The bamboos comprise Bambusoideae, one of the 12 subfamilies currently recognized within the grass family (Poaceae) (GPWG 2001, Kellogg 2015, Soreng et al. 2015, 2017). The subfamily is subdivided into three tribes; two (Arundinarieae and Bambuseae) are composed of species possessing a suite of “woody” characteristics while the third (Olyreae) is composed of species with “non-woody” characteristics (either suffrutescent or herbaceous). In molecular analyses, each of the three tribes is monophyletic, but their relationships to one another remain uncertain. Based on plastid DNA, the Olyreae are sister to Bambuseae (GPWG 2001, Bouchenak-Khelladi et al. 2008, Sungkaew et al. 2009, Kelchner & BPG 2013, Wysocki et al. 2015, Soreng et al. 2017), but initial studies with the nuclear genome infer the Olyreae as sister to a clade formed by Arundinarieae + Bambuseae (Triplett et al. 2014, Wysocki et al. 2016, Guo et al. 2019).
Regardless of its phylogenetic position, Bambuseae consistently separates into two well-supported and geographically-correlated clades based on plastid and nuclear DNA sequences (Zhang & Clark 2000, GPWG 2001, Bouchenak-Khelladi et al. 2008, Sungkaew et al. 2009, Kelchner & BPG 2013, Wysocki et al. 2015, Soreng et al. 2017, Guo et al. 2019). These clades are referred to informally as the Paleotropical woody bamboo (PWB) clade, native to the Eastern Hemisphere, and the Neotropical woody bamboo (NWB) clade, native to the Western Hemisphere (BPG 2012, Kelchner & BPG 2013), but the term Neotropical in NWB is somewhat of a misnomer. According to Morrone (2014), the Neotropical region, defined by Sclater (1858) and adopted by Wallace (1876), only corresponds to the Western Hemisphere tropics of South America, Central America, southern Mexico and the West Indies. Morrone (2014) excludes both the Andean and southern portions of South America, and northern Mexico from this region because they belong to the Austral or Holarctic realms, respectively. The entire geographical extent of the NWB clade is roughly from northern Mexico and the West Indies to south-central Chile and Argentina (Judziewicz et al. 1999, Judziewicz & Clark 2007, Kelchner & BPG 2013, Clark et al. 2015, Wysocki et al. 2015). We recognize that this geographic region encompasses tropical, temperate and alpine zones but will, for convenience, refer to it and its native bamboos as Neotropical.
Despite its molecular support, no morphological or anatomical synapomorphy has been identified for the NWB clade (Clark et al. 2015, Kellogg 2015, Soreng et al. 2017). The NWB clade consists of three monophyletic groups that correspond to the three subtribes Arthrostylidiinae, Chusqueinae and Guaduinae. Arthrostylidiinae has a sister relationship with Guaduinae and these two subtribes together are sister to Chusqueinae (Ruiz-Sanchez et al. 2008, 2011a, Fisher et al. 2009, 2014, Kelchner & BPG 2013, Tyrrell et al. 2012, 2018, Wysocki et al. 2015, Soreng et al. 2017). The three subtribes are readily distinguishable from each other by a combination of morphological and anatomical features (see Neotropical woody bamboo subtribes, below). As the last detailed review of Neotropical bamboo genera was published by Judziewicz et al. (1999) at the end of the last century, the present work reviews the current state of Neotropical woody bamboo taxonomy and provides: 1) new morphological keys (based on vegetative characters) to and synoptic descriptions of the NWB genera, thereby updating those in Judziewicz et al. (1999); 2) an updated comprehensive species list; and 3) a biodiversity analysis by country and habitat type, as well as an indication of future directions for NWB systematics, evolution and biogeography, among other types of studies in this group. The data were obtained mainly by consulting checklists, floristic studies and specialized literature on woody bamboos occurring in this region, as cited in the following sections. We also include information derived from specimens housed in herbaria (BHCB, CEPEC, COL, CUCV, HUEFS, IBUG, IEB, ISC, MEXU, MO, NY, US, VIC, XAL, WIS, Thiers 2020) or available through databases (e.g., Tropicos 2020) and of plants analyzed during fieldwork, as part of the authors’ collective taxonomic experience. Additionally, a companion update for the Neotropical herbaceous bamboos (Olyreae) is in preparation (R.P. Oliveira, pers. comm.).
Neotropical woody bamboos by the numbers.Clark & Oliveira (2018) recently documented 21 genera and 422 species of Neotropical woody bamboo, and just two years later, we currently recognize 23 genera and 446 species, 103 of which have been newly described since the year 2000 (an increase in specific richness of 29.5 %). Thus, almost a third of the total Neotropical woody bamboo species richness has been described in just the past 20 years (Figure 1). In the same period, five new genera [Aulonemiella L.G. Clark, Londoño, C.D. Tyrrell & Judz. (Clark et al. 2020), Cambajuva P.L Viana, 7L.G. Clark & Filg. (Viana et al. 2013a), Didymogonyx (L.G. Clark & Londoño) C.D. Tyrrell, L.G. Clark & Londoño (Tyrrell et al. 2012), FilgueirasiaGuala (Guala 2003), and Tibisia C.D. Tyrrell, Londoño & L.G. Clark (Tyrrell et al. 2018)] were described while one genus, Neurolepis Meisn., was synonymized (a net increase in generic richness of 26.3 %). The changes to genera also resulted in 29 new species combinations. Even since the recent world bamboo checklist of Vorontsova et al. (2016), 30 new species and two new genera have been described, representing an unprecedented rate of growth in the number of Neotropical woody bamboo taxa (Figure 1). Thus, of the 446 accepted Neotropical woody bamboo species (Appendix 1), 133 have been described or re-assessed in the 21st century.
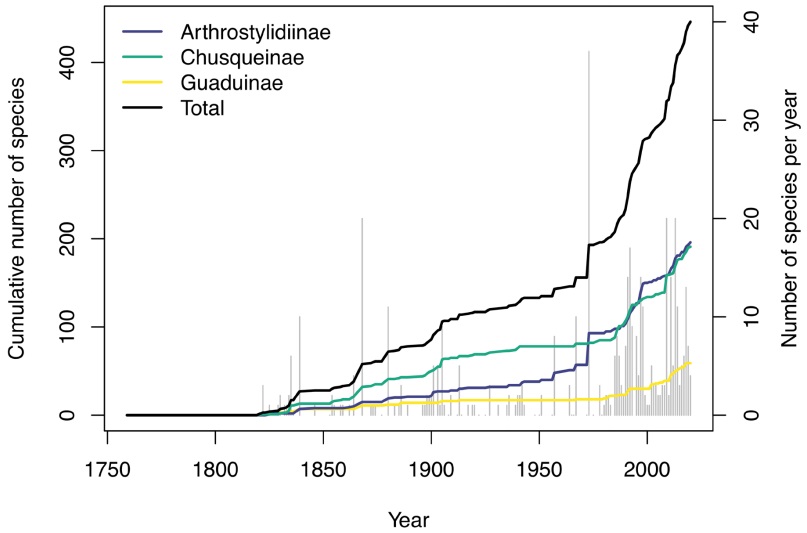
Figure 1 Number of native Neotropical woody bamboo species described and cumulative number of described species by year for Arthrostylidiinae (blue line), Chusqueinae (green line), Guaduinae (yellow line) and total (black line). Grey bars behind depict the total number of species described each year.
Among the top ten countries with the highest woody bamboo diversity are Brazil (168 spp., 17 gen.), Colombia (75 spp., 10 gen.), Venezuela (71 spp., 10 gen.), Peru (58 spp., 6 gen.), Mexico (53 spp., 8 gen.), Ecuador (48 spp., 6 gen.), Costa Rica (39 spp., 7 gen.), Bolivia (37 spp., 7 gen.), Panama (26 spp., 7 gen.), and Argentina (21 spp., 5 gen.) (Table 1). Thirty-two new species and two new genera have been described for Brazil since 2000 (prior to 2015: Sendulsky 2001, Londoño & Clark 2002a, b, Guala 2003, Clark 2004a, Filgueiras & Londoño 2006, Clark & Blong 2009, Santos-Gonçalves et al. 2011, Viana et al. 2011, Santos-Gonçalves et al. 2012, Mota et al. 2013, 2014b, Viana et al. 2013a, b, Viana & Filgueiras 2014). Greco et al. (2015) recorded 164 native woody bamboo taxa for Brazil, among them varieties, subspecies, uncertain species, and synonyms, and Filgueiras & Viana (2017) cited 18 genera and 165 species, several of them endemics and endangered with extinction. Here we record 17 genera and 168 species, 16 of them newly described since the compilation by Greco et al. (2015) (Parma et al. 2016, Santos-Gonçalves et al. 2018, Vinícius-Silva et al. 2016, Mota et al. 2017, Vidal et al. 2018, Jesus-Costa et al. 2018b, 2019, Pianissola et al. 2018, Afonso et al. 2019, Andrade et al. 2019, 2020).
Table 1 Bamboo species richness in the top ten countries with the highest diversity for each of the three Neotropical woody bamboo subtribes: Arthrostylidiinae, Chusqueinae, and Guaduinae.
| Country | Arthrostylidiinae | Chusqueinae | Guaduinae | Total |
|---|---|---|---|---|
| Brazil | 96/13 | 48 | 24/3 | 168/17 |
| Colombia | 29/7 | 36 | 10/2 | 75/10 |
| Venezuela | 40/8 | 22 | 9/1 | 71/10 |
| Peru | 18/4 | 32 | 8/1 | 58/6 |
| Mexico | 8/4 | 22 | 23/3 | 53/8 |
| Ecuador | 12/4 | 32 | 4/1 | 48/6 |
| Costa Rica | 13/5 | 23 | 3/1 | 39/7 |
| Bolivia | 16/5 | 14 | 7/1 | 37/7 |
| Panama | 9/5 | 14 | 3/1 | 26/7 |
| Argentina | 5/3 | 11 | 5/1 | 21/5 |
The numbers left of the slash are the species, to the right are the genera.
Eight new species and two new genera have been described for Colombia since 2000, for a total of 75 species (Londoño & Clark 2002a, Clark et al. 2007a, 2020, Judziewicz & Davidse 2008, Londoño & Zurita 2008, Londoño 2011, Tyrrell et al. 2012, Judziewicz et al. 2013a, Judziewicz & Londoño 2013, Ruiz-Sanchez & Londoño 2017). Eleven new species and one new genus have been described for Venezuela since 2000 (Niño et al. 2006, Clark et al. 2007a, Tyrrell et al. 2012, Clark & Ely 2013, Judziewicz et al. 2013a, Tyrrell & Clark 2013). Clark & Ely (2011) reported 67 native woody bamboo species for Venezuela, whereas we confirm a total of 71. This count includes species described since Clark & Ely (2011), but also some adjustments [e.g., they reported the occurrence of G. fascicularis Döll, but this is a synonym of G. latifolia (Bonpl.) Kunth]. Nineteen new species have been described for Mexico since 2000 (prior to 2015: Ruiz-Sanchez et al. 2011a, b, Ruiz-Sanchez & Clark 2013, Londoño & Ruiz-Sanchez 2014, Ruiz-Sanchez et al. 2014a, b, Ruiz-Sanchez 2015, Ruiz-Sanchez et al. 2015b). Ruiz-Sanchez et al. (2015a) reported 50 native woody bamboo species for Mexico, but now there are 53, with four more new species (Ruiz-Sanchez & Castro-Castro 2016, Ruiz-Sanchez et al. 2017a, 2018, 2019) since 2015, one of which was previously listed as an unidentified species of Merostachys Spreng. We expect the number of Neotropical bamboo species will continue to increase, with Bolivia, Brazil, Colombia, Ecuador, Mexico, Peru and Venezuela as the most probable locations for the discovery of new taxa.
Neotropical woody bamboo distribution and habitat. Collectively, Neotropical woody bamboos are distributed from 28 oN in Mexico and the West Indies to 47 oS in Chile and Argentina (Judziewicz et al. 1999, Judziewicz & Clark 2007). Chusquea Kunth is the most widely distributed genus in the Americas; however, the majority of its species diversity is found in the Andean montane forests of Bolivia, Peru, Ecuador, Colombia and Venezuela (Figure 2) (Judziewicz et al. 1999, Clark et al. 2015). Neotropical bamboos have adapted to a variety of habitats and can be found from lowland forests at sea level to superpáramos above 4,300 m elevation, and dry grasslands and cerrado to wet, humid forests (Judziewicz et al. 1999, Clark et al. 2015). While Neotropical bamboos can be found throughout the Americas, several regions have particularly high concentrations of bamboo richness or unique endemic taxa, for example: the Atlantic Forest region of South America, Guayana highlands, Mesoamerica, the Greater Antilles, and the central and northern Andes (Figure 2).
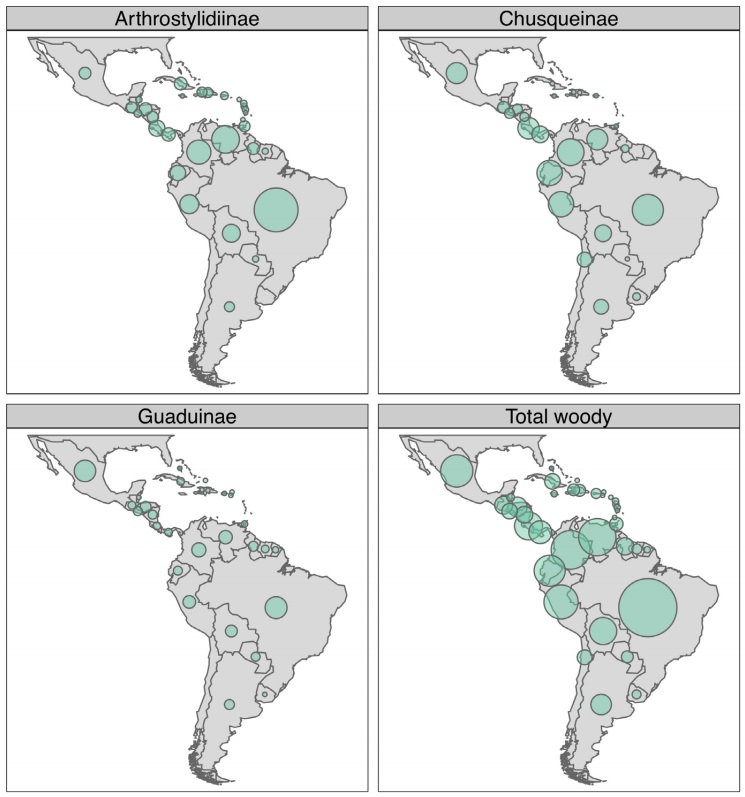
Figure 2 Maps depicting Neotropical woody bamboo species richness by country for each subtribe and summary. Circle sizes indicate relative richness; scale is equal across all panels.
Bamboos are relatively broadly distributed ecologically. Along the moisture gradient, bamboos can be found from arid (350 mm annual precipitation) to very wet (13,000 mm) habitats. Species of Otatea (McClure & E. W. Sm) C. Calderón & Soderstr., for example, inhabit xerophytic scrub in Mesoamerica while others are found in tropical dry forest (Soderstrom & Calderón 1979, Ruiz-Sanchez et al. 2011b, Clark et al. 2015, Ruiz-Sanchez 2015). In the West Indies, members of the genus Tibisia have populations found on drier serpentine or karstic soils or in habitats associated with serpentine soils (Tyrrell et al. 2018). Further south, Actinocladum verticillatum (Nees) McClure ex Soderstr., Filgueirasia cannavieira (Silveira) Guala, F. arenicola (McClure) Guala, and Guadua paniculata Munro all grow in the dry cerrado of central Brazil (Judziewicz et al. 1999). Guadua paniculata can also be found in the tropical dry forests of Mesoamerica. Aulonemia effusa (Hack.) McClure is typical of the campos rupestres of the Espinhaço Range (Viana 2010). Several species have contended for “the most drought tolerant bamboo” including Otatea acuminata (Munro) C.E.Calderón & Soderstr. and O. victoriae Ruiz-Sanchez (Ruiz-Sanchez 2015), Filgueirasia arenicola, and Actinocladum verticillatum (Judziewicz et al. 1999).
Despite the presence of bamboo in drier habitats, species richness tends to concentrate in moister habitats. Mesic sites like the grass-dominated campos de altitude in Brazil are habitat for Cambajuva, Chusquea, and Glaziophyton Franch., while the tepuis and Gran Sabana of the Guayana highlands in southern Venezuela, Colombia, Brazil, Guyana and Surinam are host to species of Aulonemia Goudot, Merostachys and Rhipidocladum McClure, as well as the regional endemic Myriocladus Swallen (Judziewicz 1998, Afonso et al. 2019). The monotypic genus Apoclada McClure grows in moist Araucaria angustifolia (Bertol.) Kuntze forests in southern Brazil (Judziewicz et al. 1999) and a few endemic Chusquea species inhabit the understory of temperate Nothofagus Blume forests of southern Chile and Argentina or grasslands in northern Argentina (Guerreiro et al. 2013, Guerreiro & Vega 2019).
The Atlantic Forest of South America is a very heterogeneous biome, including humid to dry, and montane to lowland, forests that together exhibit high generic diversity of woody bamboos. The moist Atlantic forests are inhabited by species of Athroostachys Benth., Atractantha McClure (A. aureolanata Judz., A. cardinalis Judz.), Aulonemia, Chusquea, Colanthelia McClure & E.W.Sm., Eremocaulon Soderstr. & Londoño, Guadua, and Merostachys whereas Alvimia Calderón ex Soderstr. & Londoño and most species of Atractantha are characteristic of the drier sandy restinga and mata litorânea forest types (Judziewicz et al. 1999, Londoño & Clark, 2002b, Clark et al. 2015). Species of Arthrostylidium Rupr., Aulonemia, Chusquea, and Rhipidocladum occur in the wet montane forests of the Andes from Bolivia to Venezuela, with the majority found at elevations from 2,000 to 3,500 m. These species typically live along forest edges or in canopy gaps and many of them are restricted to moist, steep ravines with running water (quebradas) (Judziewicz et al. 1999). It is common to see Guadua and Elytrostachys McClure species in some of the most hydric habitats: along rivers and streams, or in wet valleys, lower-montane forests, and tropical rainforests, especially in the Amazonian region (Londoño & Peterson 1991, Londoño 2011, Silveira 2005). In the Amazonian region, Guadua can also form extensive forests covering hundreds of thousands of km2 (Soderstrom & Calderón 1979, Nelson 1994, Judziewicz et al. 1999, Londoño 2001, Clark et al. 2015).
Some bamboos can tolerate high elevation and cold habitats, where temperatures can dip to or below the freezing point (Ely et al. 2014, 2019). Páramos, for example, are areas of heliophilous and cryophilic vegetation (open shrubby to grassland) from 3,200 to 4,700 (occasionally near 5,000) m elevation. Monthly average temperatures in páramos range from 12 °C to -2 °C with frequent precipitation (Cuatrecasas 2013). The bamboo flora of páramos and subpáramos is dominated by Chusquea, mostly species of subgenus Swallenochloa, but occasionally species of Aulonemia can also be found in these cool, moist ecosystems (Clark 1989, 1997, Judziewicz et al. 1999).
Neotropical woody bamboo subtribes. The main features of the three NWB subtribes and their taxonomic history during the last 20 years is herein updated, including revised lists of genera and species, as summarized in this section. More detailed descriptions of each subtribe are provided in Clark et al. (2015).
Arthrostylidiinae.- Arthrostylidiinae species can be distinguished from the other NWB subtribes (Chusqueinae and Guaduinae) by having a simple midrib, stomata usually only on the underside of the foliage leaf blades (hypostomatic), intercostal sclerenchyma present in the mesophyll, and an abaxial waxless stripe that appears greener than the surrounding surface along the narrow-side margin of the foliage leaves. Culm and foliage leaf oral setae are absent, but sheaths of both leaf types usually bear fimbriae or fimbriate auricles at the summit, and foliage leaf blades are reflexed at the pseudopetiole in most members of the subtribe (Tyrrell et al. 2012, Clark et al. 2015).
The species of Arthrostylidiinae exhibit a wide range of morphological characteristics, both vegetative and reproductive. In most taxa, internodes are subequal along a culm, but in some genera (Arthrostylidium, Aulonemia, Didymogonyx, Glaziophyton, and Myriocladus), the first internode may be greatly elongated with subsequent internodes successively shorter or one long internode alternates with one to a few very short internodes. Branch complements in the subtribe are highly varied and include, at the extremes, fan-shaped (apsidate) arrays with potentially hundreds of small delicate branches at a node to three or a few main branches per node to nodes with a single divergent branch equal in diameter to its main culm. Likewise, the foliage leaf blades can be linear and very narrow to lanceolate and delicate to broadly ovate and sometimes large; the mature culms of Glaziophyton do not even have foliage leaves or branches. Synflorescence forms are similarly varied and often variable within a genus, and flowering units may be standard spikelets or pseudospikelets. Species with racemose or spicate synflorescences might be compact and secund, or elongated and lax or with a straight or zig-zagged main axis. Other taxa express paniculate forms ranging from open elliptical or triangular to contracted or even capitulate synflorescences. In a few taxa, the synflorescences consist of just one or a very few spikelets. Although most members of the subtribe have a basic caryopsis, Alvimia has bacoid caryopses and Actinocladum McClure ex Soderstr. and Merostachys share nucoid caryopses. This diversity of forms is reflected in the high number of genera recognized within the subtribe.
Arthrostylidiinae is the most diverse subtribe of NWB with 196 described species in 16 genera. Since 1999, four new genera (Aulonemiella, Cambajuva, Didymogonyx and Filgueirasia), 39 new species, and six new combinations have been described in the subtribe (Supplementary material 1; Sendulsky 2001, Londoño & Clark 2002b, Guala 2003, Judziewicz & Riina 2006, Judziewicz & Tyrrell 2007, Judziewicz & Davidse 2008, Judziewicz et al. 2010, 2011, Judziewicz & Clark 2011, Lizarazu et al. 2011, Santos-Gonçalves et al. 2011, Viana et al. 2011, Santos-Gonçalves et al. 2012, Tyrrell et al. 2012, Judziewicz et al. 2013a, b, Tyrrell & Clark 2013, Viana et al. 2013a, b, Viana & Filgueiras 2014, Parma et al. 2016, Vinícius-Silva et al. 2016, Santos-Gonçalves et al. 2018, Jesus-Costa et al. 2018a, b, 2019, Ruiz-Sanchez et al. 2018, Afonso et al. 2019, Ruiz-Sanchez et al. 2019, Andrade et al. 2020, Clark et al. 2020). Tyrrell et al. (2012) found four major clades within the Arthrostylidiinae. Clade I, the earliest-diverging clade, is composed of Cambajuva and Glaziophyton. Clade II is sister to clade III and clade IV and consists of Didymogonyx, Elytrostachys, Arthrostylidium and Rhipidocladum. In clade III, Aulonemia and Colanthelia are sister genera and those sister to Aulonemiella. Finally, clade IV is formed by one subclade including Athroostachys sister to Merostachys with Actinocladum as sister, and another subclade comprising Alvimia sister to Atractantha and Filgueirasia sister to these two genera (Tyrrell et al. 2012, 2018, Viana et al. 2013a, Jesus-Costa et al. 2018a).
Among the countries with the most Arthrostylidiinae diversity are Brazil (96 species, in 13 genera), Venezuela (40 spp., 8 gen.), Colombia (29 spp., 7 gen.), Peru (18 spp., 4 gen.), Bolivia (16 spp., 5 gen.), Costa Rica (13 spp., 5 gen.), and Ecuador (12 spp., 4 gen.) (Table 1; Figure 2; Supplementary material 1). Brazil stands out for having five endemic genera belonging to this subtribe: Alvimia, Athroostachys, Cambajuva, Filgueirasia, and Glaziophyton (Viana et al. 2013a, Greco et al. 2015, Filgueiras & Viana 2017).
Included genera and number of species: Actinocladum (1), Alvimia (3), Arthrostylidum (28), Athroostachys (2), Atractantha (5), Aulonemia (49), Aulonemiella (2), Cambajuva (1), Colanthelia (10), Didymogonyx (2), Elytrostachys (2), Filgueirasia (2), Glaziophyton (1), Merostachys (55), Myriocladus (13), Rhipidocladum (20).
Chusqueinae.- Chusqueinae species can be distinguished from the Arthrostylidiinae and Guaduinae by having two papillae on each subsidiary cell of the foliar stomatal apparatus and spikelets consisting of four glumes and one fertile floret without a rachilla extension (Clark et al. 2007b, Fisher et al. 2009). Additionally, Chusqueinae species have solid culms with few exceptions, lack fimbriae and oral setae on the culm and foliage leaf sheaths, and have hypostomatic foliage leaf blades with a complex midrib and no intercostal sclerenchyma in the mesophyll (Clark et al. 2015). Foliage leaf blades are not reflexed at the pseudopetiole as in most Arthrostylidiinae.
Before 2009, this subtribe included two genera: Chusquea and Neurolepis. The morphological differences between these two genera were the absence of aerial branching and the presence of nerved inner ligules in Neurolepis and the multiple, dimorphic buds per node in Chusquea. Analysis of plastid markers revealed that although Chusqueinae is monophyletic, the two lineages of Neurolepis are paraphyletic to a monophyletic Chusquea, and therefore Neurolepis was subsumed into Chusquea (Fisher et al. 2009, 2014, Kelchner & BPG 2013, Wysocki et al. 2015). Pachymorph, leptomorph, and amphimorph rhizomes types are all found within Chusquea s.l., but the pachymorph type is the most common (Clark et al. 2007b, Fisher et al. 2009). Of note, the largest and widest foliage leaves in the grass family are found within C. subg. Magnifoliae L.G. Clark & Fisher; the blades of C. spectabilis L.G. Clark and C. nobilis (Munro) L.G. Clark can reach 370-400 cm in length, and in C. spectabilis, as much as 30 cm in width (Judziewicz et al. 1999). Among the species of Chusquea with multiple buds per node, there exists an almost species-specific array of variation based on the combination of central bud morphology, the number and arrangement of subsidiary buds, and the position of the nodal line. The central bud can be triangular (and erect), sometimes with the prophyll elongated to as much as 10 cm, or it can be dome-shaped (and horizontally oriented). Subsidiary buds can range in number from two to hundreds, and they can flank, subtend or nearly encircle the central bud; in some species, the subsidiary buds completely encircle the nodal region (e.g., C. pittieri Hack.). The nodal line varies from being horizontal to dipping slightly to markedly below the bud complement. Nodal line position is correlated to some extent with branching pattern: intravaginal branching occurs only in taxa with a horizontal nodal line, extravaginal branching is found in taxa with a horizontal or slightly dipped nodal line, and infravaginal branching occurs in taxa with a markedly dipped nodal line. Synflorescences are typically paniculate, and can vary from open and pyramidal to contracted or spicate, and in some species are capitate. Although the spikelet groundplan is invariable across Chusquea, variation in relative lengths of the four glumes to the floret apex is important in species identification.
Chusqueinae is the second most diverse subtribe among the NWB with 191 described species in one single genus. Since 1999, 42 new species and 17 new combinations have been described (Supplementary material 1; Clark & March 2000, Clark 2003, 2004a, b, Clark & Losure 2005, Niño et al. 2006, Clark et al. 2007c, Clark & Blong 2009, Fisher et al. 2011, Clark & Ely 2013, Guerreiro & Rúgolo de Agrasar 2013, 2014, Mota et al. 2013, 2014a, b, Ruiz-Sanchez & Clark 2013, Fisher et al. 2014, Guerreiro et al. 2014, Ruiz-Sanchez et al. 2014a, b, Ruiz-Sanchez et al. 2015b, Alegría-Olivera et al. 2017, Attigala et al. 2017, Mota et al. 2017, Ruiz-Sanchez et al. 2017a, Pianissola et al. 2018, Vidal et al. 2018, Andrade et al. 2019, Clark & Mason 2019, Fadrique et al. 2019, Guerreiro et al. 2019, Clark & Kaul 2020). Chusquea is divided into five subgenera: Chusquea subg. Chusquea, C. subg. Rettbergia (Raddi) L.G. Clark, C. subg. Swallenochloa (McClure) L.G. Clark, C. subg. Platonia Fisher & L.G. Clark, and C. subg. Magnifoliae (Fisher et al. 2014). Chusquea subg. Chusquea is divided into six sections and one informal group (C. sect. Chusquea, sect. Longifoliae L.G. Clark, sect. Longiprophyllae L.G. Clark, sect. Serpentes L.G. Clark, sect. Tenellae L.G. Clark, sect. Verticillatae L.G. Clark, and the C. meyeriana Rupr. ex. Döll informal group) (Fisher et al. 2014, Attigala et al. 2017, Vidal et al. 2018).
The countries with the highest diversity of Chusquea are Brazil (48 spp.), Colombia (36 spp.), Ecuador and Peru (32 spp. each), Costa Rica (23 spp.), Mexico and Venezuela (22 spp. each), Bolivia and Panama (14 spp. each), and Argentina and Chile (11 spp. each) (Table 1; Figure 2; Supplementary material 1). Included genus: Chusquea (191).
Guaduinae.- Species of Guaduinae can be distinguished from the other two subtribes by having stomata on both leaf surfaces. Adaxial surfaces often have papillae associated with the stomata, whereas papillae are frequently absent from the abaxial surface. Additionally, Guaduinae species have oral setae borne on the culm and/or foliage leaf sheaths (absent in Apoclada) and these are absent in the Arthrostylidiinae and Chusqueinae (Soderstrom & Ellis 1987, Londoño & Clark 2002a, Ruiz-Sanchez et al. 2008, Tyrrell et al. 2018). Also, intercostal sclerenchyma is absent from the mesophyll of the foliage leaf blades and the midrib is complex, as in the Chusqueinae (BPG 2012, Soderstrom & Ellis 1987). The foliage leaf blades are not reflexed at the pseudopetiole as in most Arthrostylidiinae.
Guaduinae species have hollow or solid culms, and pachymorph rhizomes with short or elongate necks. These may be only a few cm long, but rhizome necks up to 8 m long can be found in Apoclada simplex McClure & L.B. Sm., Eremocaulon aureofimbriatum Soderstrom & Londoño, Guadua sarcocarpa Londoño & P.M. Peterson, G. weberbaueri Pilg., Olmeca recta Soderstr., and Ol. reflexa Soderstr. (Soderstrom 1981, Judziewicz et al. 1999, Ruiz-Sanchez et al. 2011a, b). Most of the species are self-supporting and generally erect with arching to nodding apices, but in Olmeca Soderstr., Guadua and Tibisia some species exhibit a scandent to clambering habit (Londoño & Davidse 1991, Londoño & Clark 2002a, Ruiz-Sanchez et al. 2015a, Tyrrell et al. 2018). Internodes are subequal along the culm and usually cylindrical (Apoclada, Eremocaulon, Otatea, Olmeca, and Tibisia), or sulcate above the insertion of the bud branch complement and with infra- and supranodal bands of white hair present (Guadua). With the exception of Apoclada, which has 2-4 buds per node, all species in the subtribe have a single bud per node, and one to five dominant branches and few to many smaller secondary branches are developed in the nodal region. The foliage leaf blades can be linear and very narrow to lanceolate and ovate to broadly ovate-lanceolate; auricles on the foliage leaves are usually present in Eremocaulon and Guadua but absent in the other genera. Thorns are present only in Guadua and very rarely in Eremocaulon. Reflexed culm leaf blades are present in Eremocaulon, Olmeca, Otatea, and Tibisia. Synflorescences terminate leafy or leafless branches and are open paniculate to capitate in form. Flowering units are spikelets (Apoclada, Olmeca, Otatea, and Tibisia) or pseudospikelets (Eremocaulon and Guadua) with several florets per unit. The general fruit type is the basic dry caryopsis, but Guadua sarcocarpa, G. weberbaueri, Olmeca recta, and Ol. reflexa have a bacoid caryopsis (Londoño & Peterson 1991, Olivier & Poncy 2009, Ruiz-Sanchez et al. 2011b, 2015, 2017b, Ruiz-Sanchez & Sosa 2015, Tyrrell et al. 2012, 2018).
Guaduinae is the least diverse subtribe of Neotropical lignified bamboos with only 59 described species in six genera. Since 1999, one new genus (Tibisia), 22 new species, and seven new combinations have been described (Supplementary material 1; Londoño & Clark 2002a, Clark & Cortés 2004, Filgueiras & Londoño 2006, Londoño & Zurita 2008, Ruiz-Sanchez et al. 2011a, b, Lizarazu et al. 2013, Londoño 2013, Londoño & Ruiz-Sanchez 2014, Ruiz-Sanchez 2015, Ruiz-Sanchez & Londoño 2017, Jesus-Costa et al. 2018b, Tyrrell et al. 2018). Within Guaduinae, there are three major clades: Clade I, formed by Tibisia, is sister to all other genera (Tyrrell et al. 2018); Clade II, in which Guadua is sister to Eremocaulon and Apoclada; and Clade III, consisting of Otatea and Olmeca as sister genera, which is sister to Clade II (Ruiz-Sanchez et al. 2011a, Tyrrell et al. 2012, 2018, Wysocki et al. 2015).
The countries with the highest diversity of Guaduinae are Brazil (24 spp., 3 gen.), Mexico (23 spp., 3 gen.), Colombia (10 spp., 2 gen.), Venezuela (9 spp., 1 gen.), Peru (8 spp., 1 gen.) Bolivia (7 spp., 1 gen.), Honduras (6 spp., 2 gen.), Argentina and Guyana (5 spp. each, 1 gen.), Ecuador, (4 spp., 1 gen.), Nicaragua (4 spp., 1 gen.), Paraguay (4 spp., 1 gen.) and El Salvador (4 spp., 2 gen.), respectively (Table 1; Figure 2; Supplementary material 1). Brazil has two endemic genera, Apoclada and Eremocaulon (Greco et al. 2015, Filgueiras & Viana 2017), and Mexico has one, Olmeca (Ruiz-Sanchez et al. 2011a). Tibisia is only found in the northern islands of the West Indies (Tyrrell et al. 2018).
Included genera and number of species: Apoclada (1), Eremocaulon (5), Guadua (33), Olmeca (5), Otatea (12), Tibisia (3).
Key to the Neotropical woody bamboo subtribes (Spanish version in Appendix 1).
1. Foliage leaf blades with a simple midrib and an abaxial green stripe along the narrow-side margin of the foliage leaf blades, usually reflexed at the pseudopetiole; intercostal sclerenchyma present in the mesophyll ……………………………………………………………………………………………………………………………………………………Arthrostylidiinae
1. Foliage leaf blades with a complex midrib and the abaxial leaf surface uniform in color, pseudopetiole straight, not reflexed; intercostal sclerenchyma absent in the mesophyll ……………………………………………………2
2. Subsidiary cells of the foliar stomatal apparatus with two papillae per cell, adaxial surfaces without papillae associated with stomata, abaxial surfaces with papillae; buds multiple and usually dimorphic per node in species with aerial branching, one bud per node in those without aerial branching; culm and foliage leaf sheaths without oral setae………………………………………………………Chusqueinae (Chusquea)
2. Subsidiary cells of the foliar stomatal apparatus lacking any papillae, adaxial surface often with papillae associated with the stomata, abaxial surface usually lacking papillae; bud usually one per node, exceptionally 2 to 4 and subequal (Apoclada); culm or foliage leaf sheaths usually with oral setae……………………………………………………………………………………………………………………………………………Guaduinae
Key to the genera of the Neotropical woody bamboo subtribe Arthrostylidiinae (Spanish version in Appendix 1).
-
1. Culms regularly cross-partitioned like a rush, normally leafless; Serra dos Órgãos, Rio de Janeiro state, Brazil ………………………………………………………………………………………………………………………………………………Glaziophyton
1. Culms not cross-partitioned, normally leafy; widespread across the Neotropical region …………………………2
-
2. Mid-culm nodes with branches or secondary buds borne on two sides of a flattened, triangular plate (a fan-shaped or apsidate array) derived from a single bud per node …………………………………………3
2. Mid-culm nodes with one dominant branch and two or more smaller lateral branches (or buds) or a single branch per node derived from a single bud per node or bearing 1-8 buds or branches in a row ……………………………………………………………………………………………………………………………………………………………………6
-
-
3. Culm leaves with the blade erect …………………………………………………………….……………………………………………………4
3. Culm leaves with the blade spreading to usually reflexed …………………………………………………………………………..5
-
4. Internodes all more or less equally elongated along the length of the culm; bud triangular in outline …………………………………………………………………………………………………………………………………Rhipidocladum
4. One elongated internode alternating with one very short internode along the length of the culm; bud cordate in outline …………………………………………………………………………………………………………Didymogonyx
-
-
5. Foliage leaf blades dimorphic, those of the terminal node much larger than those of the lateral branches; culm and foliage leaf sheaths usually with distinctively ruffled margins at the apex (derived from fused fimbriae) …………………………………………………………………………………………………………………………………………Actinocladum
5. Foliage leaf blades all about the same size; culm and foliage leaf sheaths with chartaceous margins that may or may not bear usually unfused fimbriae at the apex …………………………………………………………Merostachys
-
6. Culms with the basalmost internode greatly elongated with successive internodes of variable lengths (some very short) or elongated internodes alternating with 1-4 very short internodes along the length of the culm ………………………………………………………………………………………………………………………………7
6. Internodes all more or less equally elongated along the length of the culm ……………………………………………………………………………………………………………………………………………………………………9
-
-
7. Culms with the elongated internodes alternating with 1-4 very short internodes along the length of the culm; Andes …………Aulonemia in part [A. herzogiana (Henrard) McClure, A. hirtula (Pilg.) McClure, A. queko Goudot]
7. Culms with the basalmost internode greatly elongated with successive internodes of variable lengths (some very short); Guiana Highlands ……………………………………………………………………………………………………………………………………………………………………………8
-
8. Culms 1.5-3.5 cm in diameter, up to 15 m long, initially erect then leaning on woody vegetation; foliage leaf blades linear-lanceolate, attenuate at the base; Cerro Marahuaka, Venezuela ……………………………………………………………………………………………Arthrostylidium schomburgkii (Benn.) Munro
8. Culms usually less than 1.5 cm in diameter, not more than 6 m tall, erect; foliage leaf blades lanceolate or usually broader, often with rounded or cordate bases; widespread on tepui summits in the Guiana Highlands ………………………………………………………………………………….…Myriocladus (most species)
-
-
9. Culm leaves poorly or not differentiated from the foliage leaves, or differentiated but with blades reduced to a small mucro or absent ………………………………………………………………………………………………………………………………10
9. Culm leaves usually well differentiated from foliage leaves and the blades developed ……………………………………………………………………………………………………………………………………………………………………………12
-
10. Culm leaves differentiated, the sheaths with a small to well-developed corky crest or flange present at the junction with the girdle ……………………………………………………………..………Atractantha radiata
10. Culm leaves poorly or not differentiated, the sheaths lacking a crest or flange at the junction with the girdle ………………………………………………………………………………………………………………………………………………………………….11
-
-
11. Foliage leaf blades 0.7-3.5 mm wide …………………………………………………………………………………………………………………………………………………………Myriocladus in part (M. involutus Judz. & Davidse, M. steyermarkii Swallen)
11. Foliage leaf blades 10-67 mm wide ……………………………………………………...Aulonemia in part (most species)
-
13. Culms 0.45-1.75 m tall, erect; 1-8 subequal buds or branches in a line per mid-culm node; Brazilian cerrado ……………………………………………………………………………………………………………………………………………...Filgueirasia
13. Culms 1-20 (-25) m tall, clambering or scandent or hanging or erect; one bud per node developing into (1-) 3-5 (-many) branches per mid-culm node, subequal or the central branch dominant but not in a linear array; widespread but almost entirely absent from the Brazilian cerrado …………………………………………………..14
-
14. Culms erect; foliage leaf blades erect, tessellate; high altitude grasslands in Santa Catarina state, Brazil …………………………………………………………………………………………………………………………………………Cambajuva
14. Culms clambering or scandent to hanging; foliage leaf blades lax or reflexed, not tessellate; widespread and associated with forest vegetation ………………………………………………………………………………15
-
-
15. Culms 1-2.5 mm in diameter, 1-6 m long; Atlantic forests of São Paulo state, Brazil………………….………………………………....Colanthelia in part (C. secundiflora Santos-Gonç., Filg. & L.G.Clark)
15. Culms (1-) 3-25 (-35) mm in diameter, 3-15 (-25) m long; widespread ………………………………………………16
-
16. Culm leaf sheaths with a small to well-developed corky crest or flange present at the junction with the girdle………………………………………………………………………………………Arthrostylidium scandens McClure
16. Culm leaf sheaths lacking any crest or flange at the junction with the girdle, but sometimes a ring of hairs present…………………………………………………………………………………………………..………………………………….17
-
-
17. Internodes solid, sometimes developing a narrow lumen in old culms……………………………………………………18
17. Internodes hollow, thick-walled…………………………………………………………………………………………...……………...…..19
-
18. Culm leaf sheaths with a dark, thickened girdle; foliage leaf blades apically acuminate, the sheaths bearing spreading, straight to curly fimbriae 0.1-0.66 cm long......……………………………...Alvimia in part (A. auriculata Soderstr. & Londoño, A. gracilis Soderstr. & Londoño)
18. Culm leaf sheaths with a brown to stramineous girdle similar in thickness to the sheaths; foliage leaf blades apically setose, the sheaths bearing erect, slightly wavy fimbriae 0.3-6 cm long……………...Aulonemia in part [A. setigera (Hack.) McClure, A. setosa (Londoño) P.L. Viana & Filg.]
-
-
19. Nodal line annular, corky; culm leaves papery; bud/branches borne on a weakly developed promontory; endemic to igapó in affluents of the Rio Negro in southwestern Amazonas, Venezuela and northwestern Amazonas, Brazil……………………………………………………………………………… Atractantha amazonica Judz. & L.G.Clark
19. Nodal line flattened in side view, not corky; culm leaves papery to woody; bud/branches borne on a usually well-developed promontory; widespread including the West Indies……………………………………………………………………………………………………………………………………………….………...…..20
-
20. Culm leaf sheaths with a dark, thickened girdle; endemic to the coastal forests of Bahia, Brazil…………………………………………………………………………………….……..Alvimia lancifolia Soderstr. & Londoño
20. Culm leaf sheaths with the girdle, if developed, the same color and thickness as the sheaths; widespread including the West Indies but not in Bahia, Brazil…………………………………………………………………………………………………………………….Arthrostylidium in part
-
-
21. Fimbriae on culm and foliage leaf sheaths 1-10 cm long, straight, free, more or less terete, erect on culm leaves, erect to spreading on foliage leaves; Central America and northern Andes but absent from the West Indies……………..……………………………………………………………………………………………………………………………….Elytrostachys
21. Fimbriae on culm and foliage leaf sheaths absent or 0.2-12 cm long (if greater than 4 cm long then flattened and often partially or completely connate), wavy or curly at least at the apex, sometimes straight, erect to spreading on both culm and foliage leaves; widespread including the West Indies ……………………..22
-
22. Foliage leaf blades abaxially strongly tessellate, the inner ligules 3-12 cm long ……………………………..Aulonemia in part [A. parviflora (J. Presl) McClure, A. radiata (Rupr.) McClure & L.B. Sm.]
22. Foliage leaf blades abaxially without evident cross-veins (not tessellate), the inner ligules less than 1 cm long……………………………………………………………….…………………………….……………………………………….23
-
-
23. Culm leaves lacking any corky flange or crest at the juncture of the sheathand girdle; occurring in the West Indies or the Andes…………………………………………………………………………………………………………………………...……24
23. Culm leaves with a corky flange or crest present at the juncture of the sheath and girdle; Atlantic forest of southeastern Brazil and Misiones, Argentina………..………………………………………………………………………………..…25
-
24. Culm internodes hollow, terete; occurring in the West Indies…………………………………………………………………………………………………………………...…Arthrostylidium in part
24. Culms internodes solid, often somewhat flattened in the plane lateral to the bud or branch complement; occurring in the Andes of Colombia and Ecuador….………………………………………Aulonemiella
-
-
25. Branch complement of usually 3 subequal branches; foliage leaf blades with the midrib excentric………………………………………………………………………………………………………………………………..………Athroostachys
25. Branch complement of usually 1 dominant plus 2 to many smaller secondary branches; foliage leaf blades with the midrib more or less centric……………………….………………………….………………………….……………..….26
-
26. Juncture of culm leaf sheath and girdle bearing a prominent flange; culms 0.5-2 cm in diameter, 10-20 m long; restricted to Espirito Santo and Bahia, Brazil ………………………Atractantha (most species)
26. Juncture of culm leaf sheath and girdle bearing a narrow crest; culms 0.1-0.6 cm in diameter, 1-6 m long; occurring in Misiones, Argentina and Rio Grande do Sul to Espirito Santo, Brazil ………………………………………………………………………………………………………..…..……..…..Colanthelia (most species)
-
Key to the genera of the Neotropical woody bamboo subtribe Guaduinae (Spanish version in Appendix 1).
-
1. Culms with infra- and supranodal bands of hairs and thorns almost always present on the branches ..............................................................................................................................................Guadua
1. Culms lacking infra- and supranodal bands of hairs [except Eremocaulon capitatum (Trin.) Londoño] and thorns absent on the branches .........................................................................................................2
-
3. Culm internode diameter 0.1-0.8 cm; secondary and higher order branches usually grouped into fascicles; occurring in the West Indies .......................................................................................Tibisia
3. Culm internode diameter 1-6 (-6) cm; secondary and higher order branches if present diverging simply, not grouped into fascicles; Mexico to northern South America and Brazil but not in the West Indies ........4
-
4. Foliage leaf blades with the midrib slightly to strongly excentric, the sheaths usually bearing fimbriate auricles; one dominant branch per node bearing few to several smaller secondary branches basally; occurring in Brazil .....................................................................................Eremocaulon
4. Foliage leaf blades with the midrib centric, the sheaths with auricles absent; one or three (rarely two) subequal branches per node; occurring in Mexico, Central America and northern South America but not Brazil …………………………………………………………………………………………………………….………..……..…….....5
-
-
5. Branch 1 per node, diameter of the mid-culm branch equal to or greater than one-half the diameter of the main culm……………………………………………………………………………………………………………………………..…………..Olmeca
5. Branches 3 per node (rarely 1 or 2 or 6), diameter of the mid-culm branches equal to or less than one-quarter of the diameter of the main culm………….……………………………………………………………………………………Otatea
Neotropical woody bamboo genera. Actinocladum McClure ex Soderstr. (Arthrostylidiinae). Plants erect to clambering; rhizomes pachymorph with short necks. Culms 3-5 (-6) m long, 0.8-1.5 cm diameter. Internodes thick-walled, filled with a pithy center, all subequally elongated. Culm leaf sheaths without a girdle, deciduous, without auricles, fimbriae basally fused, oral setae lacking; blades deciduous, spreading or reflexed. Branching intravaginal; branch complements with numerous branches per node arranged in an apsidate array, arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae a ruffle on the overlapping shoulders with fused bases, oral setae lacking; blades pseudopetiolate, dimorphic (the terminal complement with blades much larger and broader than those produced from the lateral branches), linear-lanceolate and broadly lanceolate or broadly lanceolate and lanceolate-ovate, the width symmetric on each side of the midrib, stiff. Synflorescences usually racemose or weakly paniculate. Spikelets long-pedicellate, laterally compressed. Glumes 2 (-3), ovate, acuminate, unequal, short, leathery, 5-11-nerved; sterile lemmas none; fertile florets 6-9 with a rudimentary terminal floret; lemmas ovate-lanceolate, acuminate. Stamens 3. Caryopsis nucoid.
Alvimia C.E. Calderón ex Soderstr. & Londoño (Arthrostylidiinae). Plants clambering/scandent; rhizomes pachymorph with short necks. Culms 8-25 m long, 0.3-1 cm diameter. Internodes usually solid, sometimes thick-walled with a small lumen, all subequally elongated. Culm leaf sheaths with a dark, 1-1.5 mm girdle, tardily deciduous, leathery, with or without auricles, fimbriae present or absent, oral setae lacking; sheath margins smooth to strigose; blades persistent, erect, strongly nerved. Branching intravaginal; branch complements with several to many branches per node arranged as one dominant and many smaller branches arising from a single bud borne on a promontory. Foliage leaf sheaths with or without auricles, fimbriae curled distally, oral setae lacking; blades shortly pseudopetiolate, linear or lanceolate-ovate, the width symmetric or asymmetric on each side of the midrib. Synflorescences paniculate (terminal or leafy). Pseudospikelets 9-29 (-40) cm long, slender, lax, subtended by a bract and a prophyll followed by 0-3 basal bud-bearing bracts (pseudospikelets may terminate in a foliage leaf or leafy shoot in A. lancifolia Soderstr. & Londoño). Glumes 1-2, ovate-lanceolate, apiculate to mucronate, equal, 5-7 mm, with an elongate rachis above; sterile lemmas 0-1; fertile florets 3-30, distantly spaced, with a rudimentary terminal floret or a bristle-like prolongation of the rachilla; lemmas ovate-lanceolate, apiculate with a twisted apex or mucronate, glabrous to puberulent. Stamens 2 (rarely 3). Caryopsis bacoid.
Apoclada McClure (Guaduinae). Plants erect; rhizomes pachymorph with elongated necks. Culms 3-13 m long, 1.9-4 cm diameter. Internodes thick-walled, hollow or sometimes solid, all subequally elongated. Culm leaf sheaths without a girdle, glabrous, without auricles, fimbriae, or oral setae; blades erect. Branching intravaginal; branch complements with 2 (vegetative) or 2-4 (reproductive) branches per node arranged as linear, subequal branches, arising from 1-2 buds per node, promontory absent. Foliage leaf sheaths without auricles, fimbriae, or oral setae; blades shortly pseudopetiolate, linear, the width symmetric on each side of the midrib. Synflorescences of one spikelet or (less commonly) two spikelets. Spikelets laterally compressed. Glume-like bracts 1-2 (“transitional glumes”), awned, unequal; sterile lemmas 1; fertile florets 7-28 with a rudimentary terminal floret; lemmas awned. Stamens 3. Caryopsis basic.
Arthrostylidium Rupr. (Arthrostylidiinae). Plants clambering/scandent; rhizomes pachymorph with short necks. Culms 3-15 m long, 0.3-3.5 cm diameter. Internodes thick- or thin-walled, hollow, subequally elongated or with a long (3-6 m) basal internode and successively shorter apical internodes (A. schomburgkii). Culm leaf sheaths without a girdle, deciduous, glabrous, without auricles, fimbriae present but not prominent, oral setae lacking; blades persistent, erect. Branching intravaginal; branch complements with 3-15 branches per node subequal to the main culm or with one larger dominant branch, all arising from a single bud borne on a promontory. Foliage leaf sheaths with or without auricles, fimbriae present, oral setae lacking; blades shortly pseudopetiolate, linear, linear-lanceolate or lanceolate-ovate, the width symmetric on each side of the midrib. Synflorescences spicate or racemose, main axis straight or zig-zagged. Spikelets subsessile, laterally compressed. Glumes 1-3, apiculate or awned, unequal, glabrous; sterile lemmas 0-1; fertile florets 2-15 with a rudimentary terminal floret; lemmas apiculate or awned, glabrous to puberulent. Stamens 3. Caryopsis basic.
Athroostachys Benth. (Arthrostylidiinae). Plants clambering/scandent; rhizomes pachymorph with short necks. Culms 0-8 m long, 0.3-0.6 cm diameter. Internodes thick-walled, nearly solid (very small lumen), all subequally elongated. Culm leaf sheaths with a dark or thickened girdle, persistent or tardily deciduous, without auricles, fimbriae present, oral setae lacking; blades deciduous, reflexed. Branching intravaginal; branch complements with 3 branches per node arranged as a central one usually slightly larger than the laterals, arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae slightly sinuous or radiating in all directions, oral setae lacking; blades pseudopetiolate, lanceolate-oblong, large, the width symmetric on each side of the midrib. Synflorescences short-peduncled, contracted-paniculate or capitate. Spikelets subsessile, laterally compressed. Glumes 2, acuminate, glume I much shorter than the spikelet and deciduous; sterile lemmas none; fertile floret 1 with an elongate rachilla extension bearing a small rudimentary floret; lemmas acuminate, fully embracing the palea. Stamens 3. Fruit not seen.
Atractantha McClure (Arthrostylidiinae). Plants clambering/scandent or apically arching/pendulous; rhizomes pachymorph with short necks. Culms 4-15 (-20) m long, 0.4-2 cm diameter. Internodes thick-walled, solid or hollow (with peripheral air canals in some species), all subequally elongated. Culm leaf sheaths with a flange-like girdle, early to tardily deciduous, without auricles, fimbriae present, oral setae lacking; blades persistent, erect or reflexed, triangular (A. amazonica) or a mucro (A. radiata). Branching intravaginal; branch complements with 2-7 branches per node arranged as one dominant branch with 1-6 secondary or tertiary branchlets at its base or three subequal branches that rebranch (A. amazonica), arising from a single bud borne on a promontory. Foliage leaf sheaths without auricles, fimbriae numerous, delicate, and curling, oral setae lacking; blades pseudopetiolate, linear or lanceolate-ovate, the width symmetric on each side of the midrib. Synflorescences capitate or racemose. Pseudospikelets (spikelets in A. amazonica), slenderly lanceolate, slightly sickle-shaped, attenuate, indurate, obscurely nerved. Glume 1, bearing a prophyll as the first lateral appendage; sterile lemma-like gemmiparous bracts 1-several; fertile florets 1 (rarely 2), occasionally ending in a rudimentary terminal floret; lemmas lanceolate, pungent, mucronate or short awned, many-nerved. Stamens 3. Caryopsis basic.
Aulonemia Goudot (Arthrostylidiinae). Plants erect or clambering/scandent; rhizomes pachymorph with short necks or amphimorph. Culms (0.3-) 1-10 (-15) m long, 0.15-8 cm diameter. Internodes thin- or thick-walled, hollow or solid, all subequally elongated or 1 long internode alternating with 2-4 very short internodes. Culm leaves well differentiated from the foliage leaves or not; sheaths with or without a girdle (up to 1-3 mm wide in A. cincta P.L. Viana & Filg. and A. pumila L.G.Clark & Londoño), persistent or tardily deciduous, coriaceous or papyraceous, without auricles, fimbriae long, sometimes flattened, oral setae lacking; blades persistent (deciduous in A. bogotensis L.G.Clark, Londoño & M.Kobay.), reflexed or less commonly erect, lanceolate, linear-lanceolate or lanceolate-ovate. Branching intravaginal; branch complements with 1 branch per node, sometimes rebranching, subequal to the main culm, arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae short to long, straight or curly, and pectinate to spreading, oral setae lacking; blades pseudopetiolate, linear-lanceolate or ovate, (1.4-)8-38 cm long, 0.2-13.5 cm wide, the width symmetric or strongly asymmetric on each side of the midrib, ascending to reflexed. Synflorescences paniculate, contracted to open, ovate or pyramidal. Spikelets long-pedicellate, laterally compressed. Glumes 2 (-3), ovate to ovate-lanceolate, obtuse to acute to mucronate to awned, unequal; sterile lemma 0 or 1; fertile florets 2-14 (-22) with 1 (-2) rudimentary terminal florets; rachilla slightly flattened or terete; lemmas lanceolate, broadly lanceolate, lanceolate-ovate or ovate-elliptical, usually awned. Stamens 3. Caryopsis basic.
Aulonemiella L.G. Clark, Londoño, C.D. Tyrrell & Judz. (Arthrostylidiinae). Plants clambering/scandent; rhizomes not seen. Culms (0.3-) 0.7-6 m long, 0.1-0.4 cm diameter, often slightly flattened in the plane lateral to the bud or branch complement. Internodes solid, all subequally elongated. Culm leaf sheaths bearing a girdle with short appressed trichomes, tardily deciduous, without auricles, fimbriae present, oral setae lacking; blades reflexed or spreading. Branching intravaginal; branch complements with 1 branch per node subequal to the main culm (this later rebranching) arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae present, oral setae lacking; blades pseudopetiolate, linear-lanceolate, the margins differentiated on each side of the midrib, reflexed to spreading. Synflorescences spicate or racemose (main axis zig-zagged at maturity in A. ecuadorensis (Judz. & L.G.Clark) L.G.Clark, Londoño & Judz.), bracteate with subtending bracts and prophylls. Spikelets subsessile, laterally compressed. Glumes 2, acute, unequal; sterile lemmas none; fertile florets 2-8 with a rudimentary terminal floret; rachilla internodes distally clavate with short, crisped, appressed hairs; lemmas acute to pungent, callus usually antrorsely bearded. Stamens 3. Fruit not seen.
Cambajuva P.L Viana, L.G. Clark & Filg. (Arthrostylidiinae). Plants erect; rhizomes pachymorph with short necks. Culms 0.2-2.5 m long, 0.2-1.3 cm diameter. Internodes thin-walled, hollow, all subequally elongated. Culm leaf sheaths without a girdle, tardily deciduous, without auricles, fimbriae present, oral setae lacking; blades not pseudopetiolate, erect, triangular or ovate-lanceolate. Branching intravaginal; branch complements with 1-7 subequal branches per node arising from a single bud borne on a promontory. Foliage leaf sheaths without auricles, fimbriae persistent or tardily deciduous, oral setae lacking; blades pseudopetiolate, lanceolate, the width symmetric to slightly asymmetric on each side of the midrib, tessellate. Synflorescences paniculate, spiciform. Spikelets ellipsoid, laterally compressed. Glumes 2, awned, unequal; sterile lemmas 1; fertile florets 2-4 with a rudimentary terminal floret; lemmas awned, puberulous. Stamens 3. Fruit not seen.
Chusquea Kunth (Chusqueinae). Plants erect, clambering/scandent or apically arching/pendulous; rhizomes pachymorph, less commonly leptomorph or sometimes amphimorph, with short necks. Culms (0.3-) 1-12 (-20) m long, (0.2-) 0.5-5.5 (-7) cm diameter. Internodes solid or rarely hollow, if hollow then relatively thin-walled, all subequally elongated. Culm leaf sheaths without a flange-like projection but in a few species with a short skirt, deciduous or persistent, rarely caducous, chartaceous to coriaceous, without auricles, fimbriae or oral setae; sheath margins sometimes fused at the base; girdle poorly developed to very well developed; blades persistent or deciduous in a few species, usually erect, spreading to reflexed in a few species, usually triangular, sometimes cordate at the base, but pseudopetiolate in a few species, usually chartaceous. Branching extravaginal, infravaginal, intravaginal or aerial branching absent (subgenera Platonia and Magnifoliae), branch complements consisting of usually one dominant central branch subtended or flanked by 2 to numerous smaller leafy subsidiary branches, these sometimes of two sizes, arising from multiple and almost always dimorphic buds, one usually larger central bud subtended or flanked by a few to numerous smaller subsidiary buds, these constellate, linear or verticillate in arrangement (subgenera Chusquea, Rettbergia and Swallenochloa) or only one triangular bud present but not developing (subgenera Platonia and Magnifoliae), promontory absent. Foliage leaf sheaths without auricles, fimbriae or oral setae; blades usually differentiated, linear, lanceolate to broadly lanceolate or less commonly ovate, variable (from ca. 1.5 cm to 4 m long and ca. 2 mm to 30 cm wide), the width usually symmetric, asymmetric in a few species on each side of the midrib. Synflorescences paniculate or rarely racemose, varying from congested to pyramidal with branches spreading to reflexed, and sometimes capitate (especially in subg. Rettbergia). Spikelets pedicellate, usually lanceolate, sometimes slightly falcate to falcate, usually laterally compressed. Glumes 4, glumes 1 and 2 usually similar, sometimes unequal, virtually absent to scale-like to well developed, sometimes longer than the spikelet body; glumes 3 and 4 developed, usually similar, often unequal, usually at least 1/3 the spikelet length to equaling the spikelet length, obtuse, acute, mucronate or awned; sterile lemmas 0; fertile floret 1, a rudimentary terminal floret absent; rachilla extension absent; lemmas acute, mucronate or awned, indument variable. Stamens 3 (rarely 2). Caryopsis basic.
Colanthelia McClure & E.W. Sm. (Arthrostylidiinae). Plants erect or clambering/scandent; rhizomes pachymorph with short necks. Culms 1-6 m long, 0.1-0.6 cm diameter. Internodes thick-walled, hollow, all subequally elongated. Culm leaf sheaths with a dark or prominent girdle, without auricles, fimbriae present, oral setae lacking; blades pseudopetiolate. Branching intravaginal; branch complements with 3-19 branches per node arranged as one dominant and divergent branch with 2-19 smaller lateral branches at the base arising from a single bud, promontory absent or weakly developed, midculm nodes prominently narrow crested at the juncture of the sheath and girdle. Foliage leaf sheaths with or without auricles, fimbriae short and often inconspicuous, oral setae lacking; blades pseudopetiolate, narrowly or broadly lanceolate, deciduous, the width asymmetrical on each side of the midrib, not tessellate. Synflorescences terminal, small, racemose or contracted-paniculate. Spikelets pedicellate, slender, laterally compressed. Glumes 2-3, unequal; sterile lemmas none; fertile florets 2-16 with a rudimentary terminal floret; lemmas lanceolate or ovate, obtuse, mucronate or awned, many-nerved. Stamens 3. Caryopsis basic.
Didymogonyx (L.G. Clark & Londoño) C.D. Tyrrell, L.G. Clark & Londoño (Arthrostylidiinae). Plants erect or apically arching/pendulous; rhizomes pachymorph with short necks. Culms 6-15 m long, 2-6 cm diameter. Internodes thin-walled, hollow, 1 long internode alternating with 1 very short internode for the length of the culm. Culm leaf sheaths without a girdle, coriaceous, with or without auricles, fimbriae present (setose when on auricles), oral setae lacking; blades erect, triangular, hispid-pubescent. Branching intravaginal; branch complements with 20-30 (-50) branches per node arranged in an apsidate array arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae present, oral setae lacking; blades pseudopetiolate, linear or linear-lanceolate, the width symmetric on each side of the midrib. Synflorescences racemose or subpaniculate. Spikelets laterally compressed. Glumes 3 (-5), triangular, short mucronate, unequal; sterile lemmas 1; fertile florets 4-9 (-12) with a rudimentary terminal floret; lemmas ovate or narrowly ovate, shortly mucronate. Stamens 3. Fruit not seen.
Elytrostachys McClure (Arthrostylidiinae). Plants erect or apically arching/pendulous; rhizomes pachymorph with short necks. Culms 10-20 m long, 3-4 cm diameter. Internodes thin-walled, hollow, all subequally elongated. Culm leaf sheaths without a girdle, coriaceous, without auricles, fimbriae long, oral setae lacking; blades deciduous, strongly reflexed, small, narrow, attenuate. Branching intravaginal; branch complements with 3-numerous branches per node arranged as one dominant branch with 2-numerous smaller branches arising from a single bud, promontory absent. Foliage leaf sheaths with or without auricles, long fimbriae present, oral setae lacking; blades pseudopetiolate, lanceolate, the width symmetric on each side of the midrib. Synflorescences comprised of capitate clusters of a few to many pseudospikelets, produced on lateral shoots. Pseudospikelets complexly bracted, with several orders of branching, each order subtended by a broad, short prophyll. Glumes 2; sterile lemmas none; fertile florets 1-2 with a vestigial terminal floret; lemmas awnless. Stamens 6. Caryopsis basic.
Eremocaulon Soderstr. & Londoño (Guaduinae). Plants erect or apically arching/pendulous; rhizomes pachymorph with short to elongated necks. Culms 3-20 m long, (0.3-) 1-5 cm diameter. Internodes solid, pithy or hollow with thick walls, all subequally elongated. Culm leaf sheaths without a girdle, persistent or tardily deciduous, leathery, with auricles, wavy to curly fimbriae, and oral setae; sheath margins smooth to ciliate; blades narrowly triangular to ovate-lanceolate, reflexed or erect when young becoming reflexed with age. Branching intravaginal; branch complements with 1-10 branches per node arranged as 1-3 subequal main branches with 2-10 smaller secondary branches arising from a single bud per node, promontory absent. Foliage leaf sheaths with auricles and fimbriae (bases fused or free) present, oral setae lacking; blades shortly pseudopetiolate, linear-lanceolate or linear, 7-45 cm, the width asymmetric on each side of the midrib. Synflorescences weakly paniculate or capitate. Pseudospikelets linear-lanceolate or ovate-lanceolate subtended by a bract (with a pseudopetiolate blade in E. triramis C. Jesus-Costa & Londoño) and a prophyll. Glume-like gemmiparous bracts 0-3 (-4), broadly ovate to ovate-lanceolate, mucronate or acute, unequal, 5-16 mm long; sterile lemmas 0-2; fertile florets 1-13 (-17) ending in 1-4 rudimentary terminal florets; rachillas usually bearing a fringe of hairs on the upper rim; lemmas ovate-lanceolate, acuminate or mucronate, many-nerved. Stamens 6 (4 in E. triramis). Caryopsis basic.
Filgueirasia Guala (Arthrostylidiinae). Plants erect; rhizomes pachymorph with short necks. Culms 0.45-1.75 m long, 0.1-0.9 cm diameter. Internodes thin-walled, hollow or (rarely) solid, all subequally elongated. Culm leaf sheaths without a girdle, glabrous, without auricles, fimbriae or oral setae; blades erect, lanceolate. Branching intravaginal, branch complements with 1-15 branches per node (all subequal or one slightly larger), each arising from a single bud (1-15 buds per node), promontory absent. Foliage leaf sheaths without auricles, fimbriae present, oral setae lacking, ciliolate or hispidulous; blades shortly pseudopetiolate, linear, the width symmetric on each side of the midrib. Synflorescences composed of 1 or (less commonly) 2 spikelets. Spikelets laterally compressed. Glumes (1-) 2, boat-shaped, acute to acuminate, awned, subequal; sterile lemmas 0-1; fertile florets 3-15 with a rudimentary terminal floret; lemmas lanceolate, awnless or short awned. Stamens 3. Caryopsis basic.
Glaziophyton Franch. (Arthrostylidiinae). Plants erect; rhizomes pachymorph with short necks. Culms 1.5-2 m long, 0.3-0.4 cm diameter. Internodes thin-walled, hollow with chambered pith, first aboveground internode extremely long, the succeeding ones very short. Culm leaf sheaths without a girdle, strongly striate with inconspicuous or absent auricles and fimbriae, oral setae lacking; blades narrowly triangular, the tip enrolled and pungent. Usually not branching, branches seen only on young shoots after burning (McClure 1973), intravaginal; branch complements, when present, with 1 branch per node arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae short, oral setae lacking; blades shortly pseudopetiolate, lanceolate or slightly cordate, small, the width symmetric on each side of the midrib, strongly tessellate. Synflorescences paniculate, produced from the uppermost node, up to 1 m long and drooping. Spikelets laterally compressed. Glumes 2, lanceolate-ovate, acuminate to apiculate, subequal; sterile lemmas 1; fertile florets (1-) 2 (-3) with a rudimentary terminal floret; lemmas obtuse. Stamens 3. Fruit not seen.
Guadua Kunth (Guaduinae). Plants erect, apically arching/pendulous or clambering/scandent; rhizomes pachymorph with short or elongated necks. Culms (5-) 10-20 (-35) m long, (2-) 5-15 (-23) cm diameter. Internodes relatively thick-walled (rarely thin), hollow or solid, all subequally elongated, usually with supra- and infranodal bands of short, soft white to tan hairs present in the nodal region, sulcate above the insertion of the bud branch complement. Culm leaf sheaths without a girdle, deciduous or disintegrating in place, coriaceous, with or without auricles, fimbriae present or absent, oral setae rarely present; sheath margins smooth or ciliate; blades persistent or rarely deciduous, erect, triangular. Branching intravaginal; branch complements with few to several branches per node arranged as 1 main central branch bearing few to several smaller secondary branches, usually armed with thorns, arising from a single bud, promontory absent. Foliage leaf sheaths with or without auricles, fimbriae present or absent, oral setae lacking; blades pseudopetiolate, linear, linear-lanceolate or ovate-lanceolate, the width symmetric or asymmetric on each side of the midrib. Synflorescences paniculate or capitate, terminating leafy and leafless branches. Pseudospikelets stalked, lanceolate, linear-lanceolate or ovate-lanceolate subtended by a bract and a prophyll. Glume-like gemmiparous bracts one to several, ovate or ovate-lanceolate, apiculate or mucronate, equal or unequal, (2-) 5-9 mm, pubescent; fertile florets 2-17 with a terminal rudimentary floret; lemmas ovate or ovate-lanceolate, apiculate or mucronate, glabrous or pubescent. Stamens 6 [3 in G. longifolia (Bonpl.) Kunth]. Caryopsis usually basic (bacoid in G. sarcocarpa, and G. weberbaueri).
Merostachys Spreng. (Arthrostylidiinae). Plants erect or apically arching/pendulous; rhizomes pachymorph with short necks. Culms (2-) 4-20 (-22) m long, (0.1-) 0.5-3.5 (-4.7) cm diameter. Internodes thin-walled, hollow or rarely pith-filled to solid, all subequally elongated, maculate or with a mottled color pattern, papillose-scabrous and usually with a band of appressed trichomes below the nodes. Culm leaf sheaths without a girdle, deciduous, without auricles, fimbriae present, oral setae lacking; blades reflexed, pseudopetiolate. Branching intravaginal; branch complements with few to numerous branches per node arranged in an apsidate array, arising from a single bud per node, promontory absent. Foliage leaf sheaths without auricles, fimbriae present, oral setae lacking; blades pseudopetiolate, lanceolate or sometimes linear-lanceolate, the width symmetric or slightly asymmetric on each side of the midrib, reflexed. Synflorescences racemose and secund, with underdeveloped spikelets at the apex and often also at the base of the main axis. Spikelets sessile or very short pedicellate, weakly laterally compressed to terete. Glumes 2, muticous, mucronate or awned, unequal; sterile lemmas 0-1; fertile florets 1 (-6) with a rudimentary terminal floret; lemmas muticous, mucronate or awned. Stamens 3. Caryopsis nucoid.
Myriocladus Swallen (Arthrostylidiinae). Plants erect; rhizomes pachymorph with short necks. Culms 0.3-5 (-8) m long, 0.1-2 cm diameter. Internodes hollow or solid, thick-walled if hollow, first aboveground internode extremely long, the succeeding ones very short, or all internodes subequally elongated (as in M. involutus and M. steyermarkii). Culm and foliage leaves not differentiated; sheaths without a girdle, persistent, coriaceous, with auricles present, fimbriae imbricate (except in M. involutus and M. steyermarkii), oral setae lacking; blades shortly pseudopetiolate, deciduous, erect when present, linear or ovate, the width symmetric on each side of the midrib, coriaceous. Branching intravaginal; branch complements with 1 branch per node arising from a single bud, promontory absent. Synflorescences terminal, large and paniculate or less commonly small and racemose. Spikelets laterally compressed. Glumes 2-3, narrowly lanceolate, awned or awnless, equal or unequal; sterile lemmas 1; fertile florets 1-7 (-11) with a rudimentary terminal floret; lemmas lanceolate to ovate, obtuse or acute. Stamens 3. Caryopsis basic.
Olmeca Soderstr. (Guaduinae). Plants erect or clambering/scandent; rhizomes pachymorph with short or elongated necks. Culms (5-) 6-15 (-18) m long, (0.5-) 1-4.5 (-5) cm diameter. Internodes thin-walled, hollow (solid in O. reflexa), all subequally elongated. Culm leaf sheaths without a girdle, deciduous, coriaceous, with auricles present or absent, if present, fimbriate, fimbriae short or long, with oral setae; blades deciduous, erect or reflexed, narrowly to widely triangular, glabrous. Branching intravaginal; branch complements with 1 branch per node arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae short to long, bases free, with oral setae; blades shortly pseudopetiolate, linear or linear-lanceolate, 9.5-32 cm long, the width asymmetric on each side of the midrib. Synflorescences paniculate. Spikelets long-pedicellate, linear-lanceolate, laterally compressed. Glumes 2, triangular, awned or awnless, equal or unequal, glabrous; sterile lemmas none; fertile florets 2-12 with a rudimentary terminal floret; lemmas lanceolate, awned or mucronate, glabrous or hispid (especially at the apex). Stamens 3. Caryopsis basic or bacoid.
Otatea (Munro) C. E Calderón & Soderstr. (Guaduinae). Plants erect, apically arching/pendulous; rhizomes pachymorph with short or elongated necks. Culms 2-8 m long, 1-6 cm diameter. Internodes thin- or thick-walled, hollow or rarely solid, all subequally elongated. Culm leaf sheaths without a girdle, deciduous or disintegrating in place, coriaceous or papyraceous, without auricles, fimbriae short or long, with or without oral setae; blades deciduous or persistent, erect or reflexed, narrowly to widely triangular, glabrous. Branching intravaginal or rarely extravaginal; branch complements usually with 3 but sometimes 1, 2 or 6 branches per node arranged as subequal branches arising from 1 bud borne on a promontory. Foliage leaf sheaths without auricles, fimbriae short or (rarely) long, bases free, with oral setae; blades shortly pseudopetiolate, linear or linear-lanceolate, 4-60 cm, the width asymmetric on each side of the midrib. Synflorescences paniculate or racemose. Spikelets long-pedicellate, linear-lanceolate, laterally compressed. Glumes 2, triangular, awned, unequal, pubescent, scabrous or glabrous; sterile lemmas none; fertile florets 3-7 with a rudimentary terminal floret; lemmas prominently awned. Stamens 3. Caryopsis basic.
Rhipidocladum McClure (Arthrostylidiinae). Plants erect, apically arching/pendulous or clambering/scandent; rhizomes pachymorph with short necks. Culms 1-15 (-20) m long, 0.1-3 cm diameter. Internodes thin-walled, hollow or pithy near the nodes, all subequally elongated. Culm leaf sheaths without a girdle, deciduous, coriaceous, lacking auricles, fimbriae, and oral setae; blades persistent, erect, triangular. Branching intravaginal (sect. Racemiflorum) or extravaginal with deciduous culm leaves (sect. Rhipidocladum); branch complements with several to numerous branches per node arranged in an apsidate array, arising from a single bud, promontory absent. Foliage leaf sheaths without auricles, fimbriae bases free (sect. Racemiflorum) or fused (sect. Rhipidocladum), oral setae lacking; blades pseudopetiolate, linear or linear-lanceolate, 4-15 cm long, the width symmetric or slightly asymmetric on each side of the midrib. Synflorescences commonly spicate-racemose or rarely subpaniculate, main axis zig-zagged (sect. Rhipidocladum) or straight (sect. Racemiflorum), when straight the spikelets sometimes tending to one side of the main axis (secund-like). Spikelets short-pedicellate, laterally compressed. Glumes 2-3, subulate or ovate-lanceolate, acute, mucronate or awned, unequal, glabrous; sterile lemmas 1-4; fertile florets 3-8 with a rudimentary terminal floret; lemmas subulate or ovate-lanceolate, mucronate, glabrous. Stamens 3. Caryopsis basic.
Tibisia C.D. Tyrrell, Londoño & L.G. Clark (Guaduinae). Plants clambering/scandent or apically arching/pendulous; rhizomes pachymorph with short necks. Culms 1-15 m long, 0.1-0.8 cm diameter. Internodes solid, all subequally elongated. Culm leaf sheaths without a girdle, deciduous or disintegrating in place, papyraceous, without auricles, fimbriae short, sometimes pectinate, with oral setae; blades deciduous, reflexed, narrowly triangular. Branching intravaginal; branch complements with numerous branches per node arranged as 1 dominant branch and numerous smaller, subequal branches grouped into fascicles, arising from 1 bud borne on a promontory. Foliage leaf sheaths without auricles, fimbriae bases free or sometimes basally fused, with oral setae; blades shortly pseudopetiolate, linear (either flattened or conduplicate), 4.4-21.5 cm long, the width slightly asymmetric on each side of the midrib. Synflorescences spicate or paniculate. Spikelets long-pedicellate, laterally compressed, spaced 1-3 cm apart. Glumes 3, ovate-lanceolate, awned, glume 1 small, glumes 2 and 3 slightly unequal, glabrous; sterile lemmas none; fertile florets 2-6 with a rudimentary terminal floret; lemmas awned. Stamens 3. Fruit not seen.
Future directions. Given the rate of species discovery in the Neotropical woody bamboos in the last 20 years, it would seem that this bamboo flora has been well cataloged. Figure 1, however, illustrates a steeply increasing discovery rate rather than a plateau. Through experiences in the field collecting and the application of molecular phylogenetics, we believe there are still many species of undescribed bamboos in the Western hemisphere. We estimate there are at least 20 new Chusquea species yet to be described in Perú and probably a similar number for Bolivia, while in Mexico and Colombia, there are several new species in Guaduinae and Arthrostylidiinae awaiting description. More fieldwork, especially in Amazonian Brazil, the central and northern Andean countries (Bolivia, Colombia, Ecuador, Perú, and Venezuela), Central America (El Salvador, Guatemala, Honduras, and Nicaragua), and Mexico, will be necessary to document these undescribed bamboos.
The lack of phylogenetic resolution below the genus level using plastid markers is evident in most phylogenetic studies to date (e.g., in Chusquea, Merostachys, and Aulonemia) (Fisher et al. 2009, Mota 2013, Jesus-Costa 2018, Vidal 2017, Vidal et al. 2018, Vinícius-Silva 2019, Vinícius-Silva et al. 2020). For that reason, we recommend the use of single-copy nuclear genes or Restriction site-associated DNA (RAD) markers or targeted capture to resolve phylogenetic relationships within genera.
Population genetics studies are scarce in the literature of Neotropical woody bamboo species. There are a few studies using microsatellite or RAMS markers for Guadua angustifolia Kunth (Torres et al. 2009, Muñoz-Florez et al. 2010, Rugeles-Silva 2011, Rugeles-Silva et al. 2012), for G. chacoensis (Rojas Acosta) Londoño & P.M. Peterson (Rossarolla 2016), for G. weberbaueri (da Silva-Almeida Leal et al. 2020), and for G. aff. chaparensis Londoño & Zurita, and G. aff. lynnclarkiae Londoño (Melo-Silva 2019). Ely et al. (2019) used ISSR and RAPD markers to assess genetic diversity in three species of Chusquea in Venezuela from cloud forest (C. multiramea L.G. Clark & Ely, C. serrulata Pilg.) and páramo (C. spencei Ernst) habitats. All three showed unexpectedly high levels of genetic diversity, indicating the likely establishment of the sampled populations by seed rather than clonal growth. Mota (2013) also performed assays of population genetics using ISSR markers and the nuclear ribosomal internal transcribed spacer (ITS) in multiple samples of C. subg. Rettbergia from eastern Brazil, emphasizing species delimitation in C. oxylepis (Hack.) Ekman and its allies.
Population genetic analyses by ERS and colleagues are ongoing for Guadua amplexifolia J.Presl, G. inermis Rupr. ex E. Fourn., G. trinii (Nees) Nees ex Rupr., G. tuxtlensis Londoño & Ruiz-Sanchez, G. velutina Londoño & L.G. Clark, and Otatea acuminata using microsatellite markers. These studies allow us to understand how populations are connected through gene flow. Additionally, the results of these genetic analyses can be used to help delimit species and propose conservation measures for the endangered ones.
No published phylogeography studies relating to Neotropical woody bamboos exist; such studies may indicate how populations are connected through gene flow and illuminate the processes that shaped the current distribution that we observe. This is an area in great need of further investigation among the Neotropical woody bamboos, as well as those studies involving ecological niche modeling. For example, Eisenlohr (2015) investigated areas of climate suitability for Merostachys species in the Atlantic forest in Brazil, and the possible effects of future climate change on the geographic distribution of this genus. Similar studies are in progress using species of Chusquea subg. Swallenochloa also from Brazil, in order to understand the potential use of those data to infer species delimitation in this group (E. M. Pianissola, pers. comm.).
Similarly, ecophysiological studies, particularly of drought-tolerant Neotropical woody bamboos, are much needed, to understand the adaptation of woody bamboos to their environments. Ely et al. (2014, 2017, 2019) have investigated the ecophysiology of species of Chusquea and Guadua in Venezuela, but these are among the few published papers investigating the topic for NWB.
Historical and ecological biogeography studies are also needed to understand the patterns of richness, endemism and geographical distribution of the Neotropical woody bamboo species. One historical biogeographical study was published by Ruiz-Sanchez (2011), with a limited number of species and one plastid marker, but since that time, quite a number of Neotropical woody bamboo species have been described and sequenced, and historical biogeographical methods have been improved. To date, only a single ecological biogeographical study has been published, by Ruiz-Sanchez et al. (2020), who studied the diversity, richness, and endemism of the Mexican woody bamboo species. Historical and ecological biogeography studies of the Neotropical woody bamboos, taking into account their complete geographical distribution and using all the sequenced woody bamboo species to date, would complement existing knowledge of their evolutionary history but also provide a firmer foundation for conservation actions.
Undoubtedly, floristic treatments or synopses continue to be important in documenting NWB diversity. Treatments since 1999, including Clark & Ely (2011), Ruiz-Sanchez et al. (2015a), Rúgolo de Agrasar (2017) and Guerreiro & Vega (2019), have provided in-depth perspectives on NWB genera and species for Venezuela, Mexico, Argentina and Chile, respectively, as well as much-needed identification resources. A treatment of the bamboos of Brazil is part of the on-going Flora do Brasil 2020 project (Flora do Brasil 2020, under construction), but a stand-alone publication is also being planned (R. P. Oliveira, pers. comm.). A treatment of the bamboos of Ecuador by LGC is nearing completion.
Additional modern monographs or taxonomic revisions by genus or subgeneric groupings are also needed and may strongly support ongoing phylogenetic studies. Among the historically important taxonomic studies that provided the basis for the modern taxonomy of the Neotropical woody bamboos we may cite “Genera of Bamboos Native to the New World (Gramineae: Bambuseae)” by McClure (1973), “The Genera of Bambusoideae of the American Continent: Keys and Comments” by Calderón & Soderstrom (1980), and “Systematics of Chusquea Section Swallenochloa, Section Verticillatae, Section Serpentes, and Section Longifoliae (Poaceae-Bambusoideae)” by Clark (1989). Although some recent revisions have been published, for example Londoño & Clark (2002b) on Eremocaulon, Attigala et al. (2017) on Chusquea sect. Tenellae and Santos-Gonçalves et al. (2018) on Colanthelia, many genera lack revisionary treatments. Modern computational tools such as proposed by Tyrrell (2019) should also be leveraged to aid in identification of difficult to identify vegetatative material.
Finally, we affirm that several other studies on systematics, phylogenetics and biogeography currently in progress involving genera of Neotropical woody bamboo (e.g., Chusquea, Guadua, Merostachys, and Otatea among others) are part of collaborative networks integrating researchers, graduate and post-graduate students from different institutions and countries in the New World. We also encourage all those who work with Neotropical bamboos to publish monographs at the generic or subgeneric levels or floristic treatments to further document the diversity of this ecologically important group and to provide identification resources for conservationists, ecologists, foresters, and others who work with these plants complementary to the floristic treatments discussed above.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2722











 nova página do texto(beta)
nova página do texto(beta)



