The amount of reactive nitrogen (Nr) species available on the planet has increased as a result of human activities, such as fossil fuel combustion and the utilization of nitrogenous fertilizers (Galloway et al. 2003, 2004). Indeed, the release of Nr to the environment increased from 33 Tg N year-1 in 1860 to 156 Tg N year-1 in 1990, and it is anticipated that this rate will double by 2050 (Galloway et al. 2004). Such alterations to the nitrogen biogeochemical cycle are a major component of global environmental change and a principal threat to the planet's biodiversity (Sala et al. 2000, Rockström et al. 2009). In particular, given that many plants evolved in environments with limited nitrogen availability (Lee & Caporn 1998), the increase of this macronutrient has enabled those species capable of attaining rapid growth rates and of tolerating the ensuing toxic ion buildup to become better ecological competitors (Bobbink et al. 1998, 2010, Stevens et al. 2004, Farrer et al. 2013). In addition, the deposition of reactive nitrogen species is related to eutrophization, soil acidity, and cation leaching (DeHayes et al. 1999, Kronzucker et al. 2001, Britto & Kronzucker 2002, Galloway et al. 2003, Conklin 2005, Gruber & Galloway 2008, Bobbink et al. 2010, Persson et al. 2010, Tian et al. 2016). However, nitrogen deposition is not only linked with ecosystem and environmental problems, as it also has noxious effects on human health, such as intoxication by a high concentration of NO3 - and NO2 in drinking water that affects the early developmental stages of our development (Carpenter et al. 1998, WHO 2003). Reactive nitrogen is thus an important component of pollution, which leads to 16 % of premature deaths worldwide (Landrigan et al. 2018, Yeo et al. 2019).
Given the multiple noxious effects of environmental pollution, it becomes necessary, and in some instances required by law, to monitor and control the levels of pollutants that are released to the environment. However, for the case of atmospheric pollution, the deployment, operation, and maintenance of monitoring systems is complicated and can be cost-prohibitive for local governments (SEMARNAT 2012, Díaz-Álvarez et al. 2018). In this respect, the utilization of naturally occurring biomonitors has been proposed as an alternative for localities where air quality monitoring systems are lacking (Arróniz-Crespo et al. 2012, Felix et al. 2016, Díaz-Álvarez et al. 2018). Indeed, various ecophysiological traits can be utilized to characterize nitrogen deposition in regions of interest, including an increase in tissue nitrogen content and a subsequent imbalance in the C/N ratio, as well as changes in the activity of enzymes related to the nitrogen metabolism, such as nitrate reductase, and the rate of 15N isotopic discrimination (Sutton et al. 2004, Arróniz-Crespo et al. 2008, Felix et al. 2016, Díaz-Álvarez & de la Barrera 2018, Díaz-Álvarez et al. 2015, 2019, 2020). In general, an increase in nitrogen availability from atmospheric deposition can improve biomass accumulation and reduce the allocation to below-ground tissues relative to aerial tissues (Li et al. 2015). Additional nitrogen can be stored in the inorganic form within organelles or as Rubisco, leading to an increase of chlorophyll content and the plant's photosynthetic capacity (Arróniz-Crespo et al. 2008, Jin et al. 2015, Tegeder & Masclaux-Daubresse 2018). However, when nitrogen reaches a species-specific threshold, plants manifest symptoms of stress, such as changes in pH, membrane function and integrity, and energy deficiencies, which can be reflected in the maximum quantum yield of photosystem II (Fv/Fm) and the chlorophyll a/b ratio (Kronzucker et al. 2001, Britto & Kronzucker 2002, Arróniz-Crespo et al. 2008).
Plant species that rely exclusively or predominantly on atmospheric sources of mineral nutrition are particularly suited for biomonitoring. Such is the case for the bromeliad Tillandsia recurvata L. that can track dry nitrogen deposition, especially NOx and particulate matter (Díaz-Álvarez & de la Barrera 2018), and various bryophytes that, in turn, are useful biomonitors of wet nitrogen deposition (Arróniz-Crespo et al. 2008, Díaz-Álvarez & de la Barrera 2018, Díaz-Álvarez et al. 2019, 2020). However, these so called "atmospheric biomonitors" cannot be utilized in all localities for various reasons, including that the abundance of epiphytes decreases away from the humid tropics (Zotz & Bader 2009), that mosses require high humidity environments to maintain physiological function (Glime 2017a), and that the prevalent pollution can be too high for certain species, as it occurs for T. recurvata in certain regions of Mexico City (Díaz-Álvarez & de la Barrera 2018).
Ruderal weeds are a group of plants that successfully establish in high-pollution environments, such as those found in cities, at least seasonally. These plants have also been utilized as pollution biomonitors, despite that their root system is anchored to the ground, having access to existing nutrients from sources different from atmospheric deposition (Norra et al. 2005, Wang & Pataki 2010).
Based on the hypothesis that plant physiological attributes will respond to an increase in the availability of reactive inorganic nitrogen species, we conducted a dose-response greenhouse experiment to screen some ruderal weeds as potential biomonitors of nitrogen deposition based on their physiological responses to nitrogen deposition. We expect that higher nitrogen availability will lead to increased biomass accumulation, tissue nitrogen and chlorophyll content, as well as higher rates of isotopic discrimination of 15N, due to the fertilization effect. Also, the BS/BA and C/N ratios, and the nitrate reductase enzyme activity are expected to decrease. In turn, as responses to stress due to an increase in the availability of N and its toxicity, the chlorophyll a/b ratio, and the Fv/Fm are expected to decrease.
Material and methods
Experimental setup. Seeds of eleven ruderal weeds that are common in the city of Morelia, Michoacán, México (Table 1) were collected in October 2017 and 2018. The seeds were placed in paper envelopes (8.8 × 16.4 cm) and stored in the laboratory (in the dark, air temperature of 23 ºC, and relative humidity of 40 %) until they were utilized in February 2019.
Table 1 Ruderal weeds common in Morelia, Michoacán, México, whose biomonitoring potential was evaluated.
| Species | Family | Class | Origina |
|---|---|---|---|
| Amaranthus hybridus L. | Amaranthaceae | Magnoliopsida | Native |
| Bidens pilosa L. | Asteraceae | Magnoliopsida | Native |
| Chloris gayana Kunth | Poaceae | Liliopsida | Exotic |
| Chloris pycnothrix Trin. | Poaceae | Liliopsida | Exotic |
| Chloris virgata Sw. | Poaceae | Liliopsida | Exotic |
| Lepidium virginicum L. | Brassicaceae | Magnoliopsida | Native |
| Melinis repens L. | Poaceae | Liliopsida | Exotic |
| Pennisetum ciliare (L.) Link | Poaceae | Liliopsida | Exotic |
| Pennisetum setaceum (Forssk.) Chiov. | Poaceae | Liliopsida | Exotic |
| Sporobolus indicus (L.) R. Br. | Poaceae | Liliopsida | Native |
| Taraxacum officinale (L.) Weber ex F.H.Wigg. | Asteraceae | Magnoliopsida | Exotic |
The experiment was conducted in a greenhouse at the Escuela Nacional de Estudios Superiores, Unidad Morelia, Universidad Nacional Autónoma de México, Morelia, Michoacán, Mexico, where the plants were exposed to an air temperature averaging 17 ºC throughout the experiment (range of 2 to 36 ºC), a relative humidity of 59 % (5-97 %), and a daily photosynthetic photon flux (wavelengths of 400 to 700 nm) of 18.6 mol m-2 day-1. Plants were exposed to four treatments that simulated nitrogen deposition rates of 10, 20, 40, and 80 Kg N ha-1 year-1 over 120 days after sowing. Each treatment had six replicates per species. The experimental units of all species were randomly distributed on a greenhouse bench (dimensions of 15 × 1.5 m) to avoid blocking effects on individual species. For each experimental unit, at least three seeds were sown in plastic pots (volume of 1.5 liters) containing agrolyte. Following germination, the most vigorous individual was kept for the experiment, while the remaining seedlings were removed. Two sets of each experimental unit were prepared, considering that part of the experiment involved destructive sampling of plant material.
Nitrogen deposition. Because ruderal weeds are predominantly active during the rainy season, the experiment evaluated responses to wet deposition simulated with aqueous solutions of NH4NO3. In particular, the total amount of nitrogen that would deposit under rates of 10, 20, 40, and 80 Kg N ha-1 year-1, were administered over four months, the typical duration of the rainy season in the study region (Morelia has a C(w) climate, i.e., temperate, subhumid, with summer rains, INEGI 2017). Taking into consideration an opening area of 9.5 × 10-3 m2, each pot was watered daily, during 120 days, with 140 ml of 0.0161, 0.032, 0.64, or 0.128 mM NH4NO3. Additionally, the plants received weekly irrigations with 140 ml of a modified 0.1 Hoagland solution lacking nitrogen, in order to avoid nutrient deficiencies (Nobel & de la Barrera 2002). At the end of the 120-days, the biological material was harvested and analyzed.
Biomass. The plants were harvested at the end of the experiment and dried at 45 ºC in a gravity convection oven until reaching constant weight. Below-ground (BS) and above-ground (BA) dry mass were determined separately, in order to calculate the BS/BA ratio, as well as the total biomass accumulation for each individual.
Elemental and isotopic analyses. Plant material was harvested, dried to constant weight in the gravity convection oven at 60 ºC, and ground to a fine powder prior to submission to the Stable Isotope Facility, University of Wyoming for elemental and isotopic analyses that were conducted with a Costech 4010 elemental analyzer (Costech Analytical Inc., Valencia, California, USA) attached to a continuous flow isotope ratio mass spectrometer (Finnigan Delta Plus XP, Thermo Electron Corp, Waltham, Massachusetts, USA). The analytical precision was 0.4 ± 0.03 (SD) for the δ15N.
Chlorophyll. Chlorophyll content was determined colorimetrically (Lichtenthaler 1987). Freshly harvested leaf samples were macerated with cold acetone (80 % v/v in distilled water), and brought to a final volume of 3.0 ml. The absorbance of filtered aliquots was measured with an EZ 301 Spectrometer (Perkin Elmer, Waltham, Massachusetts, USA).
The maximum quantum yield of photosystem II (Fv/Fm; Maxwell & Johnson 2000), was measured with a FluorPen FP 100 hand-held fluorometer (Photon Systems Instruments, Drasov, Czech Republic), for plants that had been dark acclimated for 20 min by covering the entire pot with a brown paper bag that lined with aluminum foil (DeEll & Toivonen 2011).
Nitrate reductase activity (NR). The enzymatic activity of the nitrate reductase was quantified by colorimetry, based on the Greis-Ilosuay reaction (Díaz-Álvarez et al. 2019). Leaf samples were incubated in a 3 mM KNO3 solution during 12 hr, followed by the addition of 5 ml of a potassium buffer solution (50 mM KH2PO4, 100 mM KNO3, 100 mM potassium acetate, and 1.5 % v/v propanol-1-ol). After 1-2 min, the vials were emptied and incubated in an orbital shaker at 30 ºC during 30 min, before reading absorbance at 540 nm with the EZ 301 Spectrometer.
Data analyses. Plant survival throughout the experiment was analyzed with Friedman repeated measures ANOVAs, followed by post hoc Tukey tests (P ≤ 0.05). For the rest of the parameters, i.e., biomass production, BS/BA, NR activity, chlorophyll content, chlorophyll a/b ratio, Fv/Fm, δ15N, C/N ratio, N, and C content, plant responses were analyzed one-way ANOVAs followed by post hoc Tukey or Student's t tests (P ≤ 0.05). When the normality and variance homogeneity requirements were not fulfilled, data were analyzed with Kruskal-Wallis tests followed by Tukey or Dunn tests (P ≤ 0.05). Data are shown as mean ± 1 S.E. (n = 6). Statistical analyses were conducted with SigmaStat 3.5 (Systat Software Inc., San Jose, California).
Results
Survival for the weeds considered in the present work had different responses to the experimental treatments over 120 days after sowing (Figure 1; Supplementary material). In particular, the lowest dose of 10 kg N ha-1 year-1 led to the highest survival for Pennisetum ciliare (L.) Link (Figure 1C) but to the lowest survival for Chloris pycnothrix Trin. (Figure 1G), and Melinis repens (Willd.) Zizka (Figure 1B). In turn, the survival for Amaranthus hybridus L. (Figure 1A), Lepidium virginicum L. (Figure 1I), and Pennisetum setaceum (Forssk.) Chiov. (Figure 1J), was the highest under some of the intermediate scenarios of nitrogen deposition, and the lowest survival for Sporobulus indicus (L.) R. Br. (Figure 1D). The highest dose of 80 Kg N ha-1 year-1 led to the lowest survival for P. setaceum (Figure 1J), but to the highest survival for Chloris virgata Sw. (Figure 1H) and Taraxacum officinale (L.) Weber ex F.H.Wigg (Figure 1K). Finally, the survival for Bidens pilosa L. (Figure 1E; P = 0.029) and Chloris gayana Kunth (Figure 1F) did not respond to the experimental treatments and it was very low throughout the experiment.
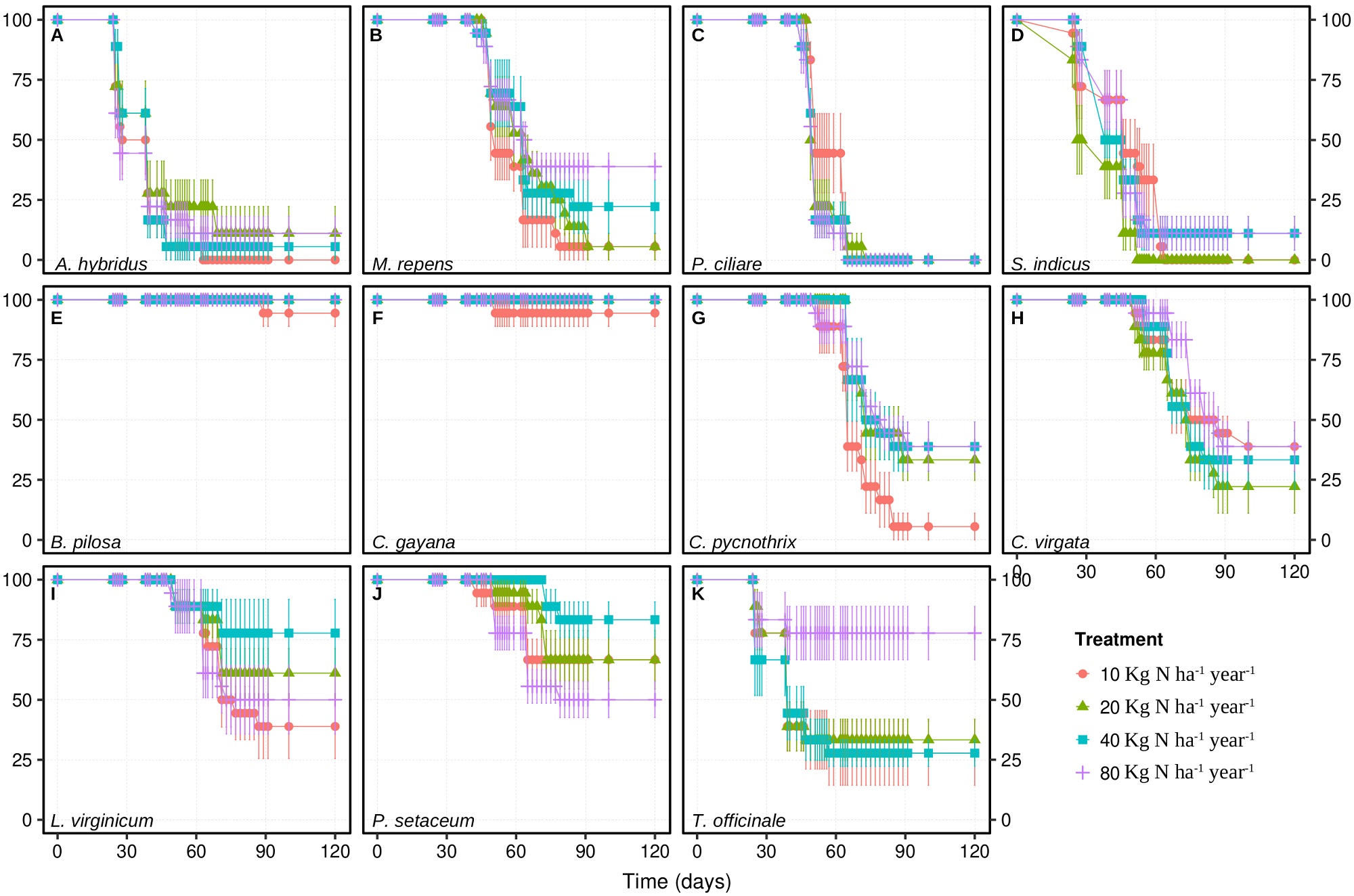
Figure 1 Survival (%) over 120 days for 11 potential ruderal weed biomonitors under experimental nitrogen deposition. Data are shown as mean ± 1 standard error (n = 6). Please refer to the Supplementary material for data analyses.
Given that only seven out of the eleven species that were evaluated had a final survival of at least 33 % after 120 days, i.e., Bidens pilosa (Figure 1E), Chloris gayana (Figure 1F), C. pycnothrix (Figure 1G), C. virgata (Figure 1H), Lepidium virginicum (Figure 1I), Pennisetum setaceum (Figure 1J) and Taraxacum officinale (Figure 1K), the remaining four species were excluded from further physiological screening.
Similar to the case for survival, the sensitivity of the various physiological parameters evaluated responded differently for each species under the different nitrogen doses (Figure 2; Supplementary material). Biomass accumulation tended to increase under the higher nitrogen doses for Bidens pilosa (Figure 2A), Chloris gayana (Figure 2B), Lepidium virginicum (Figure 2E), and Pennisetum setaceum (Figure 2F), with an ensuing decrease in the BS/BA ratio for the former two. In addition, the tissue carbon content increased with the nitrogen dose for C. gayana and P. setaceum, but it decreased for C. pycnothrix (Figure 2C).
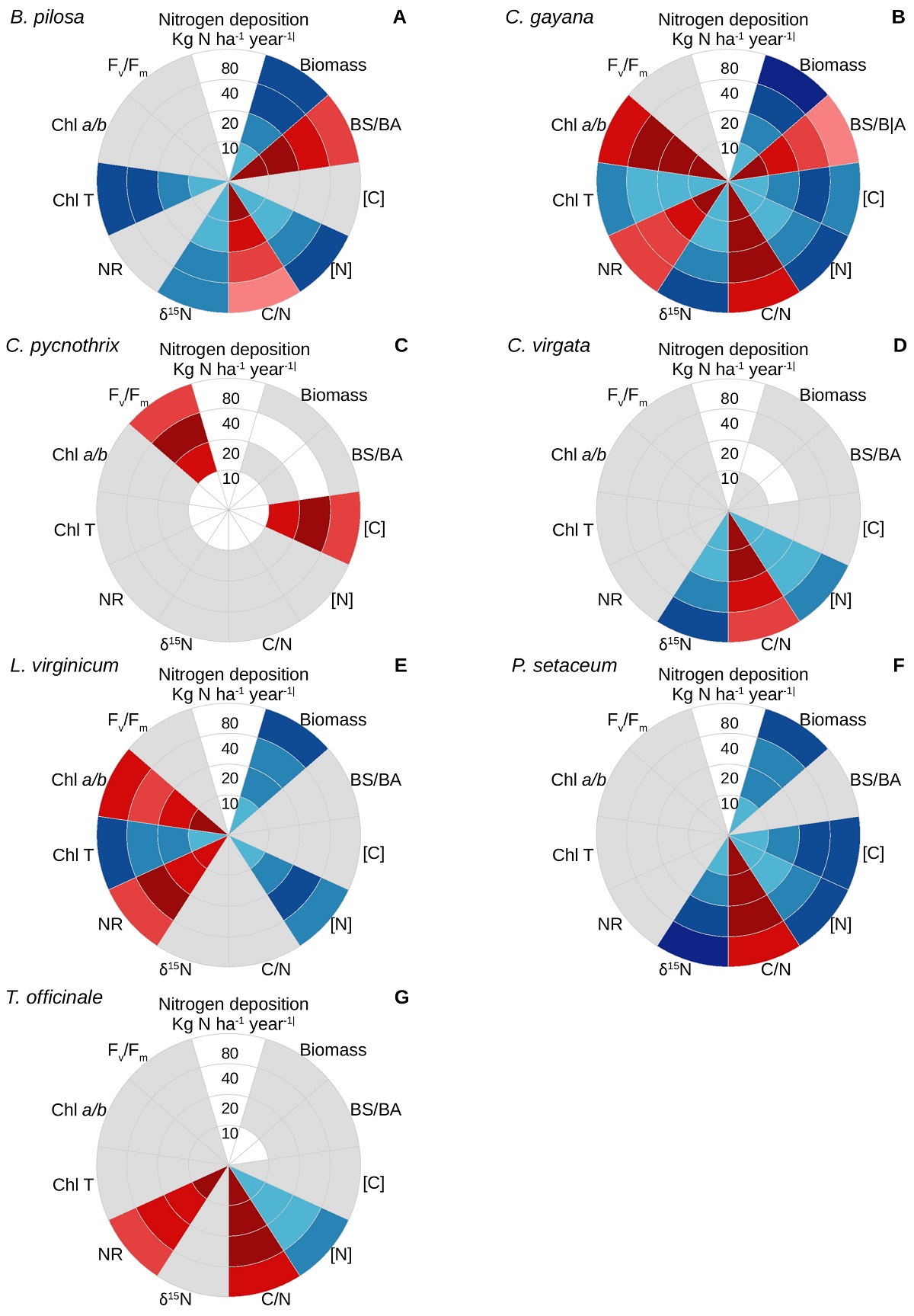
Figure 2 Physiological effects of experimental nitrogen deposition on the ruderal weeds (A) Bidens pilosa, (B) Chloris gayana, (C) C. pycnothrix, (D) C. virgata, (E) Lepidium virginicum, (F) Pennisetum setaceum, and (G) Taraxacum officinale. For each species, the nitrogen dose increases outwards. Blue indicates that a given parameter had a direct response to the dose utilized, red indicates an inverse response, and grey indicates a lack of response to the different nitrogen doses. For each parameter, different color intensities indicate statistical differences in the magnitude of the response (P < 0.05). Please refer to the Supplementary material for the specific responses and data analysis of each parameter.
In general, the nitrogen content of the seven weeds tended to increase with the nitrogen dose, except for C. pycnothrix, which did not respond to the experimental treatment, and for L. virginicum, for which the tissue nitrogen content was maximal under the intermediate dose of 40 kg ha-1 year-1. The higher tissue nitrogen content was followed by a decrease in the C/N ratio for B. pilosa, C. gayana, C. virgata (Figure 2D), P. setaceum, and T. officinale (Figure 2G). In turn, the δ15N values tended to become less negative under the higher nitrogen doses for B. pilosa, C. gayana, C. virgata, and P. setaceum, but remained unaffected for the other three species. The activity of the nitrate reductase tended to decrease with the nitrogen dose for C. gayana and T. officinale, it reached its maximum under 40 kg ha-1 year-1 for L. virginicum and remained unaffected for the other species.
The total chlorophyll content tended to increase with the nitrogen dose for B. pilosa, C. gayana, and L. virginicum. The chlorophyll a/b ratio decreased for C. gayana under the nitrogen doses above 10 kg N ha-1 year-1, and for L. virginicum growing under 80 kg N ha-1 year-1. Finally, Fv/Fm for dark-adapted leaves did not respond to nitrogen deposition, except for C. pycnothrix, for which it decreased under 80 kg N ha-1 year-1.
Discussion
While most of the species screened here responded to the experimental nitrogen deposition, a generalized pattern was not observed, neither for the sensitivity of the physiological parameters that were measured nor by groups of species. For instance, one could have expected that exotic species, especially those reported as invasive would have fared better than the natives at higher nitrogen doses (van der Maarel 2005, Perry et al. 2010). However, this was not the case, as the survival of some of the weeds, i.e., Bidens pilosa and Chloris gayana, were insensitive to nitrogen addition, others, i.e., Chloris pycnothrix and Taraxacum officinale, had a decreased mortality with increasing fertilization, while Pennisetum ciliare, a very noxious invasive weed, succumbed under all the treatments. Even C. pycnothrix, an alien species, showed stress effects due high nitrogen deposition (a reduced Fv/Fm) than some of the native species. Such an idiosyncratic response to nitrogen deposition is prevalent in nature: while the survival of species such as the grass Deschampsia flexuosa (L.) Trin., the shrub Calluna vulgaris (L.) Hull, and the trees Fraxinus americana L., Malus coronaria L., and Schima superba Gardner & Champ. are insensitive to nitrogen addition (van den Berg et al. 2005, McWhirter & Henry 2014, Han et al. 2019), plant mortality decreases with nitrogen availability for Elaeagnus umbellata Thunb., Erodium oxyrhinchum M. Bieb., and Robinia pseudocacia L. (McWhirter & Henry 2014, Horn et al. 2018, Chen et al. 2019a), but it increases for species such as Succisa pratensis L., Antennaria dioica L., Pinus massoniana D. Don, Pouteria torta (Mart.) Radlk., and 39 species of trees (van den Berg et al. 2005, Cárate-Tandalla et al. 2015, Horn et al. 2018, Han et al. 2019). Such an increased mortality in nitrogen-rich environments has been attributed to a low tolerance to NH4 +, whose accumulation leads to acidification (Britto & Kronzucker 2002, van den Berg et al. 2005), as well as to energy deficits resulting from the cost of extruding excess NH4 + out of the cell (Kronzucker et al. 2001, Britto & Kronzucker 2002, van den Berg et al. 2005). The responses of individual species lead to changes in their distribution, which combined alter the composition of plant communities (Gotelli & Ellison 2002, Horn et al. 2018).
Some of the responses observed here, however, could simply be attributed to each species' habitat preference. For instance, Melinis repens and Taraxacum officinale have been found to establish successfully in urban environments, as long as the ground has sufficient amounts of litter or nurse plants are available (Cavieres et al. 2005, David & Menges 2011). Also, a high mortality is inherent of species that produce a large number of propagules with a low investment of maternal resources (Ricklefs 2009), especially considering the high vulnerability of plants during early developmental stages (de la Barrera et al. 2009). This could be the case, for example, of Pennisetum ciliare, whose mortality reached 100 % in the present study, but which becomes insensitive to nitrogen fertilization once it has become established (Lyons et al. 2013).
As expected, a higher nitrogen availability increased biomass accumulation for some of the weeds, i.e., Bidens pilosa, Chloris gayana, Lepidium virginicum, and Pennisetum setaceum, a response that has also been documented for Agropyron cristatum (L.) Gaertn., Anthoxanthum odoratum L., Avena fatua L., Centaurea stoebe L., Hordeum murinum L., Lolium perenne L., Medicago lupulina L., Plantago lanceolata L., Poa annua L., Prunella vulgaris L., Stipa pulchra Hitchc., and Trifolium repens L. (Jiang et al. 2005, Tian et al. 2012, Stevens & Gowing 2014, Peng et al. 2016, Tulloss & Cadenasso 2016, Shen et al. 2019). Such an improvement of primary productivity in response to fertilization is common when plants develop in nutrient-limited soils (Azcón-Bieto & Talón 2008, van der Valk 2009, Taiz et al. 2014). As additional nitrogen becomes available, biomass accumulation can increase linearly until a threshold is reached, either by a saturation of the response, an intrinsic limitation of the plant, the intracellular buildup of toxic ions, or by a co-limitation of other nutrients (Azcón-Bieto & Talón 2008, Taiz et al. 2014, J. Mao et al. 2018a). This was probably the case for Chloris pycnothrix, C. virgata, and Taraxacum officinale, whose dry mass accumulation did not respond to nitrogen fertilization. Insensitivity of growth to fertilization has also been documented for species such as Amaranthus spinosus L., Elaeagnus umbellata, Elymus caput-medusae L., Eremopyrum orientale (L.) Jaub. & Spach, Fraxinus americana, Malus coronaria, Plantago virginica L., Rhus typhina L., and Schima superba (Jiang et al. 2005, McWhirter & Henry 2014, Tulloss & Cadenasso 2016, Chen et al. 2019b, Han et al. 2019).
Resource allocation to belowground biomass that increases the BS/BA ratio is also a response of plants that grow in nutrient-poor soils, as a higher root surface area improves the ability to take up nutrients (Litton et al. 2003, Taiz et al. 2014). In turn, a higher nitrogen availability usually leads to a reduction of BS/BA, a response that has been observed in species such as Nepeta micrantha Bunge, Oriza sativa L., and that is common in forest species (Li et al. 2015, Mao et al. 2018b, Chen et al. 2019b, Wang et al. 2019). Despite that a reduction in BS/BA was only significant for two of the weeds, the remaining five displayed an apparent trend in the same direction, similar to what occurs for Amarathus spinosus, Eremopyron orientale, Lolium perenne, Medicago lupulina, Poa annua, Prunella vulgaris, and Trifolium repens (Jiang et al. 2005, Stevens & Gowing 2014, Chen et al. 2019a). However, a lack of response of BS/BA can be attributed to limitation of other soil nutrients, such that an investment in root tissue still improves soil exploration, potentially conferring a better competitive capacity (Tulloss & Cadenasso 2016).
The nitrate reductase enzyme reduces the oxidation level of NO3 - to NO2 -, catalyze one of the early steps in the nitrogen assimilation (Azcón-Bieto & Talón 2008, Tegeder & Masclaux-Daubresse 2018). This enzyme was affected by the nitrogen deposition in Chloris gayana and Taraxacum officinale, where the increase of nitrogen deposition rate reduced their activity, as also we can see in Acer saccharum Marshall and the bryophytes Braunia secunda (Hook.) Bruch & Schimp., Leptodontium pungens (Mitt.) Kindb., Racomitrium lanuginosum (Hedw.) Brid., and Rhytidiadelphus squarrosus (Hedw.) Warnst. (Pearce & van der Wal 2002, Arróniz-Crespo et al. 2008, Tang et al. 2012, Díaz-Álvarez et al. 2019). This reduction in the NR activity, under ascending scenarios of nitrogen deposition, is due to the increase of reduced nitrogen compounds in the tissues that inhibit their synthesis and activity (Downs et al. 1993, Arróniz-Crespo et al. 2008, Coelho & Romão 2015, Glime 2017b). In bryophytes, the decrease in activity is attributed to the available NH4 + satisfying the nitrogen demand, while in vascular plants it is attributed to intrinsic properties of species as growth rate and state of development (Downs et al. 1993, Arróniz-Crespo et al. 2008, Tang et al. 2012, Glime 2017b). Species that kept their NR activity constant under the scenarios of nitrogen deposition as Bidens pilosa, Chloris pycnothrix, Chloris virgata and Pennisetum setaceum, perhaps the NH4 + amount, that contributed the NH4NO3 with the treatments of nitrogen deposition, plus the NO3 - reduced were enough to satisfy the nitrogen demand keeping the NR enzyme activity constant. The null effect of the nitrogen deposition in the NR enzyme has also been seen in Acer rubrum L., Ardisia quinquegona Blume, Betula alleghaniensis Britton, Blastus cochinchinensis Lour., Fagus grandifolia Ehrh., Pinus strobus L., P. rigida Mill., Tillandsia recurvata L., and the bryophytes Pleurochaete squarrosa (Brid.) Lindb. and Pseudoscleropodium purum (Hedw.) M. Fleisch. (Downs et al. 1993, Pearce & van der Wal 2002, Pearce et al. 2003, Arróniz-Crespo et al. 2008, Tang et al. 2012, Ochoa-Hueso & Manrique 2013, Liu et al. 2018b, Díaz-Álvarez et al. 2020).
The higher nitrogen content measured under higher doses for six of the weeds has also been reported for the mosses Pseudoscleropodium purum and Rhytidium rugosum (Hedw.) Kindb., and for the vascular plants Calluna vulgaris L., Eucalyptus urophylla S. T. Blake × E. grandis Hill ex Maiden, Laelia speciosa (Kunth) Schltr, Oxytropis kansuensis Bunge, and Pinus resinosa L. (Throop & Lerdau 2004, Arróniz-Crespo et al. 2008, Bobbink et al. 2010, Du et al. 2014, 2015, Díaz-Álvarez et al. 2015, Lü et al. 2016). The nitrogen level in plant tissues is driven by vacuolar accumulation of reduced nitrogen species and hydrosoluble proteins, such as Rubisco (Zhang et al. 2016, Tegeder & Masclaux-Daubresse 2018). Nitrogen fertilization can also increase the PEPcarboxilase activity for C4 and CAM species, leading to a concurrent increase of tissue carbon content, as it was observed here for Chloris gayana, C. pycnothrix, and Pennisetum setaceum (Jin et al. 2015, Flexas et al. 2016, Tegeder & Masclaux-Daubresse 2018, Zhou et al. 2020).
The decrease in the C/N ratio in response to nitrogen fertilization that we found for Bidens pilosa, Chloris gayana, Chloris virgata, Taraxacum officinale, and Pennisetum setaceum, which also occurs for Schizolobium amazonicum Ducke, Zea mays L, as well as for various species in the Cleistogenes and Stipa genera, is a direct result of the nitrogen buildup described above (Chen et al. 2009, Luo et al. 2017, Vieira et al. 2018). In turn, the fact that the C/N ratio did not change for Lepidium virginicum and Chloris pycnothrix appears to be a consequence of the development of new plant tissue, which has also been documented for Betula pendula Roth, Agrostis capillaris L., and Galium saxatile L. (Stevens et al. 2011, Harmens et al. 2017).
The increased δ15N values in response to higher nitrogen deposition rates such as those observed for Bidens pilosa, Chloris gayana, Chloris virgata, and Pennisetum setaceum, are opposite to those reported for species such as Laelia speciosa and Pinus massonia Lamb., whose leaves become increasingly impoverished in 15N under higher nitrogen availability (Jiang & Zhang 2009, Díaz-Álvarez et al. 2015). An isotopic impoverishment of plant tissues indicates an enhanced discrimination of 15N under an abundance of nitrogen resulting from increased enzyme-mediated processes (Yoneyama et al. 1991, Santiago et al. 2005, Xiao et al. 2011). In the present work, however, the negative δ15N values found under the lower nitrogen doses can be an indication of high enzymatic activity, as it was the case, for instance, for Chloris gayana whose lowest δ15N values occurred concurrently with its highest nitrate reductase activity. Thus, our results could be an indicator of enzyme saturation that prevented further nitrogen uptake. In addition, the observed isotopic enrichment may also be reflecting an increased loss of excess nitrogen in the plant by NH4 + volatilization or NO3 - leaching (Högberg & Johannisson 1993, Dijkstra et al. 2003, Jiang & Zhang 2009, Ma et al. 2012).
The higher chlorophyll content found for Bidens pilosa, Lepidium virginicum, and Chloris gayana, in response to increasing nitrogen availability is common in nature, and has been observed for species such as Ardisia quinquegona, Camellia japonica L., Fraxinus mandshurica Rupr., Lindera aggregata (Sims) Kosterm., Pleurochaete squarrosa, and Populus cathayana Rehder. (Arróniz-Crespo et al. 2008, Wang et al. 2012, Yuan et al. 2017, C. Liu et al. 2018a). However, in other cases the chlorophyll content is insensitive to the prevalent nitrogen availability, as it occurs for species like Calamagrostis angustifolia Kom., Quercus acutissima Carruth., Blastus cochinchinensis, Cryptocarya chinensis (Hance) Hemsl., C. concinna Hance, Randia canthioides Champ. ex Benth., and Populus deltoides W. Bartram ex Marshall, or as found here for as found for Chloris pycnothrix, C. virgata, Pennisetum setaceum, and Taraxacum officinale (Dou et al. 2009, Li et al. 2018, Liu et al. 2018b, Liu et al. 2018c, Mao et al. 2018b, Xu et al. 2018). For these cases, chlorophyll content may be driven by other environmental factors, especially the prevalent photon flux density, which was relatively high in our experimental setup, or that the luxury nitrogen be allocated for chlorophyll production in new tissue (Azcón-Bieto & Talón 2008, Taiz et al. 2014). A decrease in the chlorophyll a/b ratio, such as what we observed for Lepidium virginicum and Chloris gayana, is an indication of an increased resource allocation to the light harvesting complex than to the photosystem reaction centers, leading to a reduced photosynthetic capacity, as it has been described for Pseudoscleropodium purum and Rhytidiadelphus squarrosus (Arróniz-Crespo et al. 2008, Lambers et al. 2008, Ochoa-Hueso et al. 2014).
The lack of response of Fv/Fm to nitrogen that was observed for most of the weeds suggests that its increased availability did not have a fertilization effect on photosynthesis, but, conversely, it did not impose stress either, including at the higher doses (Maxwell & Johnson 2000, DeEll & Toivonen 2011). The lack of response to high rates of N deposition can be associated with a more competitive behavior and greater tolerance of plants, as it has been found for invasive alien species (Lyons et al. 2013). In contrast, for the case of C. pycnothrix, the significant decrease of Fv/Fm for plants under 80 kg ha-1 year-1 was concurrent with the lowest carbon content for this species. This indicates a decreased use of light energy and photosynthesis under high N deposition rates, a stress that can lead to the decline of plant populations (Arróniz-Crespo et al. 2008).
Three conditions are required for developing an adequate biomonitor of environmental pollution: that the species has an ample distribution, that it is tolerant to an ample range of concentrations of the pollutant of interest, and that at least one physiological trait is sufficiently sensitive to respond in a "predictable" fashion to different levels of said pollutant (Markert et al. 2003). Indeed, the eleven ruderal weeds that were screened in the present study were selected based on their ample geographic distribution, their abundance in the region of interest, and their apparent preference, or at least tolerance, for the elevated amounts of anthropogenic reactive nitrogen that are common in cities. In turn, the physiological parameters that were utilized have been shown to respond to nitrogen deposition both under experimental and field conditions. However, while most of the weeds displayed at least some biomonitoring potential, the mortality of four species was such that it precluded further physiological evaluation. This appeared to be more related to inherent characteristics of the plants, such as their ecological strategy of producing numerous seeds or low tolerance of the experimental handling, despite their successful proliferation in urban environments.
A field validation of the biomonitoring potential for Bidens pilosa, Chloris gayana, C. virgata, and Pennisetum setaceum is thus recommended, as 5-9 physiological traits of these species adequately responded to the experimental nitrogen deposition. All in all, the use of ruderal weed biomonitors, which are be abundant in urban environments appears to be promising for characterizing nitrogenous pollution for consideration in integrative biomonitoring efforts.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2789.











 text new page (beta)
text new page (beta)



