The seed´s primary function is to protect the embryo, sensing favorable conditions for the germination and nourishment of the germinating seedling (Sehgal et al. 2018). Because of climate change, seed quality will be affected mainly due to seasonal droughts, fluctuations in soil moisture acting as a selective pressure for germination, seed size and yield (Beebe et al. 2013). This effect occurs when photoassimilates uptake and their remobilization from source tissues toward the seeds is modified and alter the grain composition (Farooq et al. 2018). In particular, common beans (Phaseolus vulgaris L.) grow in a range of habitats where it is exposed to seasonal droughts, with soil moisture fluctuations. This stress may act as a selective pressure on yield, seed size and quality (Beebe et al. 2013); however, the low yield is typical result in common beans grown at low water availability (Padilla-Chacón et al. 2019).
To achieve systematic progress in breeding, it is necessary to have a broad genetic base (Singh 2001). Most of the available diversity in wild and landraces of these species remains without use due to a 'bottleneck' effect that took place during the domestication process from a small number of wild populations, there is greater diversity in the wild forms of the species in the Phaseolus genus (Jiménez-Galindo & Acosta-Gallegos 2012).
Common bean is one of the most important legume crops, providing as much as 15 % of total daily caloric intake and 36 % of total daily protein in Africa and America (Schmutz et al. 2014; http://faostat.fao.org/site/291/default.aspx). The development of high yield varieties with increased drought tolerance and high grain quality are primary objectives of a bean genetic improvement program (Acosta et al. 2004). There are available genetic resources of P. vulagris selected for drought tolerance developed at arid and semiarid areas (Bebee et al. 2013). In contrast to common bean, Phaseolus acutifolius A. Gray (Tepary bean) distributes in arid and semi-arid regions from northern Mexico to Southwestern USA; its yield under limited water availability is qualified as satisfactory, even outstanding (Gujaria-Verma et al. 2016, ILDIS 2018, Leal-Delgado et al. 2019); it is a pulse less consumed than common bean even its high nutritive value (Bhardwaj & Hamama 2004).
Carbohydrate content, as a key factor for plant growth, regulates synthesis and seed reserves accumulation during development, seed filling time and the overall metabolic activity (Sehgal et al. 2018). Starch is generally composed of amylose and amylopectin, about 98-99 % of the polysaccharide dry weight, organized as granules, can show similar or heterogeneous size and shapes (Tayade et al. 2019). In cereals like wheat (Triticum aestivum), drought decreases the total starch concentration and amylose proportion, modifying the amylose‐to‐amylopectin ratio in the grain (Li et al. 2015, Yu et al. 2016).
The genetics and molecular details of starch synthesis and granules organization are described in cereal crops, yet, little is known of their synthesis in Phaseolus; even less to the effects water restriction has in the seeds, their sugars and starch accumulation and effects on the seed growth and germination (Lemmens et al. 2019). Though, in common beans, water restriction during plant growth modifies the amylose-amylopectin ratio, digestibility and pasting properties of isolated starch in the seeds (Ovando-Martínez et al. 2011).
The objective of this research was to evaluate the effect water restriction during on seed´s development regard their carbohydrates concentration, starch granules size and amylolytic activity in seeds of P. vulgaris and P. acutifolius, which contrast in their drought tolerance. For this purpose, this study included common bean seeds from cv. “Rosa Bufa”, from Chihuahua, improved for extremely dry environments at the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, INIFAP-Chihuahua, Mexico. This common bean cultivar has a reported yield of 376 kg ha-1, higher than that of other cultivars under the same stressing conditions (Jiménez-Galindo & Acosta-Gallegos, 2012). The above contrasted with Tepary bean variant 10017 collected at Durango, Mexico, from highly dry habitats of northern Mexico. Tepary bean 10017 is registered in a core collection of the INIFAP (Cárdenas-Ramos et al. 1996). The outstanding tolerance to water restriction of the 10017 variants, during vegetative stage, was reported by Leal-Delgado et al. (2019). We hypothesized that the seed responses to water deficit in their physiology, carbohydrates concentration and starch grain morphology are species dependent.
Materials and methods
Evaluated seeds. Seeds of the Tepary bean cv. 10017 (P. acutifolius A. Gray) were donated by the INIFAP. The material was collected at Durango, Mexico, and is registered in the germplasm bank catalog for Phaseolus spp. of the INIFAP (Cárdenas-Ramos et al. 1996). The cv. Rosa Bufa (P. vulgaris) was also donated by INIFAP, Campo experimental Sierra Chihuahua (Jiménez-Galindo & Acosta Gallegos 2013). Plants from both species were cultivated between May and August 2018, in a greenhouse at the Colegio de Postgraduados, Campus Montecillo, Texcoco, State of Mexico (19° 29' N, 98° 53' W, 2,240 m altitude). During plant development, the mean daily temperature was 24 ± 4 °C, sunlight radiation of 1,259.25 μm m−2 s−1 and the relative maximum and minimum humidity were 83.17 and 9.93 %. Each plant was grown in a plastic pot (15 × 13 × 11 cm), in 5.5 kg of sandy crumb-type soil. Plants were daily irrigated (∼100 % field capacity; FC) until the third trifoliate leaf was fully expanded (∼30 days after sowing; DAS). Then, plants from each species were randomly separated into two groups. One group continued with regular irrigation to maintain soil near FC (control); the other group, was maintained at 25 % FC by irrigation restriction. Soil moisture was daily evaluated via the gravimetric method until harvest.
Morphometric traits. The mass of 100 randomly chosen seeds (Figure 1) was gravimetrically determined by weighing in an electronic balance (Hongzuan HZ-2003). Seed size was determined by measuring the length, width and thickness using a vernier caliper (HER-411, Steren, China).
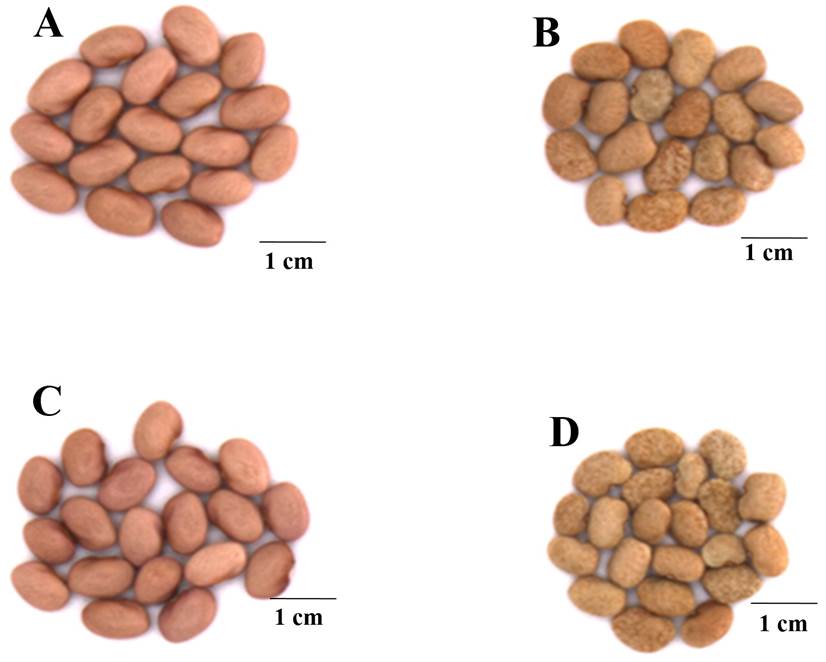
Figure 1 Representative image of seeds harvested from plants of P. vulgaris (cv. Rosa Bufa) (A-C) and Tepary bean P. acutifolius (cv. 10017) (B-D) under 100 % FC (A-B) and 25 % FC (C-D). Barr represent 1cm.
Starch grain analysis. Seeds were imbibed in water for 48 h and dehulled. Cotyledons were fixed in FAA solution (50 % ethanol, 5 % acetic acid, 10 % formol and 35 % distilled water volume ratios) for 72 h. Cotyledons were infiltrated and embedded into paraffin to obtain 10 µm transverse sections in a microtome (Erma Inc. Japan). The samples were analyzed using periodic acid Schiff reaction and evaluated by field emission in light microscopy (Carl Zeiss, Model LG, Germany). Micrographs of each sample were taken with 400 times magnification. At least 12 mature grains from three independent plants of each treatment were imaged. The starch granule number per field was counted in eight micrographs of the same size. Grain total starch concentration was quantified using Image J (Java, JVM) software. Data were collected from at least eight cotyledons with four replicates per image.
Soluble sugars and starch. Glucose, fructose, sucrose, and starch concentrations were determined in 12 h imbibed seeds. Fifty mg of triturated seed was mixed with 500 µL of 80 % ethanol, heated at 80 °C for 30 min and centrifuged at 10,000 × g for 10 min. Glucose, fructose and sucrose were enzymatically quantified from the supernatants and starch in the insoluble material as described by Bernal et al. (2005) and Leal-Delgado et al. (2019).
Water uptake and germination. Twenty-five seeds of each treatment were germinated in Petri Dishes, for triplicated, for 96 hours, in total darkness and at 25 °C, following ISTA (International Seed Testing Association 2018) standards. The seeds were blotted and placed on paper towels to remove surface water. Seed imbibition was determined at 12, 24, 36, 48, 60 hours, or up to germination. A similar seed set of each treatment was monitored every 12 h until reaching maximum germination. The criterion for germination was a radicle protrusion of > 1 mm.
Native PAGE and activity staining. Native PAGE electrophoresis for starch amylolytic enzyme detection was carried out with crude extracts from 12 h germinating seeds, as described by Zeeman et al. (1988) and Delatte et al. (2006). This method relies on the composition of a glucan substrate and amylopectin from potato (Sigma, Aldrich) incorporated into the gel and visualized by staining with an iodine solution. The native gels contained 6 % (w/v) polyacrylamide and 2 % amylopectin. After electrophoresis, gels were kept at 37 °C, for 1 h, in a medium containing buffer 50 mM sodium acetate, pH 5.5, 1 mM MgCl2, 1 mM CaCl2, 1 mM dithiothreitol. Clear bands developed in amylopectin-containing gels and were stained with Lugol solution.
Amylase activity. Amylase activity was assessed 12 h after germination started, in 100 mg of the cotyledon samples, using an amylase activity assay kit (Sigma Aldrich). Each sample was homogenized in 0.5 mL of the amylase assay buffer. The samples were centrifugated at 13,000 × g for 10 min to remove insoluble material. A standard curve was obtained using nitrophenol standards for colorimetric detection at 405 nm with 0, 2, 4, 6, 8, 10 µL from the 2 mM nitrophenol standard, placed into a 96-well plate, generating a 0 (blank), 4, 8, 12, 16, and 20 nmol/well standards. Ten µL from each sample were added into the wells, plus a 40 µL assay buffer and 100 µL of the master reaction mix. A standard and positive control wells were included. The plate was mixed for 3 min using a horizontal shaker, their initial absorbance (T initial) was then measured at 405 nm (Abs405). The samples were kept at 25 °C the plate was protected from light during the incubation. Readings were made five times every 5 min. The results were calculated from the background by subtracting the final Abs405 blank absorbance from the final Abs405 measurement of the standards and those from the samples, calculating the change in absorbance from T initial to T final for the samples. The ∆Abs405 of each sample was compared to the standard curve to determine the amount of nitrophenol generated by the amylase between T initial to T final.
Experimental design. A completely randomized factorial design was used to analyze the effects of two water conditions (well-watered or 100 and 25 % FC) on two bean species. The experimental unit was a plant, five replicates were evaluated.
Statistical analysis. For the statistical comparison of treatments, the normality test (Shapiro-Wilk) was performed for each variable as well as the equal variance test. An ANOVA was performed for each response variable using the Sigma Stat 3.5 software, followed by a comparison of means using Tukey's multiple comparison test to found significant differences (P ≤ 0.05).
Results
Seed weight, width, length, and thickness were significantly different between P. vulgaris (cv. Rosa Bufa) and P. acutifolius (10017) (Figure 2). Water restriction had no significant effect on P. vulgaris seed size or mass. In contrast, in P. acutifolius 10017 Tepary beans the seed length and width decreased 10 % each compared to its control (Figure 2). The starch granules of P. vulgaris were 27 % longer than those of P. acutifolius at 100 % FC (Figure 3). The starch granules and neutral carbohydrates were detected with periodic acid-Schiff’s (Figure 3 A-D). Interestingly, the major axis in P. vulgaris had no significant differences at 100 % FC while in P. acutifolius was decreased. However, in both species significantly decreased by 10 % the maximum and minimum axis length because of the water restriction. Regard to neutral carbohydrates no differences were detected (Figure 4 A-B).
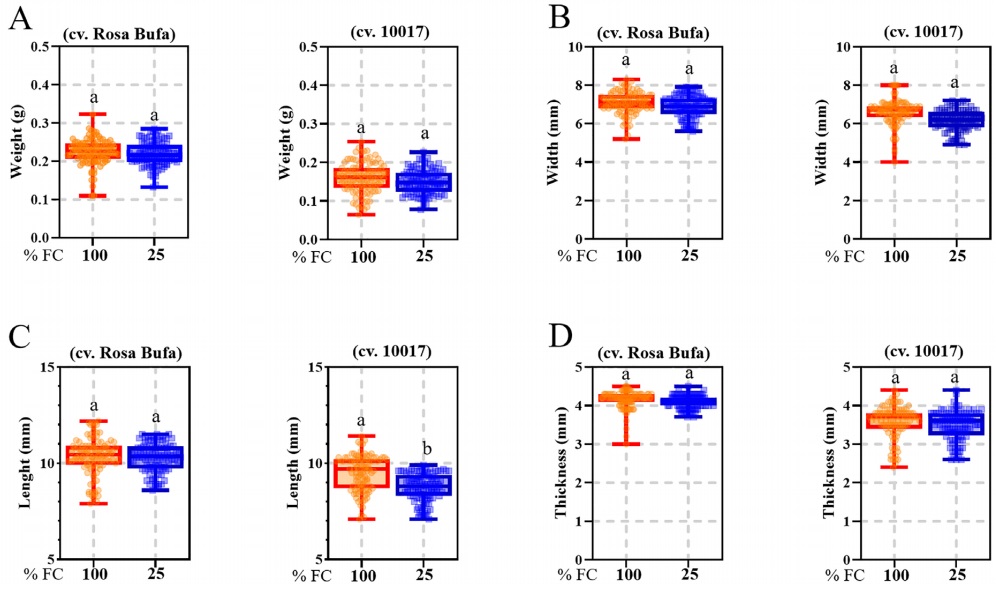
Figure 2 Seeds of Phaseolus vulgaris L. (cv. Rosa Bufa) and P. acutifolius (cv. 10017); seed weight (A), width (B), length (C) and thickness (D) at harvest. Plants were grown under two soil moisture treatments during the whole cycle, 100 % (red box) and 25 % of soil field capacity (blue box). n = 100. Different letters indicate a significant difference between moisture treatments. Tukey test (P ≤ 0.05).
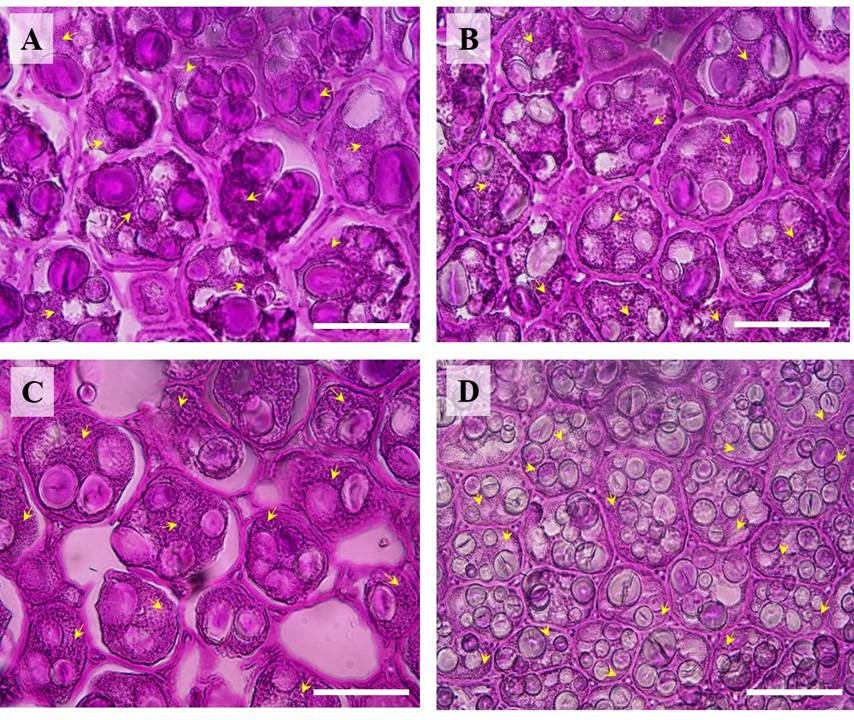
Figure 3 Light micrographs (40X) showing starch granules and neutral carbohydrates (yellow arrows) of cotyledons of Phaseolus vulgaris (cv. Rosa Bufa) (A-C) and P. acutifolius (cv. 10017) seeds (B-D) growing at 100 % field capacity (FC) (A-B) or 25 % FC (C-D) during their development. Starch granules were analyzed with a Peryodic acid-Schiff reaction. The bars represent 30 μm.
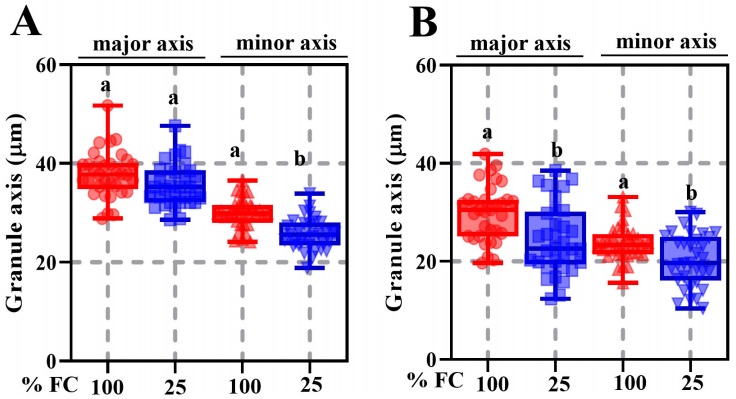
Figure 4 Maximum and minimum starch granule length ± SE in seeds of Phaseolus vulgaris (cv. Rosa Bufa) (A) and P. acutifolius (cv. 10017) (B) growing at 100 % (red box) and (25) % soil field capacity (blue box) during plant development; n = 8. Different letters indicate a significant difference between moisture treatments. Tukey test (P ≤ 0.05).
The glucose concentration ranged, in seeds of both species, between 180 to 230 μmol g−1 at 100 % FC. The glucose concentration increased to 219-294 μmol g−1 as an effect of 25 % FC (Figure 5A). The fructose concentration in both species and at both FC was less than 10 % than that of the glucose concentration, there were no significant differences between species and watering levels (Figure 5A). The sucrose and starch concentration had no difference between species or water treatment (Figure 5B).
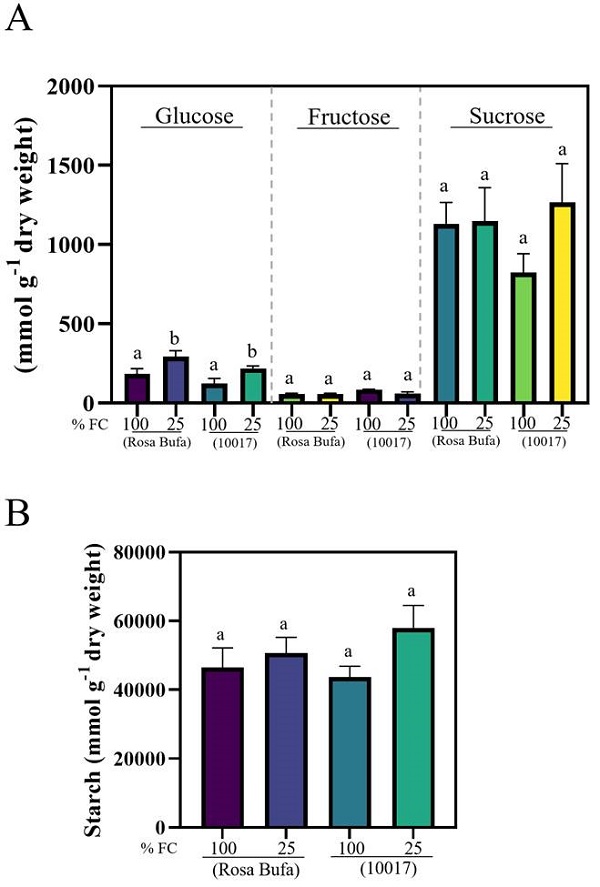
Figure 5 Glucose, fructose, sucrose (A) and starch concentration (B) (± SE) in seeds of Phaseolus vulgaris (cv. Rosa Bufa) and P. acutifolius (10017) grown at 100 and 25 % FC during seed development; n = 5. Different letters indicate a significant difference between moisture treatments. Tukey test (P ≤ 0.05).
Seed imbibition increased by the effect of water restriction (Table 1). Results show that P. acutifolius in both FCs had similar maximum imbibition. However, in the first 12 h, seeds grown at 25 % FC of cv. Rosa Bufa imbibed water six-folds more than those grown at 100 % FC (Table 1).
Table 1 Comparison of the amount of water (g) imbibed by seeds of P. vulgaris cv. Rosa Bufa and P. acutifolius 10017 Tepary bean.
| Specie | Cultivar | Humidity (%) |
DWS | g/12h | g/24h | g/36h | g/48h | g/60h | g/72h | g/84h | g/96h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. vulgaris | Rosa Bufa | 100 | 0.24 ± 0.02a |
0.02 ± 0.0 a |
0.15 ± 0.04 a |
0.28 ± 0.08 a |
0.30 ± 0.06 a |
0.36 ± 0.07 a |
0.44 ± 0.08 a |
0.64 ± 0.11 a |
0.74 ± 0.13 a |
| 25 | 0.22 ± 0.01 b |
0.12 ± 0.0 b |
0.18 ± 003 b |
0.22 ± 0.07b |
0.28 ± 0.07 b |
0.4 ± 0.12 a |
0.47 ± 0.13 a |
0.65 ± 0.20 a |
0.77 ± 0.23 a |
||
| P. acutifolius | 10017 | 100 | 0.17 ± 0.02 c |
0.004 ± 0.02 c |
0.03 ± 0.06 c |
0.13 ± 0.03c |
0.16 ± 0.03 c |
0.21 ± 0.05c |
0.27 ± 0.06c |
0.41 ± 0.09 c |
0.51 ± 0.11 c |
| 25 | 0.16 ± 0.02d |
0.002 ± 0.04 c |
0.03 ± 0.05 c |
0.12 ± 0.06b |
0.15 ± 0.08 d |
0.2 1 ± 0.08c |
0.27 ± 0.07 c |
0.41 ± 0.11c |
0.47 ± 0.13c |
Means (± ES) in the same column followed by the different lower case letters (a, b, c, d) are significantly different at (P < 0.05) using Tukey test. n = 50. DWS = dry weight seed (g).
The water restriction did not modify maximum germination in either species, both species began germination at 24 h (Figure 6A-B). However, P. vulgaris seeds from plants grow at 25 % FC reached 100 % germination at 36 h. In contrast, the seeds developed at 100 % FC (control), total germination was reached by 60 h. In P. acutifolius no changes were observed as an effect of FC (Figure 6B).
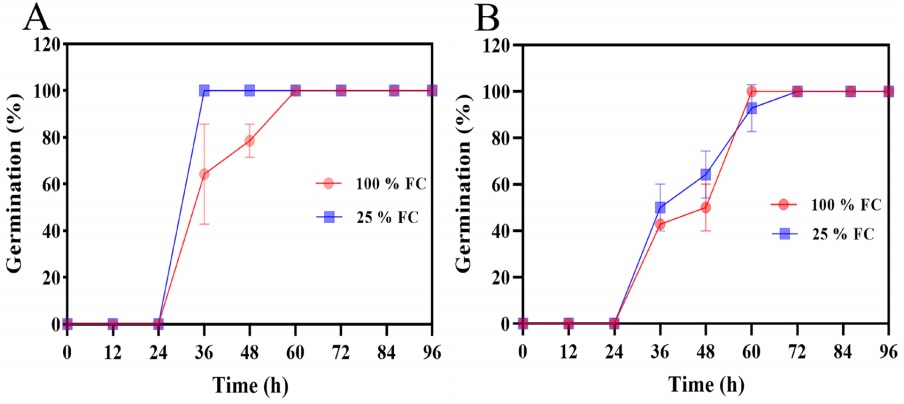
Figure 6 Seed germination percentage of P. vulgaris cv. Rosa Bufa (A) and P. acutifolius (10017 Tepary bean) (B) at 100 % (FC) red circle, and 25 % (FC) blue square during seed development; n = 50.
Amylolytic enzyme activity using semiquantitative native PAGE (zymogram) showed three major bands in crude extracts from both species. The clear colorless bands were putative amylases (Figure 7A) represented as bands A1 and A2, the third one putative clear or pale blue, A3 leads, also suggested some activity (Zeeman et al. 1988) attributed to amylolytic enzymes. One minor band was visible when gels were incubated for a longer period (Figure 7A).
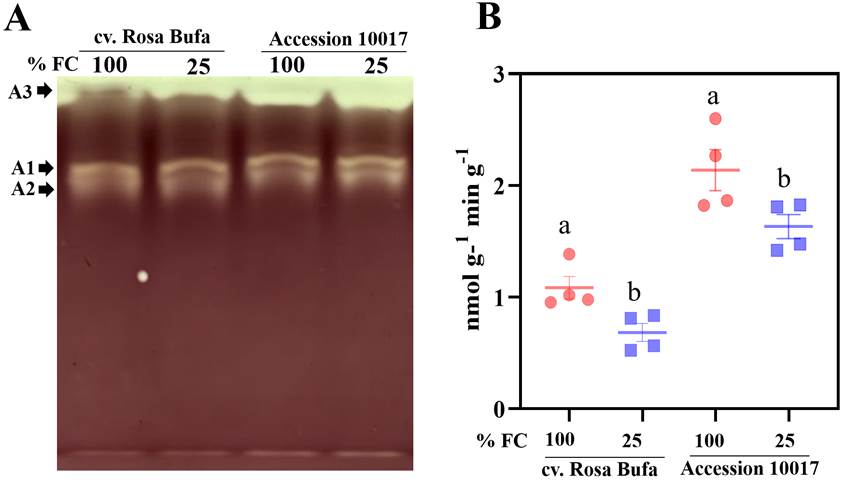
Figure 7 Native PAGE of starch-degrading enzymes (A). A1, A2, A3 represent different band with amylolytic activity in the gel. Changes in enzymatic activities of α-amylases (B). The experiments were carried out from crude homogenates of seeds at physiological maturity germinated for 24 h of P.vulgaris cv. Rosa Bufa and P.acutifolius (10017) at 100 % (FC), red circle and 25 % (FC) blue square. Different letters indicate a significant difference between moisture treatments. Tukey test (P ≤ 0.05).
The enzymatic activity of the α-amylases was quantified in crude extracts from both species (Figure 7B). Reduction between 15 and 20 % in their activity was observed as pNP-G7 production in seeds grown at 25 % FC (Figure 7B) in both species, but in Tepary beans the activity was twice as high compared to cv. Rosa Bufa.
Discussion
Starch is the most abundant nutrient in Phaseolus seeds, accounting for up to 70 to 80 % of their total carbohydrates. Nevertheless, little research has been focused on the impact drought has on the starch content of legume seeds. This research focuses on evaluating the water restriction effects on the seeds' developmental process. The seed weight of Tepery beans was ̴ 40 % lower than P. vulgaris. These observations concur with previous reports of grain legumes with seed size variation between species (Blessing et al. 2018). Our results indicate that under water restriction (25 % FC), both species do not significantly modify their seed morphological traits (weight, thickness, and width) except for length in P. acutifolius (Figure 2). However, the number of seeds per plant decreased 40-50 %, due to the restriction, respect to the control (data not included) because of reproductive tissue abortion. Seed abortion can be a response to reduced resource availability because of low rates of carbohydrate remobilization from vegetative tissue and pod wall to seeds. Seed size increases under water deficit before or at the early reproductive stage (Fang et al. 2011). However, on chickpeas (Pushpavalli et al. 2015), lentils (Shrestha et al. 2006), grass peas (Gusmao et al. 2012) and common beans (Sehgal et al. 2018), it is reported that seed size was not affected by water deficit despite yield decrease. This also points to a relative increase in carbon allocation to reproductive tissues. Thus, our results indicate that 25 % FC has no effect on seed size in P. vulgaris and P. acutifolius.
At water restriction conditions and nitrogen deficiency cotyledons of maize (Zhou et al. 2018) and wheat (Zhang et al. 2017) alter their starch synthesis and grain weight. It is well documented that moisture regulates the grain's length and surface area, thus influencing grain yield. Starch granules of P. vulgaris are partially modified under moisture restrictions, but the seed morphology was not altered. In contrast to P. acutifolius, drought affects starch synthesis by regulating the enzymatic activity for starch biosyntheses, such as soluble starch synthase and ADP-glucose pyrophosphorylase (Lu et al. 2019). Our results indicate that 25 % FC in P. vulgaris could accelerate grain filling in a reduced number of seeds, maintaining grain weight. The observation would have great significance in achieving the dual goal of increasing crop yield and saving water for final product quality.
Information in the literature regards a differential role of starch granules in beans as a drought response is limited. The available data regard plants grown under optimal conditions indicate that enzymes as starch synthase and ADP-glucose pyrophosphorylase in rice grains contribute to establishing sink strength in seeds by distinctively participating in different stages of seed development (Prathap et al. 2019). The starch accumulation in common beans pre‐filling pods analyzed in this research concurs with other crop data, where the AGPase activity increases as a response to drought treatments (Cuellar-Ortiz et al. 2008). However, results contrast with those obtained in storage organs, where a high sink strength is distinctive; as in stem rice, in which drought induces a decrease in starch accumulation and AGPase activity (Yang et al. 2001).
Although P. acutifolius yield was significantly reduced under water restrictions, the number of seeds per plant was three times higher than that in P. vulgaris cv. Rosa Bufa (data not published). This could be explained by the fact that Tepary bean plants have an indeterminate growth habit that may attenuate temporary drought effects, following deep rooting strategies which are more capable to withstand drought than shallow-rooted annuals (Ye et al. 2018). Thus, the reduction of the maximum and minimum axis of starch granules in Tepary beans compared to P. vugaris suggests that a high proportion of small starch granules in Tepary bean is a consequence of the enzymes involved in starch synthesis playing key roles in grain size, which could improve the starch molecular structure and functional properties in the grain. These results are consistent with analyses in two wheat varieties. On them, the authors report visual evidence of ultra-structural changes on granule surfaces induced by drought, with differential reduction of granule sizes, leading to an increased susceptibility of the starches to drought-induced hydrolytic enzymes (Li et al. 2015).
The differences between both bean species in the present study indicate the capacity of P. acutifolius seeds to buffer drought impact and maintain a similar nutrient concentration under both, irrigated and drought conditions. Nevertheless, this study supports the fact that genetic variation in bean species in response to drought exists not only for their yield, as previously detected but also for their nutrient concentration (Herrera et al. 2019).
Regard their soluble sugars, glucose concentration in cotyledons increased as a response to water restriction. Fructose levels were maintained without changes in levels 10 to 20 times lower than glucose. These results contrast with those reported for wheat plants exposed to drought stress during grain filling, in which, glucose, fructose, and sucrose concentrations significantly declined in grains of a drought-sensitive genotype accompanied by a sharp reduction in cell wall invertase activity and soluble invertase activity (Koch 2004, Ruan 2012). Certain concentrations of soluble sugars in the cotyledons of cereals are associated with protection to stresses and drought response (Lotfi et al. 2010). Regard sugar signaling in reproductive organs, it has been shown that hexoses stimulate cell division, while sucrose promotes cell endoreduplication and starch accumulation in the cotyledons in Vicia faba (Weber et al. 1996). These authors further propose that cell wall invertase (CWIN) in the seed coat of V. faba affects the developmental processes of seeds by regulating sugar signaling in the embryo. In maize, the mutation of a CWIN gene (INCW2) resulted in a miniature seed phenotype by blocking cell division in the endosperm (Vilhar et al. 2002). Our results indicate that a possible regulatory network modulates the sucrose metabolism and signaling in seeds set under abiotic stresses. In this study, we detected neutral carbohydrate in the light micrographs (Figure 3A-D). However, further work is needed to extend the method of analysis to other oligosaccharides, currently underway in our laboratory.
Seed quality traits depend on the accumulation of various storage molecules during seed development. Drought stress during the initial stage of seed development reduces the capacity of seeds to sink strength, by decreasing the number of endosperm cells and formed amyloplasts (Sehgal et al. 2018), thus reducing grain weight with a decline in endosperm competence to gather starch, in terms of both, rate and duration. In the present study, a similar effect was observed in P. acutifolius.
To test if water restriction affects the physiological seed quality of Phaseolus species, we tested the hypotheses that 25 % FC during seed development affects seed imbibition and germination percentage (Table 1). Results showed that in both cultivars, water absorption increased four-folds with respect to dry seeds at 72-96 h (Table 1). This result concurs with those reported by Aliu et al. (2016) where the time limit for the imbibition of seeds began to reduce after 72 h and even 48 h.
Surprisingly, in cv. Rosa Bufa at 25 % FC, imbibition significantly increased at 12 h respect to the 100 % FC (Table 1). These results correlate with the germination percentage reached at 36 h at 25 % FC while at 100 % FC was at 60 h. Some reports demonstrate that germination depends on water as the main factor that stimulates germination and the chemical composition of the seeds (Saux et al. 2020). We do not discard that in cv. Rosa Bufa the water restriction during seed development impacts the imbibition rate thus, germination as a consequence of alterations in the biochemical components associated with the water adsorption. The differences in seeds imbibition among the species may relate to seed coat thickness, the number of seed coat pores and the size of the micropyle and hilum (Borji et al. 2007).
Several reports indicate that α-amylases are key in the degradation of stored starch in the cotyledons (Ali & Elozeiri 2017, Damaris et al. 2019, Sehgal et al. 2018, Liu et al. 2018). These enzymes account for 40-60 % of de novo protein synthesis in grains (Liu et al. 2018). Although α-amylase is necessary to initiate starch degradation in cereal grains (Beck & Ziegler 1989), little is known about the activity in legumes.
The water restriction effects during grain development on amylolytic activity and α-amylase activity were investigated in this study (Figure 7). The native gels show three putative starch hydrolyzing enzymes involved in reserves remobilization during germination (Figure 7A). This indicates that bean extracts from P. vulgaris and P. acutifolius are capable of starch degradation and that endo amylases activity is present in the amyloplast. Therefore, we conducted a quantitative assay to assess α-amylase activity on crude extracts from cotyledons. Results show that the enzymatic activity reduced ̴10 to 15 % in seeds from plants grown at 25 % FC in both species, compared to seeds from plants in 100 % FC. However, a two-fold increase in activity was observed, twice as higher, in Tepary beans compared to P. vulgaris (Figure 7B). It might be possible that in Tepary beans an increase in the phosphorylase activity enables the phosphorolysis of starch partly to accelerate the hydrolysis (Chen et al. 2017). Srivastava & Kayastha (2014) report that in seeds of Trigonella foenum-graecum β-amylase was the major starch degrading enzyme, depending on the amount of enzyme present, compared to α-amylase and on its localization at the periphery of amyloplasts. That might reflect their different roles among various amylolytic enzymes in a complex regulation. Further experiments are necessary to elucidate the role of each amylase in starch stored degradation in the cotyledons, particularly during seed germination, to facilitate crop breeding as an efficient biomarker in legumes.











 nueva página del texto (beta)
nueva página del texto (beta)



