Dioecy, the coexistence of individuals that produce either female or male flowers, is extremely rare in the family Cactaceae; only 24 of the 1,438 known species (ca. 2 %) have evolved toward dioecy or one of its variants - subdioecy, gynodioecy, or trioecy (Fleming et al. 1994, Hunt et al. 2006, Mandujano et al. 2010, Orozco-Arroyo et al. 2012). Dioecy arose from an ancestral hermaphroditic condition as a mechanism to either avoid inbreeding depression or optimize resource allocation to the male and female function (Barrett 2002). With the evolution towards dioecy, sex morphs acquire distinct roles, and sex-specific selection gradually leads to dimorphism in primary and secondary sexual traits (Lloyd & Webb 1977, Geber 1999, Barrett & Hough 2013). Studies describing sexual dimorphism in cacti are scarce (Hoffman 1992, Fleming et al. 1994, Díaz & Coccuci 2003). Nevertheless, the first step toward understanding reproductive diversity in the family Cactaceae is to characterize dimorphism in breeding systems morphologically.
Dioecy has evolved independently in several cacti lineages, and hermaphroditism is regarded as the ancestral condition since Pereskia, the most primitive cactus genus, has bisexual flowers and unisexual flowers of the genera Consolea, Cylindropuntia, Opuntia, Echinocereus, Pachyecereus, and Mammillaria show vestiges of a non-functional verticil (Fleming et al. 1994, Rebman 1998, Strittmatter et al. 2002, Del Castillo & Trujillo-Argueta 2009, Hernández-Cruz et al. 2018). Differences in primary and secondary characteristics in cacti populations with various gender combinations (female, male, and hermaphrodite) include the number of ovules and pollen grains, which are usually significantly higher in unisexual flowers than in hermaphrodites (Díaz & Cocucci 2003, Del Castillo &Trujillo-Argueta 2009) and are absent or insignificant in unisexual flowers (Baker 2006, Gutiérrez-Flores et al. 2017); female flowers are smaller than hermaphroditic or male flowers (Baker 2006, Fleming et al. 1994), have shorter stamens (Hoffman 1992, Díaz & Cocucci 2003, Baker 2006) or styles (Fleming et al. 1994), and lower nectar production (Díaz & Cocucci 2003). Differences in fertility and performance of the progeny, such as seed weight and viability, and seedling growth have also been reported (Del Castillo & Trujillo-Argueta 2009).
In gynodioecious or trioecious populations of Mammillaria blossfeldiana Boed., M. neopalmeri R.T. Craig, and M. dioica K. Brandegee, female flowers have been identified based on the morphology of the non-functional vestigial androecium, which has small, entirely indehiscent anthers, with malformed pollen or no pollen at all (Lindsay & Dawson 1952, Parfitt 1985, Rebman 2001). So far, only in M. dioica has gynodioecy been confirmed by means of an embryological study, which showed that male sterility is due to the abortion of anthers (Sánchez & Vázquez-Santana 2018). However, sexual dimorphism has not been described in any other species in this genus, nor have morphometric analyses of sexual characteristics been performed.
In their classic work, Bravo-Hollis & Sánchez-Mejorada (1991) described the flowers of Mammillaria magnimamma Haw. as hermaphrodites, with a pistil longer than the stamens. However, our observations in a population at Valle del Mezquital, State of Hidalgo, during the 2018 reproductive season, revealed the coexistence of individuals bearing hermaphroditic flowers and individuals whose flowers had a vestigial androecium; both types of individuals were able to produce fruits and seeds. These observations led us to propose the existence of a gynodioecious population in the semi-arid region of Valle del Mezquital. Thus, the general objective of our study was to determine the breeding system of M. magnimamma, with the following specific objectives: 1) characterize sexual differences in floral morphology in the population by means of scanning electron microscopy (SEM) and morphometric analysis, and 2) compare pollinator frequency, seed size and germination success between floral morphs.
Materials and methods
Study Site and Species. The study was conducted near Huichapan (20° 22' 47.2" N, 99° 36' 23.9" W), Valle del Mezquital, State of Hidalgo. The climate type is BS1kw, that is, semi-arid temperate with a summer rainy season; maximum temperatures occur in April and May (27° C), and the minimum in January (4 °C); precipitation ranges from 500 to 700 mm and phaeozem haplic soils predominate in the area (UMAFOR 2010, SEMARNAT 2013). The M. magnimamma population is located at an elevation of 2,358 m, forming part of a xerophytic shrubland together with Acacia farnesiana (L.) Willd, Coryphanta cornifera Lem., C. octacantha Britton & Rose, Cylindropuntia imbricata (Haw.) F.M. Knuth, Echinocereus cinerascens Lem., Ferocactus latispinus Britton & Rose, Myrtillocactus geometrizans Console, Opuntia engelmannii Salm-Dyck ex Engelm., O. hyptiacantha F.A.C. Weber, O. lasiacantha Pfeiff., O. robusta H.L. Wendl. ex Pfeiff., and Yucca filifera Chabaud (UMAFOR 2010).
Mammillaria magnimamma grows as a single-stemmed plant or as a cluster of multiple stems that grow from the base (Bravo-Hollis & Sánchez-Mejorada 1991). Flowers are 25 mm long and 20 mm wide, bell-shaped with the external segments of the perianth reddish, and inner segments with a deep pink midrib; stamens and style are whitish to deep pink, and a wide range of colours can be found in populations (Bravo-Hollis & Sánchez-Mejorada 1991). Flowers of the genus Mammillaria exhibit features associated with melittophilic syndrome such as white to pinkish colour, short floral tube, and copious amounts of pollen (Bravo-Hollis & Sánchez-Mejorada 1991, Mandujano et al. 2010). M. magnimamma flowers are diurnal, start opening around 10:30 a.m., are fully open between 1:30 and 3:30 p.m., and close around 6:00 p.m. Most flowers last only one day, except for a few flowers with indehiscent anthers that can remain open for up to two days provided the tepals remain hydrated (Callejas-Chavero et al. in review). Flowering takes place during the dry season of the year, peaking in late March and mid-April (Valverde et al. 2004); fruiting takes place in the rainy season, from June to August. The clavate fruits are 20-35 mm long and contain some 100 seeds each (Valverde et al. 2004).
In situ Determination of Sex and Sex Ratios. A total of 107 individuals of the population were geo-referenced with a Garmin eTrex 30 GPS during the 2019 reproductive season. The external morphology of flowers on each plant was observed throughout anthesis to determine their floral type and confirm that the individuals observed in 2018 kept the same sexual condition. One flower per plant was collected and cut longitudinally to corroborate its sex (Gutiérrez-Flores et al. 2017). The gynoecium and androecium were examined to detect deformed organs and the presence of ovules or pollen grains (Sánchez & Vázquez-Santana 2018). Individuals whose flowers bore a robust style, ovules, and pollen grains were labelled as hermaphrodites; plants whose flowers showed a robust style and ovules plus a vestigial androecium with small anthers that remained completely indehiscent throughout anthesis were labelled as females (Gutiérrez-Flores et al. 2017, Sánchez & Vázquez-Santana 2018). The relative frequency of sexes was determined by dividing the number of each sex by the total number of plants. A χ2 test was used to assess whether the frequency distribution of plants with different flower types departed from a homogeneous distribution; the test was carried out with the software PAST v3.26 (Hammer et al. 2001).
Micro-morphological and Morphometric Characterization of Flower Morphs. To characterize the micro-morphology of each floral morph, one to five fully open flowers were collected from 15 hermaphroditic and ten female plants (for a total of N = 17 hermaphroditic and N = 25 female flowers) and kept in FAA fixative solution (five parts of formaldehyde, five parts of glacial acetic acid and 90 parts of 50 % ethanol). In preparation for examination of the gynoecium (ovary, style, and stigma) and androecium (filaments, anthers, and pollen grains) with scanning electron microscopy (SEM), the flowers were longitudinally cut and washed with distilled water twice for 30 min. These flowers were examined with a Zeiss EVO MA15 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) using the variable pressure mode at above-freezing temperature, using a cold sample stage at -10 °C. Micrographs were captured with a high-definition backscattered electron detector, with 39 X, 22 X, 71 X, and 2.00K X magnification for the stigma lobes, ovary chamber, anthers, and pollen grains, respectively. This procedure was repeated for each flower type for comparison.
Morphological differences observed between the two flower types were evaluated by means of a morphometric analysis of the variables describing primary and secondary sexual traits (Hoffman 1992, Gutiérrez-Flores et al. 2017). One mature, closed flower bud and one fully open flower were collected from each of 25 hermaphroditic and ten female plants (Gutiérrez-Flores et al. 2017) and preserved in FAA. These flowers were cut longitudinally and photographed in the Olympus SZX10 stereomicroscope; sexual traits were measured using the software Olympus cellSens Entry 1.17 (Olympus Corporation, Hamburg, Münster, Germany), following the criteria proposed by Gutiérrez-Flores et al. (2017). Primary traits, i.e., those directly related to the female and male sexual organs, include the gynoecium (ovary, stigma, and style) and androecium (stamens and anthers), as well as the production of ovules and pollen (Lloyd & Webb 1977, Geber 1999). We measured the ovary polar and equatorial diameters as well as the length of the style, stigma, and pistil at 1X magnification. The number of stamens in one half of each flower was counted and doubled to estimate the total number of stamens; the length of basal, middle, and distal stamens (9 stamens/flower) was measured at 2.5X magnification. The number of ovules in one half of the ovary chamber was counted at 2X magnification and doubled to estimate the total number of ovules. To count the number of pollen grains, 15 anthers per mature closed bud were collected and their pollen suspended in 50 µL of FAA solution; the pollen grains in a 10 µL aliquot of this suspension were counted in a Neubauer Improved Superior chamber (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). The total number of pollen grains was estimated by extrapolating to the total volume and number of anthers per flower. Secondary sexual characters are those related to pollinator attraction and pollen dispersal; these include the corolla and perianth width, flower length, and length and width of the nectarium. These traits were measured at 1X magnification (Lloyd & Webb 1977, Geber 1999).
To test for differences in morphological characteristics between flower types, a non-parametric permutational multivariate analysis of variance (PERMANOVA, suitable for non-normally distributed data) was first conducted to identify which morphometric variables of primary and secondary characteristics best discriminate the flower types; for this analysis, we used the Euclidean distance and ran 9,999 permutations to estimate the significance of the between-group differences (Hammer et al. 2001). The individual variables thus identified were tested using either Kruskal-Wallis test followed by pairwise Mann-Whitney tests (non-normal distribution) or one-way ANOVAs followed by Tukey’s multiple comparison tests (normal distribution). All the analyses were carried out using the software PAST v3.26 (Hammer et al. 2001). Finally, we calculated the pollen:ovule ratio (P:O) proposed by Cruden & Lyon (1985) to determine the mating system and whether hermaphroditic flowers show self-fertilization.
Pollinators. Flower visitors were observed, and potential pollinators were collected. Those insects that immersed themselves into the flower, made contact with anthers and stigma, emerged with the body covered with pollen grains, and then flew to another flower on either the same or another plant, were regarded as pollinators. Pollinators and their visiting frequency to five flowers of each of 16 hermaphroditic (N = 80 flowers) and eight female (N = 40 flowers) individuals were recorded during anthesis. Pollinators visiting each plant were recorded in two 20-minute observation periods per hour from 10:30 a.m. to 5:30 p.m., on two consecutive days, for a total observation time of 159.2 h. At the end of the pollination activity, the pollinators were caught and preserved in 70 % alcohol for identification in the laboratory using specialized taxonomic keys (Michener et al. 1994) and additional corroboration by specialists. A χ2 test was used to determine whether the frequency of pollinator visits differed between flower types.
To evaluate nectar production, 15 hermaphroditic and nine female individuals were isolated throughout anthesis using fine nylon mesh netting. At the onset of anthesis (10:30 a.m.), five hermaphroditic and three pistillate flowers per individual were collected and dissected to quantify nectar volume with 10 μL micropipettes and measure sugar concentration with a refractometer. This procedure was repeated every half hour until the end of anthesis (5:30). A total of 75 hermaphroditic and 45 pistillate flowers was examined.
Seed Size and Germination. Fruits from 29 hermaphroditic and seven female plants - as practicable, depending on fruit availability - were collected to estimate the average number of seed per fruit. A total of 176 and 40 fruits were collected from hermaphroditic and pistillate plants, respectively. From this sample, a random subsample of three fruits per individual was taken from six hermaphroditic (N = 18 fruits) and three female (N = 9 fruits) plants to prepare a composite sample of seed from each flower type (N = 806 and 430 hermaphroditic and pistillate seeds, respectively). The length (r1) and width (r2) of each seed were measured under the microscope, and the seed area was calculated as A = r1 × r2 × (. A Kruskal-Wallis test was used to determine the significance of differences in the average number of seeds and mean seed area, using the software XLSTAT v2019.4.1 (Addinsoft 2019) and PAST v3.26 (Hammer et al. 2001).
Ten fruits from hermaphroditic (N = 500 seeds) and five from female (N = 250 seeds) plants were selected, and 50 seeds from each fruit were germinated. The seeds were disinfected with 2 % sodium hypochlorite and sown on filter paper in Petri dishes; they were kept in the greenhouse at 26 ºC and 70 % humidity and watered twice a week (Ruedas et al. 2000). Seed germination of each flower type was recorded daily for 41 days, and the percentage of seeds germinated per day was calculated to evaluate the germination response. The standard error for a binomial distribution was calculated for each day and each flower type (Zar 2010), and the distributions were compared with a non-parametric Wilcoxon signed-rank test using the software PAST v3.26 (Hammer et al. 2001).
Results
Morphological Analysis. The in-situ morphological examination of M. magnimamma plants confirmed the coexistence of two flower morphs, 94 individuals (87.85 %) were hermaphrodites, and 13 (12.15 %) were male-sterile individuals (Figure 1). The frequencies of floral morphs in the population departed significantly from a homogeneous distribution (χ2 = 27.2, df = 1, P < 0.0001). Our observations on the external morphology of flowers confirmed that the plants did not change their sexual condition from one year to the other.

Figure 1 Floral morphs and colour variation in Mammillaria magnimamma in the Valle del Mezquital population. Frontal view of a hermaphroditic flower with dehiscent anthers and abundant pollen grains that can be seen on the perianth and the filaments of stamens A, C, E. Frontal view of a pistillate flower showing the non-functional androecious with shriveled filaments and collapsed yellow anthers B, D. Pistillate flower with whitish anthers lacking pollen F. Longitudinal section of hermaphroditic and pistillate flowers showing the difference in flower length G, H.
The SEM analysis results were consistent with the morphological variation observed in the field (Figure 2). Hermaphroditic flowers showed a functional gynoecium and androecium; a robust style and stigma with papillated lobules (Figure 2A); a unilocular ovary filled with mature ovules (Figure 2C); and dehiscent anthers with numerous, well-developed, apparently fertile, spheroidal, tricolpate pollen grains (Figures 2E, G). Female flowers showed a conspicuous style and stigma (Figure 2B); the ovary filled with mature ovules (Figure 2D); a non-functional androecium with collapsed, indehiscent anthers and a constriction at the filament-anther union (Figure 2F); and small pollen grains with malformations and undifferentiated walls (Figure 2H).

Figure 2 Micromorphology of reproductive structures of different floral morphs of Mammillaria magnimamma from Valle del Mezquital population. Longitudinal section of hermaphroditic and female flowers. Papillae stigma of hermaphroditic (A) and female (B) flowers. Ovary chamber filled with ovules of hermaphroditic (C) and female (D) flowers. Dehiscent anthers of hermaphroditic flower (E); collapsed, indehiscent anthers and constriction at the union of the filament (arrow) of a female flower (F). Pollen grains from hermaphroditic flowers (G); abnormal pollen grain from female flowers (H). 39 X, 22 X, 71 X, and 2.00K X magnifications for the stigma lobes, ovary chamber, anthers, and pollen grains, respectively.
The primary sexual traits that best differentiated between floral types were those associated with the androecium (PERMANOVA F = 3.281, P = 0.031; Table 1). Even though some differences between morphs were found in traits associated with the female function, such as the ovary polar diameter and the number of ovules, these were not statistically significant (PERMANOVA F = 2.308, P = 0.127). The non-functional condition of the androecium of female flowers was confirmed by their significantly shorter stamens (mean length of basal stamens: 4.064 ± 0.150 mm; middle: 4.103 ± 0.163 mm; and distal: 3.971 ± 0.176 mm) compared to those of hermaphroditic flowers (4.684 ± 0.171; 4.805 ± 0.199; and 4.892 ± 0.184 mm, respectively; Table 1), and significantly fewer pollen grains (F 1,26 = 4.422, P = 0.045; Table 1). Although the atrophied anthers of female flowers may contain pollen grains, these were small and malformed, collapsed, or fragmented.
Table 1 Means and standard errors (SE) of sexual characteristics, average number of seed per fruit and average seed length, and ANOVA or Kruskal-Wallis results (F or Hc* values, respectively and significance level) for two flower types of Mammillaria magnimamma in the Valle del Mezquital population.
| Variable | Hermaphrodite (N = 18) |
SE | Pistillate (N = 10) |
SE | F or Hc* | P |
|---|---|---|---|---|---|---|
| Primary characters | ||||||
| Gynoecium | ||||||
| Style length (mm) | 8.924 | 0.488 | 9.139 | 0.239 | 0.745 * | 0.388 |
| Stigma length (mm) | 1.358 | 0.110 | 1.176 | 0.064 | 2.355* | 0.125 |
| Number of stigma lobules | 6.556 | 0.372 | 6.300 | 0.335 | 0.279* | 0.598 |
| Equatorial diameter of ovary (mm) | 2.081 | 0.106 | 2.060 | 0.122 | 0.148 | 0.704 |
| Polar diameter of ovary (mm) | 2.764 | 0.120 | 2.407 | 0.188 | 2.927 | 0.100 |
| Number of ovules per ovary | 171.667 | 12.678 | 140.100 | 14.876 | 2.608 | 0.119 |
| Androecium | ||||||
| Number of stamens per flower | 167.889 | 9.680 | 139.200 | 12.828 | 2.992* | 0.084 |
| Length of basal stamens (mm) | 4.682 | 0.171 | 4.064 | 0.150 | 5.833 | 0.023 |
| Length of middle stamens (mm) | 4.805 | 0.199 | 4.103 | 0.163 | 5.672 | 0.025 |
| Length of distal stamens (mm) | 4.692 | 0.184 | 3.971 | 0.176 | 6.620 | 0.016 |
| Number of pollen grains | 176,831.1 | 13,110.8 | 125,380 | 22,961.049 | 4.422 | 0.045 |
| Distance stigma-stamens (mm) | 3.528 | 0.176 | 3.884 | 0.239 | 1.453 | 0.239 |
| Secondary characters | ||||||
| Corolla width (mm) | 8.074 | 0.303 | 7.159 | 0.372 | 3.235 | 0.072 |
| Perianth diameter (mm) | 5.994 | 0.325 | 5.029 | 0.325 | 3.773 | 0.051 |
| Flower length (mm) | 15.821 | 0.450 | 14.007 | 0.742 | 5.380 | 0.031 |
| Floral tube length (mm) | 10.491 | 0.344 | 10.307 | 0.349 | 0.383 | 0.543 |
| Length of nectary chamber (mm) | 2.194 | 0.090 | 1.856 | 0.151 | 5.257 | 0.033 |
| Width of nectary chamber (mm) | 1.523 | 0.105 | 1.440 | 0.092 | 2.830* | 0.108 |
| Seeds | ||||||
| Mean number of seeds per fruit | 97.541 | 12.946 | 120.036 | 6.022 | 8.320 | 0.003 |
| Seed area (mm2) | 1.021 | 0.027 | 1.113 | 0.042 | 4.637* | 0.031 |
Regarding secondary sexual characteristics, there were significant differences in floral display (PERMANOVA F = 3.069, P = 0.037) and floral reward (PERMANOVA F = 6.286, P = 0.016) between the two flower types. Female flowers were significantly shorter (15.821 ± 0.450 mm) and had smaller nectar chambers (1.856 ± 0.151 mm) than hermaphroditic flowers (14.007 ± 0.742 and 2.194 ± 0.090 mm; respectively) (Table 1, Figure 1G-H). The P:O ratio was 1,030.08, which corresponds to facultative xenogamy and indicates that some of the crosses in M. magnimamma can occur by autogamy.
Pollinators. Mammillaria magnimamma seems to be pollinated primarily by bees. They land directly on the stigma and enter to reach the nectar chamber making contact with the anthers. This ensures the collection and subsequent deposition of pollen on flowers in the same or another plant and suggests that small local bees, particularly in the genus Ceratina, are potential pollinators of M. magnimamma (Table 2). However, nitidulids in the genus Meligethes sp. were frequently observed crawling at the base of flowers and along the floral tube, stamens, and stigma, and moved between flowers, mainly in the same plant, with their body covered with pollen (Table 2). Hermaphroditic flowers received twice as many visits (124) as female flowers (60 visits; χ2 = 27.30; P = 0.0012). Hermaphroditic flowers were visited by bees (61 visits) and beetles (63 visits) in similar frequency; the bees most frequently visiting were Apis mellifera (Linnaeus, 1758), Chelostoma rapunculi (Lepeletier, 1841), and Ceratina (Zadontonemus) nautlana (Cockerell, 1897) sp. 3 (Table 2). Pistillate flowers were more frequently visited by bees (35 visits) than by Nitidulids (25 visits); the bees most frequently visiting were Ashmeadilella prosopidis (Timberlake, 1916), Ceratina (Zadontonemus) nautlana, sp. 1, and sp. 5 (Table 2).
Table 2 Pollinators of Mammillaria magnimamma and visit frequency per flower type in the Valle del Mezquital population.
| Family | Subfamily | Species | Hermaphrodite | Pistillate |
|---|---|---|---|---|
| Apidea | Apinae | Apis mellifera (Linnaeus, 1758) | 15 | 3 |
| Halictinae | Lasioglossum dialictus (Robertson, 1902) | 3 | 0 | |
| Megachilinae | Chelostoma rapunculi (Lepeletier, 1841) | 13 | 4 | |
| Ashmeadilella prosopidis (Timberlake, 1916) | 2 | 5 | ||
| Xilocopinae | Ceratina (Zadontomerus) nautlana (Cockerell, 1897) | |||
| Ceratina (Zadontomerus) nautlana sp. 1 | 9 | 8 | ||
| Ceratina (Zadontomerus) n. sp. 2 | 4 | 3 | ||
| Ceratina (Zadontomerus) n. sp. 3 | 12 | 3 | ||
| Ceratina (Zadontomerus) n. sp. 4 | 3 | 2 | ||
| Ceratina (Zadontomerus) n. sp. 5 | 0 | 7 | ||
| Nitidulidae | Meligethinae | Meligethes sp. | 63 | 25 |
| Total | 124 | 60 |
We were unable to quantify nectar production, as it was absent in some flowers or was produced in such extremely low quantity that it remained attached to the micropipette tip.
Nectar produced by those flowers that did so maybe consumed by small ants and nitidulids, as these were found inside the nectaries of approximately 30 % of the flowers dissected. Nitidulids likely consume the nectar before the flowers open, as they can enter mature buds.
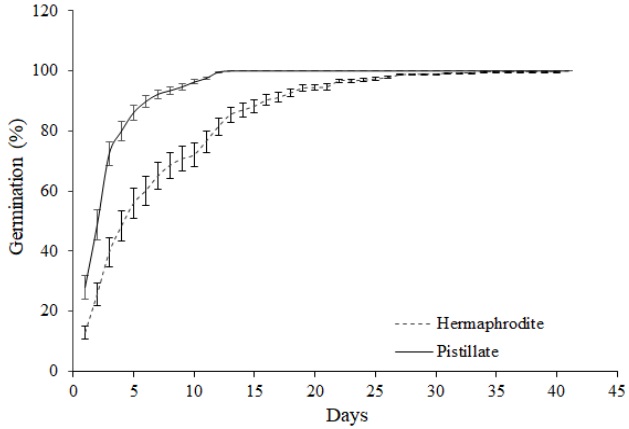
Seed Size and Germination Rate. All flower types set fruits and seeds, indicating that the female function remains active in both flower types of M. magnimamma (Table 1). However, the average number of seeds produced by hermaphroditic (97.541 ± 12.946 seeds) and female (120.036 ± 6.022 seeds) flowers differed significantly (F 1,215 = 8.320, P < 0.001); female flowers produced 23.06 % more seeds than hermaphrodites. Seeds produced by female flowers were significantly larger (mean area: 1.1 mm2) than those produced by hermaphroditic flowers (1.0 mm2; Hc = 4.637, P = 0.031). Seeds from female flowers had a germination rate (in the early 20 days) significantly higher than those from hermaphrodites (Hc = 70.59, P < 0.0001, Figure 3).
Discussion
The morphological analysis confirmed that Mammillaria magnimamma shows a gynodioecious breeding system. Hermaphroditic flowers have fully functional gynoecium and androecium, while female flowers bear a sterile androecium. Male sterility is evidenced by the failure to form functional anthers, pollen, or male gametes (Kaul 1988). According to our micromorphological analysis, the presence of tetrasporangiate indehiscent anthers with small, deformed pollen grains and the distal region of the filament being collapsed at its union with the anther are indicative of male sterility in M. magnimamma. These micromorphological features are similar to those described in pistillate flowers of Pachycereus pringlei (S. Watson) Britton & Rose (Gutiérrez-Flores et al. 2017), Mammillaria dioica (Sánchez & Vázquez-Santana 2018), and Opuntia robusta (Hernández-Cruz et al. 2019). The morphometric analysis of M. magnimamma showed that the non-functional androecium (basal, middle, and distal stamens) of pistillate flowers is smaller than that of hermaphrodites and their collapsed anthers retain scarce malformed pollen grains. Similar differences were found in the flower and nectar chamber lengths (Table 1). Our results are consistent with the dimorphic patterns observed in dioecious cacti. In dioecious populations of Echinocereus coccineus Engelm., female flowers were significantly shorter, had a narrower corolla, shorter and fewer stamens, and smaller anthers than male flowers (Hoffman 1992). In dioecious populations of Echinocereus yavapaiensis M.A. Baker (Baker 2006), trioecious populations of Pachycereus pringlei (Fleming et al. 1994), and a gynodioecious population of Mammillaria dioica (Sánchez & Vázquez-Santana 2018), female flowers are shorter and have a narrower corolla than the other floral types; pistillate flowers of Opuntia quimilo K. Schum. have lower biomass than hermaphroditic flowers (Díaz & Coccuchi 2003).
The presence of a sterile androecium in pistillate flowers suggests that the evolution towards dioecy in M. magnimamma follows the gynodioecy path; male sterility and its dispersal across the population constitute the earliest stage in the evolution of hermaphroditism towards dioecy (Charlesworth & Charlesworth 1981, Spigler & Ashman 2012). Male sterility seems to have successfully established in the Huichapan population since the sex of individuals remained unchanged from one year to the other. The gynodioecy-evolutionary pathway has been commonly observed in members of the family Cactaceae (Strittmatter et al. 2002, Del Castillo & Trujillo-Argueta 2009), and it is highly likely to occur when both the inbreeding rate and inbreeding depression are high (Käfer et al. 2017). The clonal habit of M. magnimamma may have induced inbreeding crosses as many flowers open simultaneously, and pollen transfer between flowers of the same plant is common (Barrett 2002). The P:O = 1,030.08 suggests that M. magnimamma has a mixed breeding system and that some of the crosses might occur by autogamy (Cruden & Loyn 1985) so that inbreeding might have been present in the isolated Huichapan population and has been a factor enabling the evolution towards dioecy.
As indicated by the short floral tube and nectarium, as well as by the amount of pollen, M. magnimamma exhibits a melittophilic pollination syndrome (Wyatt 1983, Mandujano et al. 2010). Local bees, particularly in the genus Ceratina, might be potential pollinators of M. magnimamma and contribute to both outcrossing and autogamy since they transfer pollen from the same or a different clone (Mandujano et al. 2010). The bees Ceratina sp. 1 and sp. 2, as potential pollinators of Mammillaria pectinifera F.A.C. Weber, facilitate outcrossing as their foraging ranges (57.05 ± 9.28 m and 79.98 ± 18.01 m, respectively) allow the transfer of pollen between different individuals in the population (Valverde et al. 2015). This M. magnimamma population occupies an 11,050 m2 area and, thus, Ceratina bees could ensure interbreeding between hermaphroditic individuals and between the two floral types. At the same time, the simultaneous opening of flowers allows bees to visit several flowers in the same clone, thus promoting autogamy (Charlesworth & Charlesworth 1987). On the other hand, beetles in the genus Meligethes sp. might also contribute to autogamy (facilitated autogamy and geitonogamy), as they enter the mature buds before the onset of anthesis and remain in the same clone for a long time moving between flowers. In the process of stealing pollen and nectar of Opuntia tomentosa Salm-Dyck, nitidulids can facilitate self-pollination (Mandujano et al. 2014). Our results suggest that both inbreeding associated with the clonal habit and pollination by generalist small-sized insects can act as a stimulus for the evolution of dioecy in M. magnimamma (Bawa 1980, Barrett 2003). Further studies are required to estimate selfing rates and clarify the role of pollinators in the evolution and maintenance of the breeding system of M. magnimamma.
Seed production by pistillate flowers of M. magnimamma suggests higher fertility than in hermaphrodites since only a few visits are required to maximize female fertility (Spigler & Ashman 2012, Barrett & Hough 2013). Pistillate flowers produced 1.23 more seeds than hermaphrodites, despite the 6:1 ratio of hermaphroditic to female individuals in the population and the 2:1 ratio of pollinator visits in favour of hermaphroditic flowers. The number of seeds produced per plant is maximized when, for a given number of ovules, the plant receives a sufficient amount of good quality pollen (Haig & Westoby 1988). We can think that bees do provide pollen in adequate amount and quality to pistillate flowers of M. magnimamma (Burd 1994), since they are abundant and pollinate both floral types indistinctly as their coloration is similar (Barrett & Hough 2013; Figure 1). The longer-lasting flower display of pistillate individuals also helps to ensure that enough pollen is received (Yakimowski et al. 2011). Seeds from pistillate flowers had a significantly higher quality than those from hermaphrodites, judging by the average number and size of seeds produced and their germination response. This difference may be related to pollination by bees, particularly wild bees in the genus Ceratina, which predominantly visited pistillate flowers (Table 1). It has been experimentally demonstrated in several crop species that pollination by wild bees can increase seed production and weight, as well as germination rates (Bommarco et al. 2012, de Oliveira et al. 2020).
The faster germination of seeds from M. magnimamma pistillate flowers might be related to differences in resource allocation as female plants of dioecious species invest considerably more resources to reproduction to ensure producing optimal numbers of high-quality offspring (Barrett 2002, Cepeda-Cornejo & Dirzo 2010). Whereas in hermaphrodites some seeds could derive from self-fertilization, which would reduce the fitness and germination response of the progeny compared to seeds from pistillate plants (Fleming et al. 1998, Barrett 2010, Del Castillo & Trujillo-Argueta 2009). Seeds from female Opuntia robusta were found to be significantly heavier, more viable, and produced faster-growing seedlings than seeds from hermaphrodites, thus reflecting a higher quality progeny and the reproductive advantage of gender specialization (Del Castillo & Trujillo-Argueta 2009). The maintenance of pistillate plants in the Huichapan population is likely to be explained by the higher quantity and better quality of their seed (Díaz & Cocucci 2003).
Mammillaria magnimamma is one of the few widely distributed species in this genus. It can be found forming discontinuous patches in various types of xerophytic vegetation across the Mexican Plateau and central Mexico (Hernández & Gómez-Hinostrosa 2015). We have located two isolated populations - in xerophytic shrublands at Valle de Metztitlán, eastern State of Hidalgo, and in Cadereyta de Montes, State of Querétaro - in which some individuals exhibit the morphology of a non-functional androecium. Further field studies and morphometric analyses are necessary to elucidate the diversity of breeding systems of M. magnimamma throughout its distribution range.











 nova página do texto(beta)
nova página do texto(beta)