of the following three, and 48 h in 100 % ethanol). Samples were then dehydrated to a critical point in a CPD-030 Bal-tec desiccator. Finally, they were covered with gold in a Desk-II ionizer (Denton Vacuum, Moorestown, N.J., USA), and scanning electronic microscope (SEM) micrographs (JSM-5310 LV, Jeol Inc., Tokyo, Japan) were produced to examine micromorphology.
Data analysis. The assessment of pollination success in the three species was done by evaluating the male and the female functions of the flowers. Male function success was calculated through the pollinia removal ratio (number of flowers with their pollinia removed/number of flowers produced); similarly, female function success was calculated through the pollinia deposition ratio (number of flowers with deposited pollinia in the stigmatic cavity/number of flowers produced) (Parra-Tabla & Vargas 2007).
To assess the effect of floral display on the probability of pollinia removal and deposition for each species, we constructed generalized linear mixed models (GLMM) with binomial distribution with the lme4 package (Bates et al. 2015) in R (R Core Team 2020); these models included the observation period as a random effect (i.e., year in the case of C. dodsoniana and C. macrobulbon, and early and late flowering season of 2013 in B. whartoniana). Floral display was assessed through the following variables: (1) number of open flowers, (2) number of inflorescences, and (3) inflorescence length. These variables were standardized to make parameter estimates comparable.
For each response variable we constructed a null model and alternative models that included the effect of one, two or three explanatory variables, and their pairwise interactions (Tables A1 and A2, Appendix 1). Insufficient sample sizes prevented the construction of some of the most complex models; the maximum number of parameters in a model was set to 1/10 of the number of flowers. Model selection was based on the sample-size-corrected Akaike Information Criterion (AICc); models with (AICc < 2 were considered to be equally supported (Burnham & Anderson 2002).
Results
Pollination success. We monitored a total of 106 reproductive individuals and 1,338 flowers for the three species, distributed as follows: 45 plants (84 flowers) for Barkeria whartoniana, 18 plants (290 flowers) for Clowesia dodsoniana, and 43 plants (964 flowers) for Cyrtopodium macrobulbon. In all cases, male function success of the flower was higher than female function success. Pollinia removal was highest in C. dodsoniana (32.07 %), followed by B. whartoniana (22.62 %), and considerably lower in C. macrobulbon (5.60 %). Notably, for the two former species there was a strong variation between observation periods (Table 3). For pollinia deposition we recorded very low rates; the largest female success (6.21 %) corresponded to C. dodsoniana, followed closely by B. whartoniana (4.76 %), but distantly by C. macrobulbon (0.62 %) (Table 3).
Table 3 Pollination success in the three orchid species studied. The information is presented by flowering season (2013 or 2014), and for the total study period (2013-2014), except for Barkeria whartoniana, for which the data correspond to two observation periods in a single flowering season (2013), namely its beginning (b) and end (e). N is the number of reproductive individuals observed. Factors affecting pollination success are total number of inflorescences and total number of flowers in anthesis. Pollination success for flowers in anthesis was assessed by counting pollinia removal and deposition events, on which the rates of male and female pollination success were respectively based. For each species, the third line shows the totals for the entire study period.
| Flowering season | N | No. of inflorescences |
No. of flowers |
Pollinia removal |
Pollinia deposition |
Male success (%) |
Female success (%) |
|---|---|---|---|---|---|---|---|
| Barkeria whartoniana | |||||||
| 2013b | 15 | 18 | 32 | 15 | 4 | 46.88 | 12.50 |
| 2013e | 30 | 34 | 52 | 4 | 0 | 7.69 | 0.00 |
| 2013b, e | 45 | 52 | 84 | 19 | 4 | 22.62 | 4.76 |
| Clowesia dodsoniana | |||||||
| 2013 | 7 | 9 | 153 | 27 | 8 | 17.65 | 5.23 |
| 2014 | 11 | 14 | 137 | 66 | 10 | 48.18 | 7.30 |
| 2013-2014 | 18 | 23 | 290 | 93 | 18 | 32.07 | 6.21 |
| Cyrtopodium macrobulbon | |||||||
| 2013 | 8 | 11 | 107 | 6 | 0 | 5.61 | 0.00 |
| 2014 | 35 | 49 | 857 | 48 | 6 | 5.60 | 0.70 |
| 2013-2014 | 43 | 60 | 964 | 54 | 6 | 5.60 | 0.62 |
Floral display effect on pollen removal and deposition. For B. whartoniana and C. macrobulbon, the null models best explained the data, both for pollinia deposition and removal (Tables A1 and A2, Appendix 1), suggesting that both male and female functions may be unaffected by the number of flowers, and by the length and number of inflorescences. In turn, pollinia deposition in C. dodsoniana was positively influenced by inflorescence length, but negatively by the number of flowers (Table A1, Appendix 1); by contrast, the best supported model for pollinia removal in this species included the three flower display variables (inflorescence length, number of inflorescences, and number of flowers), as well as the interaction between inflorescence length and number of flowers (Table A2. Appendix 1).
Floral visitors. Flower Visitation Rates were not only very low for the three species, but also highly variable among species and observation periods (Table 4). C. dodsoniana had the highest Flower Visitation Rate (0.0124 visits·flower-1·h-1), in contrast with the much lower (and similar to each other) rates recorded for B. whartoniana and C. macrobulbon (0.0053 and 0.0052 visits·flower-1· h-1, respectively). Visitors of B. whartoniana included insects and birds, while insects were the only visitors to the flowers of the two other species. Insect and hummingbird visits to B. whartoniana flowers were almost identical (0.0027 vs. 0.0026 visits·flower-1· h-1, respectively; Table 4). The hummingbird species that visited B. whartoniana was Archilochus colubris (Linnaeus).
Table 4 Flower visit rates (visits·flower-1·100 h-1) in three orchid species from the region of Nizanda, Oaxaca, Mexico. Bold typeface indicates the highest visitation rate recorded for each orchid species. Dashes indicate lack of visits. Superscripts: a, visitor recorded in video; b, visitor recorded by direct observation. For Barkeria whartoniana, the letters b and e next to the year indicate the beginning and the end of the flowering season, respectively.
| Visitor | Barkeria whartoniana | Clowesia dodsoniana | Cyrtopodium macrobulbon | |||
|---|---|---|---|---|---|---|
| 2013b | 2013e | 2013 | 2014 | 2013 | 2014 | |
| Aves (Apodiformes) | ||||||
| Archilocus colubris (Linnaeus)a | 0.45 | 0.07 | - | - | - | - |
| Insecta (Diptera) | ||||||
| Unidentified Nematoceraa | - | 0.02 | - | 0.61 | 0.01 | - |
| Insecta (Hymenoptera) | ||||||
| Apis mellifera (Linnaeus)ab | - | - | - | - | - | 0.02 |
| Euglossine spp.a | 0.09 | 0.02 | - | 1.48 | - | - |
| Melipona sp.b | - | - | - | - | 0.01 | - |
| Mesocheira bicolor (Fabricius)b | - | - | - | - | 0.01 | - |
| Partamona bilineata (Say)b | - | - | 0.02 | 0.06 | 0.01 | - |
| Plebeia moureana (Ayala)b | - | - | - | - | 0.01 | - |
| Trigona fulviventris (Guérin-Méneville)b | - | - | - | - | 0.13 | 0.03 |
| Trigona nigra (Cresson)ab | - | - | - | 0.12 | - | 0.08 |
| Trigonisca mixteca (Ayala)ab | - | 0.02 | - | - | 0.05 | - |
| Xylocopa sp.b | 0.09 | 0.07 | - | - | - | 0.01 |
| Unidentified Apoideaa | 0.06 | 0.02 | - | - | 0.58 | - |
| Polybia sp. (Vespidae)ab | - | - | 0.12 | - | 0.06 | 0.01 |
| Unidentified Formicidaea | - | - | - | 0.06 | 0.04 | - |
| Insecta (Lepidoptera) | ||||||
| Unidentified Papilionoideaab | 0.12 | 0.05 | - | - | - | - |
| Total | 0.81 | 0.25 | 0.14 | 2.33 | 0.89 | 0.16 |
Bees were the insect group that made most visits to orchid flowers, whereas other groups (wasps, ants and butterflies) were rather occasional visitors (Table 4). Importantly, the large majority of floral visitors did not perform effective visits, i.e., they neither removed nor deposited pollinia. Only for C. dodsoniana were we able to document pollinia removal through videorecording; the insects that removed the pollinia were Euglossine bees, which also displayed behaviors of scent collection. Given the uncertainty in their species identities due to the lack of collected specimens, all the activity for Euglossine bees was lumped in Table 4. Nonetheless, when a specialist in this insect group (Dr. Ismael Hinojosa, Instituto de Biología, Universidad Nacional Autónoma de México) watched the videorecording, he suggested two potential identifications for the species that removed the pollinia: Eufriesea caerulescens (Lepeletier) and Euglossa cf. imperialis. Future work will need to confirm these identities.
Floral micromorphology. SEM micrographs of the epidermis of the three floral zones examined (labellum, column and the labellum-column intersection) revealed the presence of potentially secretory structures in the three species, although their shapes and locations varied among floral zones and species. For B. whartoniana we observed signs of secretion in intercellular spaces of the lateral zone of the labellum apex (Figure 2B, C). Moreover, open stomata were observed on the anther (Figure 2D, E), which had very likely lost the gas exchanging function, but which could represent a way to release secretions (Figure 2F). Moreover, in the labellum-column intersection (Figure 2G, H) we observed a series of grooves along the fringe of the column adjacent to the labellum (Figure 2I) that may also be interpreted as a secretion route (Figure 2J, K).
In the labellum of the flowers of C. dodsoniana we also observed secretory structures (Figure 3B). At the apex of the labellum there were small openings on the epidermis surface (Figure 3C), whereas in its middle portion only epicuticular detachments were perceptible (Figure 3D, E). At the labellum base other structures without openings were apparent, some of them with secretions apparently flowing out through cuticular cracks (Figure 3F, G); these granular secretions were uniformly distributed across this zone (Figure 3H). Epicuticular detachments were also observed in the area near the labellum base and they were often associated with spherical structures and granular material (Figure 3I, J), in addition to other substances (Figure 3K, L).
In C. macrobulbon, secretory structures occurred in the three areas of the examined flowers, although they were more evident on the labellum (Figure 4B). In the central portion of this structure the presence of trichome globose cells with uniformly distributed openings was noteworthy (Figure 4C, D), some of which were associated with possible secretions (Figure 4E). Trichome globose cells also occurred near the labellum base but these lacked openings (Figure 4F). In the column we only identified stomata located between the stigmatic cavity and the anther (Figure 4G, H). Finally, at the labellum-column intersection we recorded the presence of stomata uniformly distributed across this area (Figure 4I, J).
Discussion
Pollination success. In agreement with previous studies on various orchid species (e.g., Ackerman 1981, 1989, Zimmerman & Aide 1989, Roberts 2003, Aragón & Ackerman 2004, Sun et al. 2009), our results show that pollination success is extremely low in the three tropical dry forest orchid species studied here. This result is worrisome, particularly because some of the very few pollen depositions recorded may represent cases of geitonogamy (i.e., pollinia coming from the same plant), which may result in a higher proportion of non-viable seeds (Emeterio-Lara et al. 2018). Pollination failure seems to be exacerbated in the tropics, as orchid species from these regions have very low-density populations, with their individuals being more widely spread across the landscape (Ackerman 1986). This finding contrasts with reports of higher pollination success for orchids from temperate regions, with fruit set values in natural conditions as high as 60-80 % in these environments (Tremblay et al. 2005) vs. a mean value of 11.15 % in the tropics (Neiland & Wilcock 1998). Interestingly, Cyrtopodium macrobulbon, apparently the most abundant and spatially aggregated species among the three study orchids, and the one having the largest number of flowers per plant, had the lowest pollination success. The low fruit formation in other Cyrtopodium species supports the idea that this is a common phenomenon within the genus (Pansarin et al. 2008). Therefore, it is likely that the permanence of this species in the region is not based on efficient pollination, but rather on a large longevity of individuals associated with vegetative propagation and fruit production with tens of thousands of viable seeds (Pansarin et al. 2008, Sonkoly et al. 2016).
Environmental factors including temperature, precipitation, wind speed, solar radiation and humidity are known to affect pollinators’ foraging activity (Sabat & Ackerman 1996, Ferdy et al. 1998, Castillo et al. 2002, Jersáková et al. 2006, Sun et al. 2009). In this regard, the fact that visits to flowers were often interrupted by the strong winds that are frequent in the study site (Brennan et al. 2010, Jaramillo & Borja 2004) is revealing. Apparently, pollinator abundance is high in this region due to a continuous availability of floral resources all year round (Maldonado-Romo 2014). Besides, an ongoing study in the same region found that the abundance of one potential pollinator of Clowesia dodsoniana, the bee Euglossa viridissima Friese, is relatively common in this locality (S. Javier pers. comm.). Therefore, pollinator limitation is not a likely explanation for the low pollination success in our study species.
Floral display effects on pollination success. In the absence of rewards for pollinators, floral display may represent the most important source of variation in attracting pollinators (Calvo 1990). Nevertheless, for the two species studied that do not seem to offer floral rewards in large amounts, namely Barkeria whartoniana and C. macrobulbon, floral display is likely unimportant both for pollen deposition and removal. Thus, these results do not support the hypothesis that floral display determines pollen removal and deposition success in these two species. Unlike them, for C. dodsoniana, the only species for which we recorded potential floral rewards, the results for pollen removal supported the existence of a positive relation between floral display and pollen removal. Intriguingly, although the best supported model showed a positive effect of inflorescence length on pollen deposition, it also showed a negative effect of the number of flowers on this response variable. Further examination of pollination success for this species is required in order to clarify this issue.
Earlier studies on orchid pollination have shown highly variable effects of floral display on pollination success (Zimmerman & Aide 1989, Vale et al. 2011). For example, in Neotropical epiphytic orchids like Brassavola nodosa (L.) Lindl. (Schemske 1980), Ionopsis utricularioides (Montalvo & Ackerman 1987) and Lepanthes wendlandii Rchb. f. (Calvo 1990), fruit set is directly related to the number of flowers in the inflorescences. By contrast, in other tropical species like Aspasia principissa (Zimmerman & Aide 1989), Psychilis krugii (Bello) Saudela (Ackerman 1989) and Comparettia falcata (Rodríguez-Robles et al. 1992), reproductive success was unaffected by the number of flowers. These contradictory results may be explained, among other things (i.e., incompatible pollinations, resource limitations and predation) by the behavior of some pollinators, particularly of social bees. These insects can learn to differentiate flowers that provide them with rewards from those that fail to do so (Sabat & Ackerman 1996, O’Connell & Johnston 1998). Therefore, the precise duration of most studies, which normally does not include the entire flowering season and thus may fail to account for the insect learning period (Smithson & Macnair 1997, Castillo et al. 2002, Johnson et al. 2003), can influence the observed results. In our case, we found some evidence that this is the case for B. whartoniana, as pollen removal was higher at the beginning than at the end of the same flowering season. Future studies should attempt to cover the entire flowering periods of the different species to better understand this phenomenon.
Male vs. female pollination success. In the three study species male success (pollinia removal) was larger than female success (pollinia deposition), suggesting that fruit set is more strongly limited by the latter than by the former (Nilsson 1992, Brys et al. 2008, Li et al. 2011). Moreover, despite the large variation observed between seasons (B. whartoniana) or years (C. dodsoniana), it was evident that pollination success was contrasting among the three species: C. dodsoniana had the highest success both for the male and the female functions, whereas C. macrobulbon was considerably less successful, with B. whartoniana being in an intermediate position. These results are consistent with the recorded flower visitation rates and could be related to the potential presence of rewards in C. dodsoniana, in the shape of fragrances. Species not offering floral rewards are twice as likely to suffer pollination limitation as those species that do offer such rewards (Neiland & Wilcock 1998, Smithson & Gigord 2001, Johnson et al. 2005).
Floral visitors and pollinators. Only a few floral visitors recorded in this study acted as pollinators; yet, at least for C. dodsoniana there seems to be a correspondence between flower visitation rates and pollination success. The three studied species differ substantially with respect to their pollinator attracting mechanisms, which was reflected in the number of visits to the flowers, as well as in the visitors’ taxonomic affiliation. In selecting the study species, an important criterion was that they needed to share a melitophilic pollination syndrome, and in fact, bees were the most frequent and diverse group of visitors.
Despite the time spent in field observations, we could not identify the pollinator of B. whartoniana. Some studies have documented the role of the genus Xylocopa as potential pollinators of Barkeria species (van der Pijl & Dodson 1966, Stebbins 1970), and although these bees were not the most frequent visitors of B. whartoniana, they were the insects that seemed to stay longer on its flowers (but permanence time was not quantified). An unexpected result for B. whartoniana was the recurrent visits by a single hummingbird species, although no pollinia removal or deposition resulted from these visits. This is noteworthy as floral morphology in this orchid does not match the ornithophily syndrome (Stpiczyńska et al. 2004).
Bees were the most frequent visitors to C. macrobulbon flowers, especially those of the genus Trigona, but their small sizes make them unlikely pollinators. Although for this species we also failed to detect the effective pollinator, we suspect that it may be either Centris or Xylocopa bees, as species of these taxa are the confirmed pollinators of other Cyrtopodium species (Pansarin et al. 2008, Pemberton & Liu 2008, Dutra et al. 2009). Interestingly, our results suggest a weak relation between the diversity of floral visitor and pollination success given that this orchid species, which had the lowest pollination success, was visited by the largest number of animal species.
Likewise, for C. dodsoniana we were unable to identify the pollinators. However, through direct observation and videorecording we detected two Euglossine species that removed pollinia successfully (but without any record of pollinia deposition). The preliminary identifications for these species, namely Eufriesea caerulescens and Euglossa cf. imperialis, provided by the expert taxonomist in this insect group await confirmation by collection of specimens. Flowering in C. dodsoniana normally occurs from late May to early June, at the onset of the rainy season, in coincidence with the wet season activity peak of Euglossinae bees reported by Dressler (1982). However, considering that this species had the highest floral visitation rates and that it offers floral rewards, its flowers were relatively little visited compared to other reward-offering species from other tropical regions (Tremblay et al. 2005).
Floral micromorphology and pollination. This study provided evidence for the existence of potentially secretory structures in the three study species. Structures with secretory potential have been previously recorded for flowers of these genera, such as stomata in the column of Barkeria (Valencia-Nieto 2012), as well as elaiophores (oil or resin secreting glands) and osmophores (glands secreting aromatic compounds) in Cyrtopodium (Pansarin et al. 2008, 2009) and Clowesia (Warford & Harrell 1996). However, these secretory structures probably do not produce large amounts of substances to be considered as true rewards. Only for C. dodsoniana can the offer of floral rewards be confidently stated, as its flowers release intense fragrances, similar to those confirmed to be rewards offered by other congeneric species (Aguirre-León 1979). This possibility is further supported by the fact that over half of all floral visitation records for this species are from Euglossine bees, as well as by the fragrance collecting behavior displayed by them (Eltz et al. 1999).
The production of oils and resins has been only recorded in five orchid subtribes: Bifrenariinae (Davies & Stpiczyńska 2006), Catasetinae (Davies et al. 2006, Mickeliunas et al. 2006, Franken et al. 2016), Cyrtopodiinae (Pansarin et al. 2008, 2009), Maxillariinae (van der Pijl & Dodson 1966, Davies & Turner 2004) and Oncidiinae (Stpiczyńska & Davies 2008). In C. macrobulbon, SEM micrographs led to the detection of possible osmophores or elaiophores. As said, the presence of these cellular structures in its flowers does not necessarily imply the production of large volumes of rewards, as suggested by the findings for other species of the genus (Chase & Hills 1992, Pansarin et al. 2008, 2009, Dutra et al. 2009, Franken et al. 2016). That these plants are likely to produce oils is also supported by the behavior of the eusocial Trigona bees, which often visit flowers in searching for wax and resins that they use to construct their hives (Michener 1974, Roubik 2000). Some authors have suggested that Cyrtopodium flowers can be mimetic of Malpighiaceae flowers (Pemberton 2008, Pansarin et al. 2008, Maciel et al. 2020), as these flowers also produce wax collected by nest-constructing bees; therefore, this is a possibility worth investigating in the future for C. macrobulbon.
With a naked eye, we did not perceive the presence of any floral rewards in B. whartoniana; however, the use of SEM produced three types of evidence that led us to assume the existence of secretory structures in flowers: (1) intercellular secretions in the labellum; (2) stomata; and (3) grooves between the cells of the column adjacent to the labellum. SEM micrographs also revealed the presence of secretions with a crystalline appearance on the labellum; if they were sugars, they could be interpreted as small portions of floral rewards for pollinators (Fahn 1988).
The occurrence of stomata is uncommon in the anthers of Angiosperm flowers (Rudall 2007). Yet, atrophic stomata like those observed in B. whartoniana have been related to the secretion of nectar in other plant families (Razem & Davis 1999, Nepi et al. 2003). Among orchids, earlier reports of the presence of stomata in anthers exist for the genera Barkeria, Maxillaria and Acampe, and in the latter two, stomata seem to have a secretory function (Stpiczyńska et al. 2004, Davies et al. 2005, Telepova-Texier 2009). Within Barkeria, the presence of actinocytic stomata in anther lobes has been reported for B. uniflora Dressler & Halb., but the lack of scents and nectar in its flowers suggests that they do not offer any reward (Valencia-Nieto 2012).
Final remarks. The complexity of the orchid-pollinator interaction underscores the need to make comprehensive assessments of different aspects of both orchid floral biology and pollinator behavior. For example, in this study it was revealing that the visits by hummingbirds to the flowers were exclusively observed through video recording. This implies that the observer’s presence near orchid plants may discourage some pollinators from visiting their flowers, thus pointing to the need of using video recording to strengthen orchid pollination studies. More importantly, examination of flower microstructure revealed the presence of potentially secretory structures in the three species, though only C. dodsoniana seems to offer true floral rewards. Yet, it is likely that the secretion of small amounts of rewards in B. whartoniana and C. macrobulbon are actually a component of a pollination by deception mechanism (Dafni 1984, Jersáková et al. 2006). Taken together, these results call for the need to reformulate the notion of “all-or-nothing” regarding the production of floral rewards in establishing a potential pollination mechanism.
The ecology of the orchid-pollinator interaction is a multifaceted topic that involves different population dynamics that need to be finely synchronized if the system is to function properly. In the case of our study species, their future permanence in the seasonally dry tropics, which are marginal habitats for epiphytic and lithophytic orchids, will entirely depend on the existence of large numbers of reproductive individuals, as well as on the continuous availability of abundant pollinators (Coombs et al. 2009). Reductions in the population size of either partner of this interaction, i.e. either the plant or its pollinators, are worrisome in the face of pervasive processes currently threatening these ecosystems, including deforestation and land use/land cover change, the unchecked extraction of plants from the wild, the indiscriminate use of insecticides and the resulting pollinator decline, and ultimately, the relentless global change (Schweiger et al. 2010, González-Varo et al. 2013, Kaye et al. 2019).











 nueva página del texto (beta)
nueva página del texto (beta)



