Seed fate following seed ripening affects the regeneration of plant populations (Sarukhán 1974). After dispersal, some seeds remain in the soil, forming seed banks (Roberts 1981, Baker 1989,Baskin & Baskin 2014). Other seeds remain on or inside the mother plant, forming aerial seed banks, a process called “serotiny” (Barker et al. 2004,Radeloff et al. 2004, Battersby et al. 2017). Seed banks keep seeds safe for when the environmental conditions are adequate for germination and/or seedling establishment and growth. Serotiny keeps seeds on the mother plant away from predators until either the death of the mother plant or some environmental factors promote dispersal, after which seeds can germinate or become part of the soil seed bank (Lamont et al. 1991, Rodríguez-Ortega et al. 2006).
The number of seeds in the soil seed bank is a result of the balance between inputs (mainly caused by seed dispersal) and outputs (caused by germination, depredation or death) (Harper 1977). Seasonality and/or spatial heterogeneity can modify the total of inputs and outputs of seeds, at the microsite level, causing differences in the soil bank development (Thompson 2000, López 2003, Montiel & Montaña 2003, Cano-Salgado et al. 2012). For example, the slope and the type of vegetation can favor seed bank inputs by seed accumulation after dispersal or seed retention due to soil depth (Marone et al. 2004, Caballero et al. 2005). Soil moisture during the rainy season may increase outputs through germination (Rivas-Arancibia et al. 2006, Sotomayor & Gutierrez 2015).
Some seed traits like large seed number, small seed size, light requirements and dormancy, can affect the persistence in soil of many cacti seeds (Thompson 2000, Rojas-Aréchiga & Vázquez-Yanes 2000, Rojas-Aréchiga & Batis 2001). Furthermore, serotiny has been reported in some cactus genera such as Mammillaria (Contreras & Valverde 2002,Rodríguez-Ortega et al. 2006,Peters et al. 2009,Santini & Martorell 2013). However, few studies have investigated the presence and formation of cacti seed banks (Bowers 2000, 2005,Rojas-Aréchiga & Batis 2001,Montiel & Montaña 2000, 2003,Goodman et al. 2012,Ordoñez-Salanueva et al. 2017).
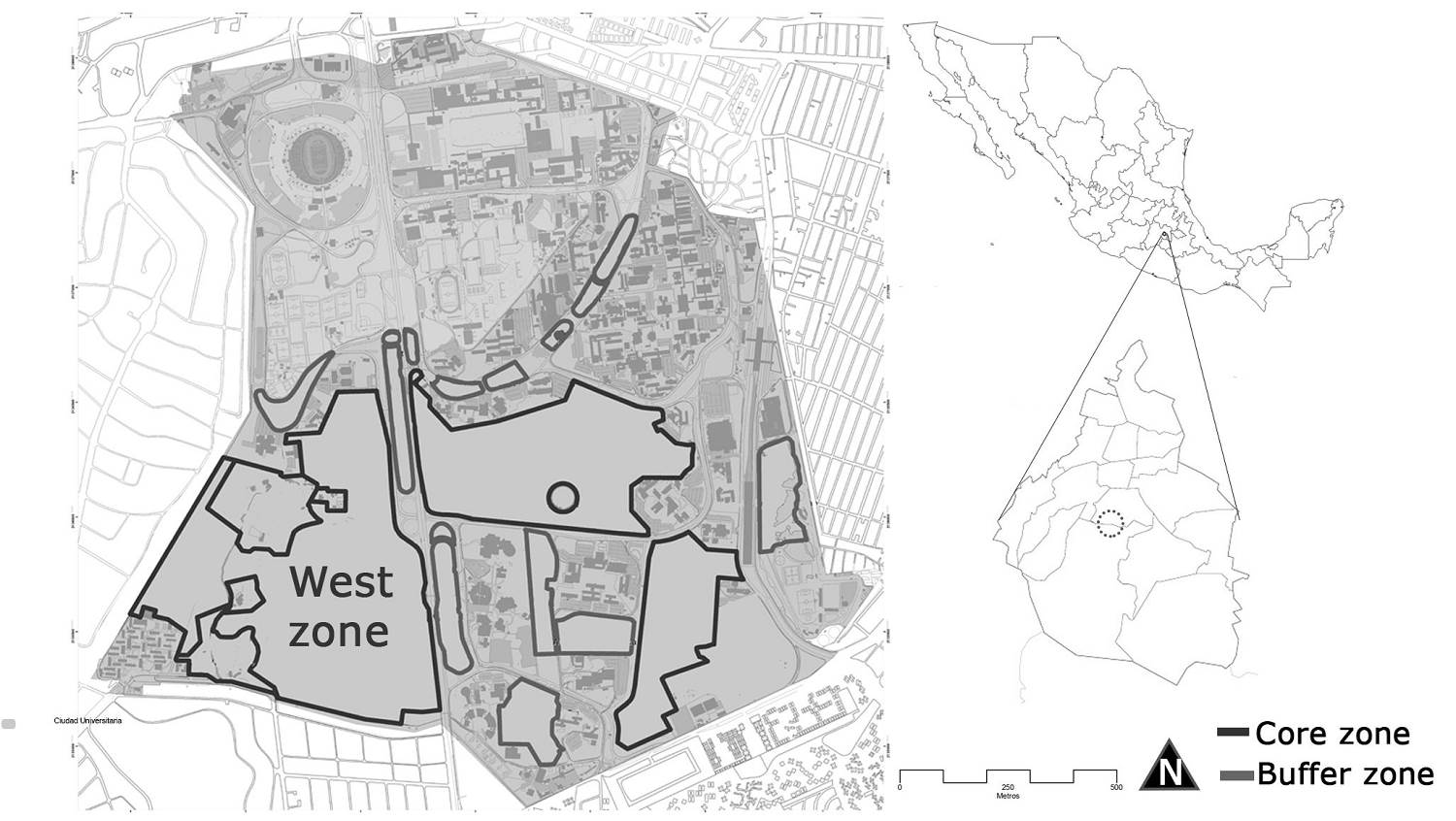
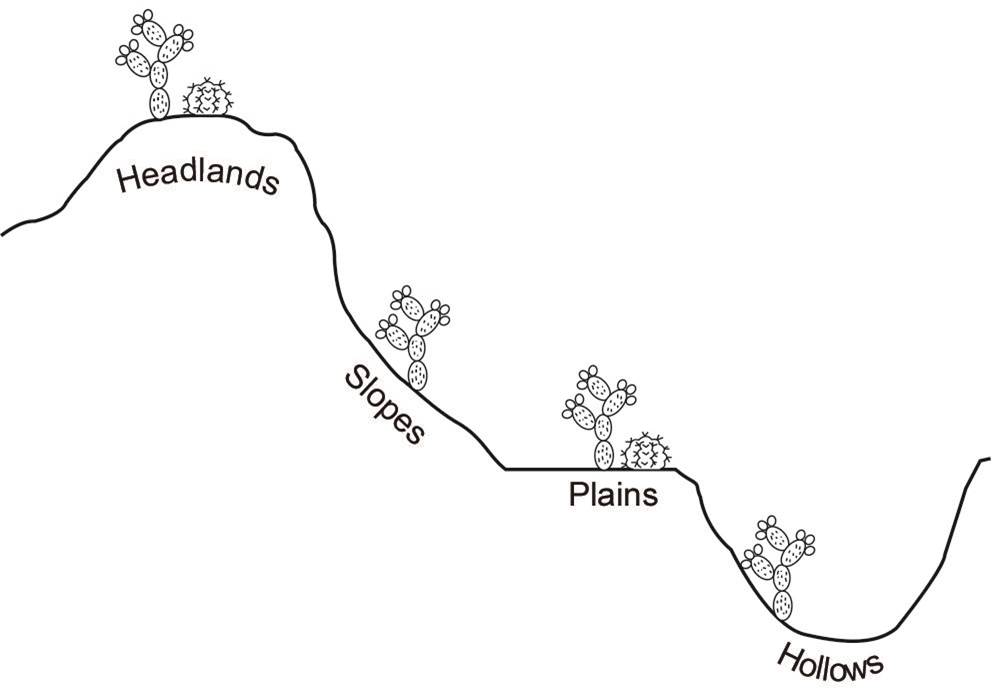
The Reserva Ecológica del Pedregal de San Angel (REPSA) is located on a lava field in Mexico City. This ecological reserve exhibits notable spatial and temporal heterogeneity due to the presence of different microenvironments created by the lava substrate and a marked rainy season during summer (Castillo-Argüero et al. 2007). Some microenvironments are hollows (concave areas of variable depth), slopes (areas with an inclination of 20o or more), plains (areas without inclination, with or without exposed rocks), and headlands (elevated areas with exposed rocks that stand out from the parent material) (Rzedowski 1994). Two genera of the Cactaceae family inhabit in the REPSA, both with contrasting distribution patterns: Opuntia inhabits in all the microenvironments while Mammillaria is restricted just to plains and headlands.
We expect that seasonality and microenvironmental heterogeneity in the REPSA may cause differences in the cacti soil seed bank due to germination associated to the rainy season and/or to the soil depth. Additionally, ecological traits of each cactus associated to their dispersal strategies and contrasting distribution patterns may affect their seed bank dynamics. Our hypothesis is that if season and microenvironment affect seed development, thus we will expect that in the rainy season seed inputs will be larger than in the dry season due to fructification events of the two genera. We also expect more seeds in the microenvironments with the deepest soils depending on species distribution, for Opuntia, we expect that seed banks will be mostly in hollows and for Mammillaria, in the plains. We also expect that due to its growth type, limitation in distribution and lower seed production, Mammillaria will have another seed strategy besides a seed bank formation, such as an aerial seed bank formation. The aims of this study were to determine (1) the characteristics of Opuntia and Mammillaria seeds banks; (2) the influence of seasonality on their seed banks; and (3) the differences in the inputs and outputs of the soil seed bank in different microenvironments.
Materials and methods
Study site. The REPSA is located in a lava field inside the main campus of Universidad Nacional Autónoma de México in Mexico City. The reserve covers about 237.33 hectares, including 13 buffer zones and three core zones (called East, Southeast, and West) (19º 18’ 50.317” N, 99º 11’ 34.861” W). This study took place in the West zone (Figure 1). The vegetation type is a xerophytic shrubland; Pittocaulon praecox is dominant and in some areas is the exotic grass Pennisetum clandestinum. The climate is temperate (at an elevation of 2,250 m) to sub-humid, with summer rains (Cb (wl) (w)). The annual precipitation is 870 mm, and the annual mean temperature is 15.5 oC, with a minimum of -6 oC and a maximum of 34.6 oC (Valiente-Banuet & Luna-García 1990,Castillo-Argüero et al. 2007). The rainy season occurs from May to October, and the dry season from November to April. The lava field originated approximately 1,670 years ago due to the eruption of the Xitle volcano, which created different microenvironments such as headlands, hollows, slopes, and plains (Siebe 2000).
Study species. Mammillaria is represented by two species: M. haageana subsp. san-angelensis (Sánchez-Mej.) D.R. Hunt (Figure 2A, B) and M. magnimamma Haw. (Figure 2C, D). Constant theft has reduced populations of M. haageana almost to extinction, but there are now approximately 200 reintroduced individuals to microenvironments where this species is usually distributed (Valverde & Chávez 2009). M. magnimamma grows more widely in sites with exposed rocks, including some plains and especially headlands. Density varies from 638 to 662 organisms/ha in disturbed and conserved areas (Valverde et al. 2004). During the rainy season, plants may produce from one to six fruits with an average of 80 seeds each, seed number per fruit varies from 0 to 582 (Valverde et al. 2004, Valverde & Chávez 2009). Seeds from both species are small (1-1.6 mm long) and develop a brown or pale brown testa (Bravo-Hollis & Sánchez-Mejorada 1991) (Figure 2D and have light requirements for germination (Ruedas et al. 2000).

Figure 2 Study species and seeds. A-B) Mammillaria haageana subsp. sanangelensis. C-D) M. magnimamma. E-F) Opuntia tomentosa. G-H) O. lasiacantha.
Opuntia can be found in every microenvironment in REPSA. O. tomentosa, a bushy to arborescent cactus, has cladodes with conspicuous short hairs (Figure 2E, F) that grows profusely, and O. lasiacantha, a shrubby cactus with glabrous cladodes (Figure 2G, H), it grows moderately. Opuntia density varies from 1,200 to 50 plants/ha (Romo-Campos et al. 2010). Their fructification occurs mostly during the rainy season, in average each plant produces 50 fruits with 80 seeds each one (Olvera-Carrillo et al. 2003). Both species develop similar small seeds (3-6 mm long) enclosed in a funicular envelop, of beige to pale brown color (Figure 2F, H), and light and post-dispersal time requirements for germination (Olvera-Carrillo et al. 2009). In the soil, temperature fluctuation or microorganisms can soften and break the seed coat (Orozco-Segovia et al. 2007,Olvera-Carrillo et al. 2009, Sánchez-Coronado et al. 2011). Due to their morphological similarities, it is not possible to clearly distinguish between these seeds at the species level.
Soil sampling. Samples were taken in two seasons: one during the rainy season (October 2016) and the other during the dry season (March 2017). We selected 21 sites each one of 100 m2 separated 10 m one from each other. In each site we marked a central point around which we made five transects in different directions of 1 m long. At the end of each transect we took a sample of the soil with a hand gardener shovel. According to Santibañez-Andrade et al. (2009) we took the first five centimeters of soil surface. For every soil sample we registered the corresponding microenvironments: hollows, slopes, plains and headlands (Figure 3). We also measured the soil depth in each sample with a ruler, if soil was absent, we registered it as cero. In each season we collected 105 soil samples (210 in total).
Seed search. To homogenize the soil samples from the different sites, we take samples and formed mixed samples by microenvironment and season (4 microenvironment × 2 seasons = 8 conditions), then sieved the soil by using different mesh sizes (Mont-Inox: 4.76 mm, 3.36 mm, and 1.68 mm). Then, using a Zeiss stereoscopic microscope, we searched for seeds in 30 g of sieved soil (10 g of soil sifted through each sieve opening) and counted the inputs (total seeds), outputs (incomplete seeds and non-viable seeds), and counted the viable seeds (seed bank) in three repetitions for each condition (24 samples of 30 g in total). We also observed if complete seeds had a wear down.
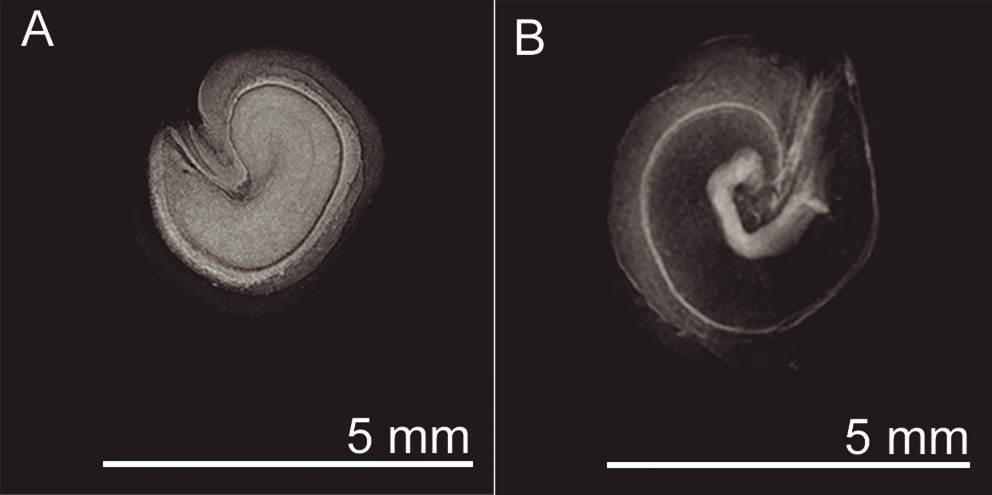
Viability tests. We tested seed viability using x-ray equipment (Faxitrom, Ultrafocus, Tucson, Arizona, USA). Seeds with completely white embryos or embryos whose darkened areas did not exceed 50 % of the total area were labeled as viable; seeds without these characteristics were labeled non-viable (Figure 4).
Aerial seed bank. From June 2017 to January 2018, we registered the fruits produced by 17 M. magnimamma plants in a 100 m2 site with 24 plants of the species. Each month, we counted the ripe, unripe, removed, and remaining fruits. We also searched for fruits remaining on the plants and seeds from previous seasons in between tubercles and in samples of axillary hairs using a Zeiss stereoscopic microscope (SP-STEMI508-TS-K-EDU, Ontario, New York, USA).
Data analysis. To identify any significant differences between the inputs (total seeds), outputs (incomplete and/or non-viable seeds), and seed banks (viable seeds) in the microenvironments and seasons, we performed a Kruskal-Wallis (χ²) using the “stats-package” test in R version 3.4.3 (α = 0.05). We also performed a principal component analysis including soil depth, inputs, outputs and viable seed in each microenvironment and each season with PCOrd version 7 (Peck 2016).
Results
Soil seed bank. In the soil we found a total of 3,675 seeds of cacti and other different species, of which 1,756 were intact seeds. We distinguished 66 different types of seeds. None of these seeds belonged to Mammillaria but smaller seed were found; and 186 seeds belonged to Opuntia (83 intact and 103 incomplete) (Table 1). The intact Opuntia seeds represented 4.73 % of the total seeds found in the soil. Opuntia was the genus with the eighth highest number of intact seeds that can be divided into two groups depending on if the surface seed coat was wear down or not. Seeds with high wear were found in both seasons, and seeds with low wear were found only in the dry season (Figure 5A, B). Seeds that lacked an embryo were considered incomplete. Two groups of incomplete seeds can be distinguished according to the aperture position: (1) near the hilum-micropylar zone or (2) in the lateral areas. The first type often showed an operculum detachment around the radicle zone (Figure 6A, B).
Table 1 Total number of Opuntia seeds (Tot), incomplete seeds (Inc), viable seeds (Via) and non-viable seeds (NV) in each microenvironment by season.
| Microenvironment | Rainy | Dry | ||||||
|---|---|---|---|---|---|---|---|---|
| Tot | Inc | Via | NV | Tot | Inc | Via | NV | |
| Hollow | 41 | 9 | 0 | 32 | 12 | 11 | 0 | 1 |
| Slope | 23 | 10 | 4 | 9 | 7 | 5 | 1 | 1 |
| Plain | 22 | 20 | 1 | 1 | 28 | 23 | 2 | 3 |
| Headland | 12 | 3 | 3 | 6 | 40 | 21 | 6 | 13 |
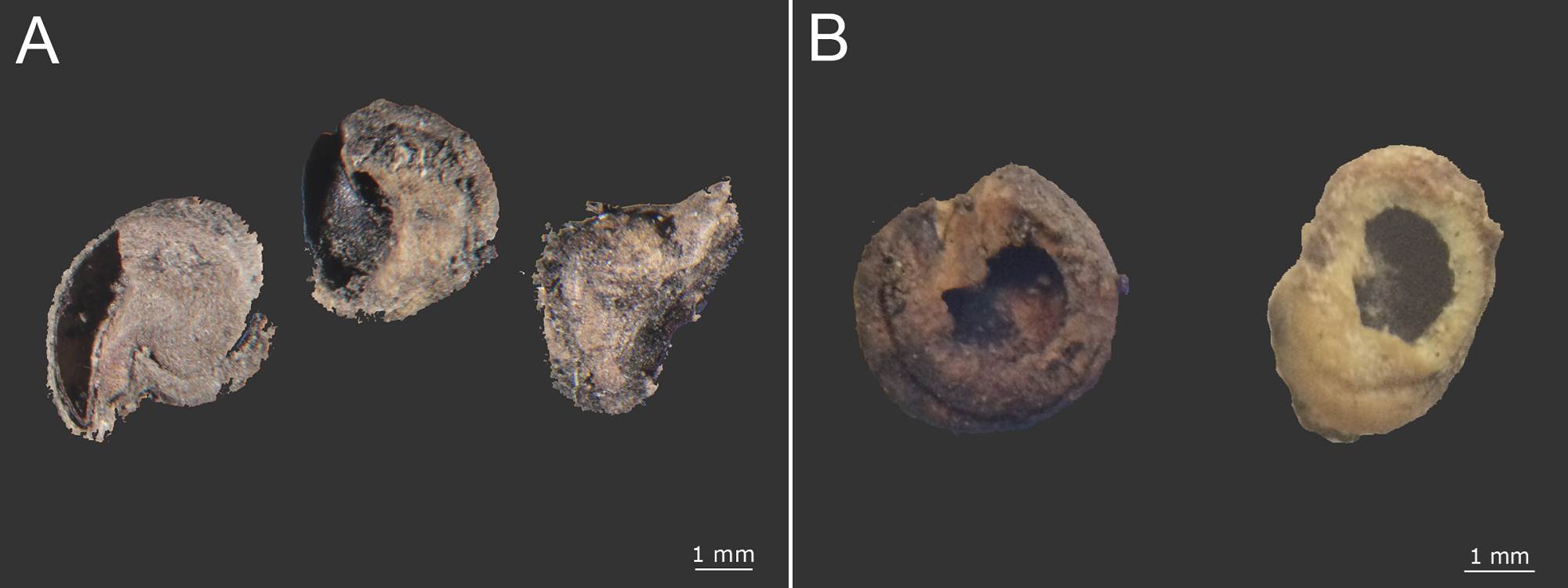
Figure 6 Opuntia incomplete seeds with aperture position: A) near the hilum micropillar region and B) in the lateral areas.
The season had a significant effect on the seed inputs by microenvironment (χ² = 18.42, df = 7, P-value = 0.01). Total inputs in the rainy season were 98 and in the dry season were 87. In the rainy season, we recorded the maximum mean value for inputs in hollows (20.5 ± 0.7) and the minimum mean value in headlands (2 ± 2.8) (Figure 7A). The number of seeds in hollows in the rainy season was four times greater than in headlands. Both microenvironments showed opposite trends in the dry season: the mean input value decreased in hollows and increased in headlands, where the maximum mean value was recorded (10 ± 3.9) (Figure 7A). In the dry season, the number of seeds in headlands was twice times greater than in hollows. In hollows inputs reduced four times from rainy to dry season, while in headlands inputs increased two times.

Figure 7 (A) Opuntia inputs, (B) soil bank and (C) outputs during rain and dry seasons in microenvironments: hollows (Hw), slopes (Sl), plains (Pl) and headlands (Hd). The boxplot represents the interquartile range, minimum and maximum values are at the extremes. Outliers are represented by points and the median by the thick line inside each box.
Viability test revealed that only 17 Opuntia seeds were viable (Table 1). No viable seeds in any season were found in hollows. Seed viability (seed bank) in headlands during the dry season showed significant differences (χ² = 16.84, df = 7, P-value = 0.01). The maximum mean value of viable seeds (seed bank) was recorded in headlands during the dry season (1.5 ± 0.5) (Figure 7B). The number of seeds in headlands in dry season was three times greater than in the other microenvironments (Table 1). Headlands were the only microenvironment in which the viable seeds showed the two levels of wear.
Outputs showed similar trends and number as inputs. Like in the case of inputs, the season had a significant effect by microenvironment (χ² = 18.12, df = 7, P-value = 0.01) (Figure 7C). The maximum mean value for outputs was in hollows during the rainy season (20.5 ± 0.7) Hollows presented the same number of inputs and outputs in the rainy and the dry season. In hollows, the number of outputs in the rainy season was three times greater than in dry season. In headlands, the outputs were two times greater in the dry season than in the rainy season (Table 1).
The soil bank was not favored in the deepest soils. The deepest soils occurred in hollows, a microenvironment with the greater number of inputs but the absence of viable seeds. Viable seeds were found in the other microenvironments with shallow soil, particularly in headlands during the dry season (Figure 8).
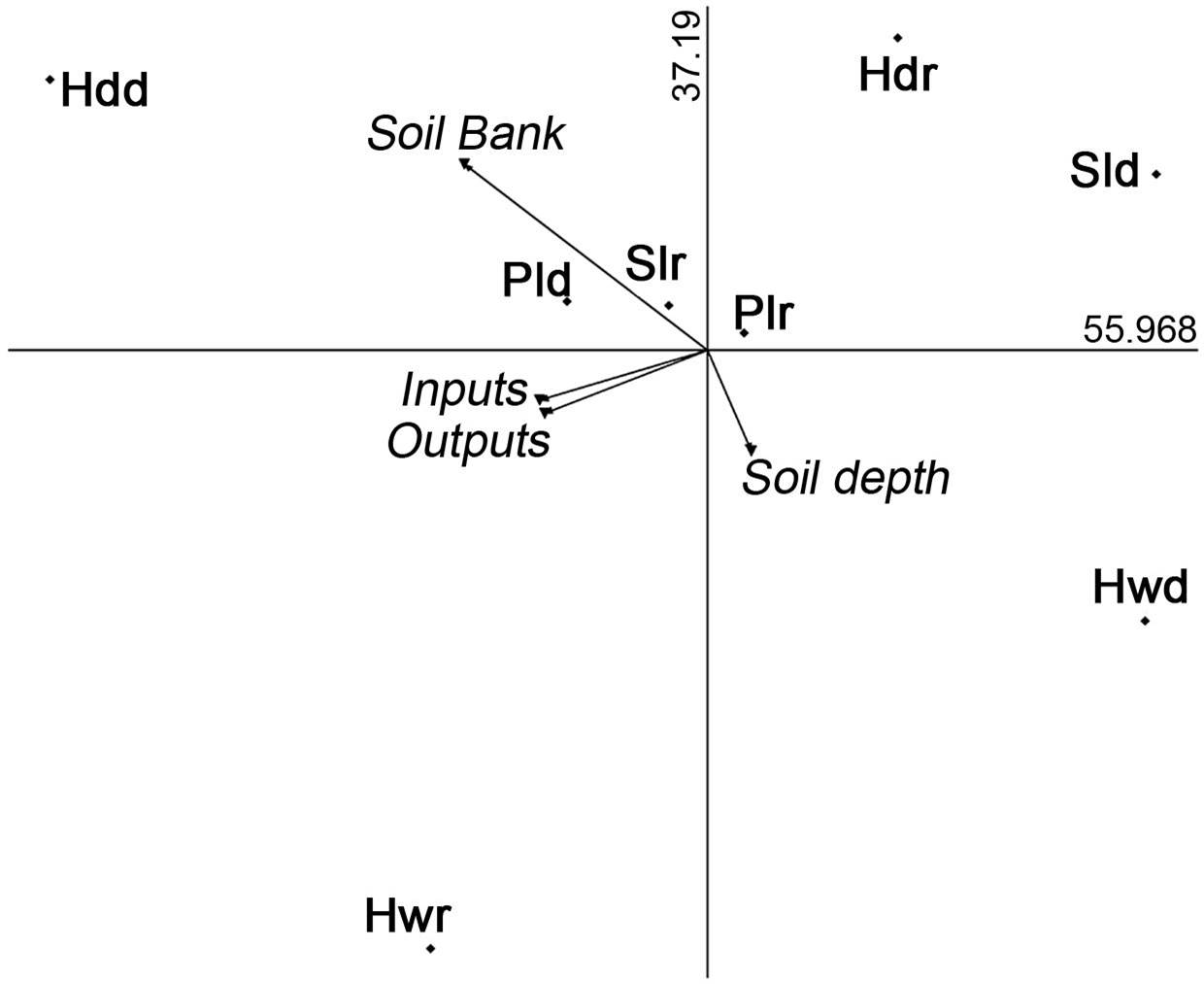
Figure 8 Principal component analysis graph displays the pattern of inputs, outputs and soil bank of Opuntia seeds. Soil bank occurs in headlands during dry season. The relationship between bank formation and soil depth is inverse. Hollow, plain, slope, headland during dry season (Hwd, Pld, Sld, Hdd) and hollow, plain, slope, headland during rainy season (Hwr, Plr, Slr, Hdr).
Aerial seed bank. At the beginning of the fruiting season, we registered 296 fruits. Most were unripe, and only 13 were red, indicating that they were ripening. The highest rate of fruit removal occurred at the beginning of the rainy season, during May and June, and fruits continued decreasing throughout the season. At the beginning of the dry season, less than 5 % of the registered fruits remained on the plants. Ripe fruits were observed in lower numbers than unripe ones on mother plants (Figure 9). Fruits remained on the mother plants for less than a month, with the exception of one mother plant on which unripe fruits remained from May 2017 to January 2018.
Seeds were scarce among the tubercles of M. magnimamma, resulting of excessively turgid fruits that exploded, releasing their seeds. This releasing was also observed in partially predated fruits (Figure 10). In no case did seeds remain on the mother plant for more than a month.
Discussion
We found that the studied genera had different seed bank dynamics. Although seed traits of both genera, such as a small size that facilitates burial and light requirements for germination (Ruedas et al. 2000,Olvera-Carrillo et al. 2009), may promote a seed bank formation, we only found seeds of Opuntia in the soil. We discarded that the absence of Mammillaria seeds was due to our sampling technique, since during our search we found smaller seeds from other species. Nevertheless, the absence of seeds does not necessarily imply the Mammillaria incapability to form a seed bank; it can be to the low number of individuals of this genus in the REPSA. One important difference between the studied genera is the hardness of the seed coat and the after-ripening requirement for germination in Opuntia (Orozco-Segovia et al. 2007,Olvera-Carrillo et al. 2009), traits that may favor seed longevity in the soil promoting a soil bank formation (Rojas-Aréchiga & Vázquez-Yanes 2000, Rojas-Aréchiga & Batis 2001) in the REPSA as it has been reported in other arid environments (Montiel & Montaña 2003, Bowers 2005).
For Opuntia, we found that their seed bank dynamics differ by season and microenvironments. It was expected that the largest number of inputs was related to the fructification event during rainy season and that the largest seed number in the soil bank was in the microenvironments with the deepest soil. We observed this pattern in the rainy season, but in the dry season in headlands the number of inputs was almost four times bigger than in the rainy season, and also was almost four times bigger than in hollows. According to Soberón et al. (1991) and Castellanos-Vargas et al. (2017), this can be explained by gravitational water runoff transport that carries particles from microenvironments at high elevations (like headlands) to microenvironments at lower elevations (like hollows), which is consistent with our results observed in the rainy season. During the dry season, the absence of rain reverses this phenomenon, since there is no rain that promotes the seeds secondary dispersal. This suggests that in the REPSA the rain is an important factor that determines seed inputs in each microenvironment by secondary seed dispersal events.
Animals dispersal also contribute in an important way to the inputs. Fleshy fruits produced by Opuntia are attractive to frugivores, as birds (González-Espinosa & Quintana-Ascencio 1986, Montiel & Montaña 2000). In the REPSA, the activity of small mammals (like Didelphis virginiana californica and Bassariscus astutus) that use open areas (like headlands) as latrines (Castellanos-Morales & List 2005,Castellanos-Morales et al. 2008) may favor to the inputs in headlands. Secondary dispersal can happen latter also by ants (García-Chávez et al. 2010).
Outputs in the seed bank are due to germination events, predation and loss of viability (Harper 1977). Both predation and germination can be inferred through the seed coat aperture position when the output occurs by predation the aperture is in the lateral areas and when occurs by germination the aperture is near the hilum-micropylar zone. Although it has been suggested that granivores activity depends on spatial heterogeneity (Montiel & Montaña 2003, García-Chávez et al. 2010), we observed predated seeds in all the microenvironments.
Opuntia germination depends on the presence of light and fungi that reduces mechanical resistance of the testa (Olvera-Carrillo et al. 2009, Sánchez-Coronado et al. 2011, Delgado-Sánchez et al. 2013), the available humidity amount (Monroy-Vázquez et al. 2017) and the after-ripened period (Mandujano et al. 2005). Because it was frequently found germinated seed coats, we supposed that all the microenvironments can meet Opuntia germination requirements.
The loss of seed viability also contributed to the seed bank outputs. Many of the non-viable seeds showed a high wear level, suggesting that they had been in the soil for more than one season and that their viability is rapidly reduced in natural conditions. Other studies have reported viability times of up to two years for Opuntia (Montiel & Montaña 2003, Bowers 2005). The viability of the seeds is associated with microenvironmental conditions such as temperature and humidity (Pukacka & Ratajczak 2005), our results show that in hollows, the microenvironment with the deepest soil, that can retain more moisture amount, favored the viability loss.
The presence of viable seeds in both seasons indicates that Opuntia may form soil banks capable of remaining throughout the year, but not in every microenvironment. Heterogeneity causes different accumulation and conservation distribution patterns of seeds in the soil (López 2003,Montiel & Montaña 2003). During the dry season, headlands accumulated the highest number of viable seeds, which either remain in the headland or are subject to secondary dispersal (Chambers & Macmahon 1994). The formation of the Opuntia spp. seed bank was not related to the highest seed input value or to the deepest soils but rather to some other biotic and abiotic factors in each microenvironment, such as humidity or the presence of microorganisms (Chambers & Macmahon 1994,Sánchez-Coronado et al. 2011,Hegazy et al. 2014). In REPSA, the prevailing conditions in headlands during the dry season represent the “safe site” for seed conservation in the soil. As well as the microenvironmental conditions, seed traits such as dependence on light for germination and coat hardness might promote their permanence in the soil once buried (Olvera-Carrillo et al. 2009,Rojas-Aréchiga & Mandujano-Sánchez 2017).
The limited distribution of Mammillaria means fewer reproductive individuals contributing to the soil bank inputs, reducing the chance of finding Mammillaria’s seeds in the soil. There is the possibility that during fruit removal, the seeds may be completely consumed without subsequently entering to the soil bank, although it has been suggested that the consumption of cactus fruits by vertebrates favors seed germination (Gomes et al. 2014). However, we also observed the activity of ants in fruits with exposed seeds, which could represent a granivore pressure that limits the seed bank formation (Montiel & Montaña 2003, García-Chávez et al. 2010). The time that the seeds remain without germinating under natural conditions can also limited the seed bank formation, for M. magnimamma a rapid germination of the seeds after dispersal has been reported in the REPSA (Valverde et al. 2004).
For M. magnimamma, the absence of seeds in the soil was not related to its ability to form aerial banks. Unlike other species (such as M. solisoides, M. hernandezii, M. napina, M. pectinifera and M. crucigera) in which serotinia has been reported (Contreras & Valverde 2002, Rodríguez-Ortega et al. 2006, Peters et al. 2009, Santini & Martorell 2013), M. magnimamma morphological traits do not promote fruit retention, such as fruit with excerted development, the fruit/tubercle ratio size and the low number or complete absence of radial spines. The lack of a strategy for the formation of banks, both aerial and soil bank, may be one of the causes of the genera low recruitment rate in the REPSA (Valverde et al. 2004) and an indication of population vulnerability in the face of environmental disturbances like fires (Martínez-Orea et al. 2012).
Our results show that the largest number of inputs to the seed bank does not coincide with the species fructification in all the microenvironments. In fact, seed bank dynamics in hollows and promontories are contrary between seasons, which indicates the importance of rain as an element that promotes secondary dispersion between microenvironments. Contrary to what we expected, the largest number of viable seeds was not associated with the deepest soil, mainly because the viability loss is a major cause of seed outputs. We point out the need to increase the time of this type of study to verify the suggested patterns in different years; to evaluate secondary dispersion as the main source of inputs into the soil; to include more biotic and abiotic variables; and to evaluate the seeds ecological longevity in the different microenvironments of the REPSA.











 nueva página del texto (beta)
nueva página del texto (beta)