Oxidative stress is the process resultant from an imbalance between the generation of free radicals and the antioxidant defenses. This oxidative stress can induce cell damage and trigger physiologic alterations involved in the origin of pathologic processes like aging, inflammation, carcinogenesis, diabetes, neurodegenerative and autoimmune diseases among others (Sindhi et al. 2013). In such a way, when the endogenous antioxidant system cannot protect the body against the damage caused by free radicals, it is necessary an external supply of antioxidants (Sarmadi et al. 2011). Therefore, the use of natural antioxidants may contribute to the prevention of several diseases and be applied as promising therapeutic agents (Tohma et al. 2017).
Plants are an important source of phytochemicals, among which stand out the phenolic compounds (Mayouf et al. 2019). These compounds are considered secondary metabolites that are produced as a defense mechanism against an attack to the vegetal tissue, the aggression of pathogens and a stressful environment (unfavorable conditions of light, temperature, pH, among others) (Dias et al. 2016). Generally their concentrations depend on the type of organ, the condition of the crops and the stage of development of the plant (García-Mier et al. 2015). Likewise, they are largely responsible from the sensory characteristics of the fruit and vegetable products, such as the color, astringency, flavor and aroma (Gómez-Romero et al. 2010). Additionally, it has been demonstrated that the phenolic compounds possess antimutagenic, anticancer, anti-inflammatory, antioxidant and anticoagulant properties (Souyoul et al. 2018). For the above, the recovery of these phytochemicals from the different tissues or plant organs may generate functional ingredients and/or therapeutic agents with potential application in the design of nutraceuticals, functional foods, cosmetics and phytomedicines (Vu et al. 2018).
Heliopsis longipes (commonly known as chilcuague) is a perennial herbaceous plant endemic of Sierra Gorda, comprising regions of the states of Guanajuato, Queretaro and San Luis Potosí in the center of Mexico (García-Chávez et al. 2004). This species belongs to the Asteraceae family and is the species with the most economic importance of its genus, as its root is widely used by the traditional Mexican, Central and South American medicine for its anesthetic, analgesic, antiulcer, anti-inflammatory and antiparasitic properties; furthermore it is also used as a culinary seasoning and insecticide (Hernández et al. 2009). Up to date, the studies realized with extracts obtained from the H. longipes root, have demonstrated that the diverse pharmacological effects are due to the presence of a bioactive alkamide, called affinin (Escobedo-Martínez et al. 2017, Willig et al. 2019). The diverse traditional uses of the root, as well as the affinin extraction imply the collection of wild plants and the total destruction of the plant, which has caused the reduction in its native populations (Cilia-López et al. 2014). So that in regions of Guanajuato and Queretaro plots are found exclusively dedicated to the H. longipes crops (Escobedo-Martínez et al. 2017), Likewise, there is an effort of the government for the establishment of crops of this species in the aforementioned regions (García-Chávez et al. 2004). Besides, it has been achieved to establish its crop under greenhouse conditions (Parola-Contreras et al. 2020). Because the utility of the H. longipes plant has been limited to the use of the root as the main raw material, it is necessary to focus studies that show that other organs of the plant can represent an important source of phytochemicals. It is known that the leaves of this species contain sterols, terpenes and flavonoids (Cilia-López et al. 2008); however, its bioactivity is still unknown. Therefore, the aim of this study was to determine the total content of polyphenols, flavonoids and tannins of methanolic extracts from leaves and flowers of H. longipes collected in different regions of Sierra Gorda and their relationship with the antioxidant effect.
Materials and methods
Plant material. Wild plants of H. longipes with homogeneous height were randomly selected and collected during the spring (April 2017 and May 2018) in three locations of Sierra Gorda, in the center of Mexico: Conca and San Juan Buenaventura (SJB) located in the municipality of Arroyo Seco of the State of Queretaro and Beltran located in the municipality of Xichu from the State of Guanajuato (Table 1). The voucher specimens were identified and deposited in the Ethnobotanical collection of the Herbarium of Queretaro “Jerzy Rzedowski” (QMEX) with catalog numbers 1084, 1114 and 1112 for samples from Conca, SJB and Beltran, respectively. Soil samples from the three locations were collected simultaneously with the biological material. The chemical analysis of the soil (Table 2) was performed according to the methods of the Official Mexican Norm (SEMARNAT 2002).
Table 1 Geographic location of the wild plants of Heliopsis longipes collected in the Sierra Gorda, Mexico.
| Municipality | Location | Longitude | Latitude | Altitude (m asl) | Climate classification | Average annual temperature (°C) |
|---|---|---|---|---|---|---|
| Arroyo Seco | Conca | -99.63 ° | 21.44 ° | 560 | Warm sub-humid with rains in summer (Aw) | 23.8 |
| Arroyo Seco | SJB | -99.47 ° | 21.35 ° | 1,400 | Semi-warm sub-humid with rains in summer (ACw) | 23.3 |
| Xichu | Beltran | -100.08 ° | 21.27 ° | 1,594 | Warm semi-arid (BS1h) | 16.8 |
SJB (San Juan Buenaventura); masl (meters above sea level).
Table 2 Chemical parameters and texture of soil from different collection sites of Heliopsis longipes.
| Location | Calcium | Potassium | Phosphorus | Magnesium | Sodium | Electrical conductivity | Organic matter | pH | Soil texture |
|---|---|---|---|---|---|---|---|---|---|
| (mg/Kg) | (dS/m) | (%) | |||||||
| Conca | 548.3 | 149.20 | 5.35 | 204.37 | 4.85 | 0.192 | 1.93 | 7.92 | Clay |
| SJB | 9278.23 | 216.83 | 1.71 | 172.76 | 4.21 | 0.258 | 3.99 | 8.35 | Clay |
| Beltran | 3219.86 | 67.73 | 16.24 | 276.80 | 6.58 | 0.067 | 1.14 | 7.63 | Loam |
SJB (San Juan Buenaventura); dS = decisiemen
Preparation of methanolic extracts. Fifty grams of fresh leaves and flowers of the H. longipes plant from each locality were separated (Figure 1). Subsequently, some leaves and flowers of every locality were randomly selected and were crushed in liquid nitrogen. Then, crushed samples of leaves and flowers were macerated with methanol in a 1:10 ratio (w/v). The suspensions were collocated in a sonicator (Bransonic M2800-CPX-HE, Emerson, Ferguson, Missouri, USA) for 3 h and centrifuged at 10,000 rpm for 15 min at 4 °C. The resultant supernatants (soluble phenolic extracts) were denominated methanolic extracts of leaves (LME) and flowers (FME) and stored in vials at 4 °C in darkness conditions until use.

Figure 1 Leaves and flowers of Heliopsis longipes collected in the Sierra Gorda, Mexico. A: Conca, B: San Juan Buenaventura (SJB) and C: Beltran.
Total polyphenol content (TPC). The total polyphenol content (TPC) of LME and FME obtained from each locality was determined spectrophotometrically by the Folin-Ciocalteu method according to Vergara-Castañeda et al. 2010, using gallic acid as standard. Briefly, 140 µL of the LME or FME extract (0.5 mg/mL) were mixed with 460 µL of distilled water and 250 µL of the Folin-Ciocalteu (1 N) reagent. After 5 min, 1,250 µL of 20 % (w/v) sodium carbonate were added. The mixture was shaken in the vortex and incubated for 2 h in darkness. The absorbance was measured at 760 nm. The results were expressed as mg equivalent of gallic acid per gram of fresh weight (mg GAE/g).
Total flavonoid content (TFC). The total flavonoid content (TFC) of LME and FME obtained from each locality was determined spectrophotometrically according to the method described by García-Mier et al. (2015), using rutin as standard. A 50 µL aliquot of the LME or FME extract (0.5 mg/mL) was mixed with 180 µL distilled water and 20 µL of a 1 % (w/v) 2-aminoethyldiphenylborate solution. The absorbance was measured at 404 nm. The results were expressed as mg equivalents of rutin per gram of fresh weight (mg RE/g).
Condensed tannins content (CTC). The condensed tannins content (CTC) of LME and FME obtained from each locality was determined spectrophotometrically according to the method described by Feregrino-Pérez et al. (2008), using (+)-catechin as standard. Briefly, 50 µL of the LME or FME extract (0.5 mg/mL) were mixed with 200 µL of vanillin reagent (0.5 % vanillin, 4 % HCl in methanol). The absorbance was measured at 495 nm. The results were expressed as mg of (+)-catechin equivalents per gram of fresh weight (mg CE/g).
Determination of antioxidant activity (AOx). The antioxidant activity (AOx) of LME and FME obtained from each locality was evaluated through the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) and 2,2´-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) assays.
DPPH•radical scavenging activity (DRSA). The DPPH• radical scavenging activity (DRSA) of LME and FME was determined according to the method described by Feregrino-Pérez et al. (2011). A 20 µL aliquot of extract (LME or FME) was added to 200 µL of DPPH• radical (0.1 mM) in methanol solution. The mixture was shaken vigorously and incubated for 30 min in the darkness at room temperature; the absorbance was measured at 515 nm. Methanol was used instead DPPH• for the blank, while distilled water was used instead of extract for the control. The DRSA was calculated through the following equation:
ABTS•+radical scavenging activity (ARSA). The ABTS•+ radical scavenging activity (ARSA) of LME and FME was determined according to the method described by Feregrino-Pérez et al. (2011). Initially, the cationic radical ABTS•+ was generated by mixing the ABTS stock solution (7 mM) with potassium persulfate (2.45 mM); the resulting mixture was left to stand in the darkness for 12 h at room temperature. Then, the ABTS•+ radical solution was diluted in phosphate buffered saline (PBS, 0.15 M, pH 7.4) to obtain an absorbance of 0.70 ± 0.02 at 730 nm. For the assay 230 µL of this diluted solution were mixed with 20 µL of the extract (LME or FME). The absorbance was measured at 730 nm from 1 to 6 min after the initial mixture, in darkness conditions. PBS was used instead of ABTS•+ for the blank, while distilled water was used instead the extract for the control. The ARSA was calculated through the following equation:
Trolox equivalent antioxidant capacity (TEAC). Both DPPH and ABTS assays, trolox (6-hidroxi-2,5,7,8-tetramethyl chromane-2-carboxylic acid, 0-800 µM, final concentration) were employed to generate a standard curve and the trolox equivalent antioxidant capacity (TEAC) was obtained for each extract. The results were expressed as µmol trolox equivalents per gram of fresh weight (µmol TE/g).
EC50determination.- The EC50 value, defined as the effective concentration of the extract required to scavenge 50 % of radical activity (DPPH• or ABTS•+) was calculated from a linear regression plot of radical scavenging (%) versus extract concentration (µg/mL). Trolox was employed as reference antioxidant compound.
Ultra Performance Liquid Chromatography (UPLC) analysis. The H. longipes extracts were concentrated in a SavantTM SpeedVacTM vacuum concentrator system at 4 °C for two days and resuspended (10 mg) in 1 mL of methanol (HPLC grade). The samples were filtered using an Acrodisc of 0.45 µm pore mesh and 25 mm diameter (Agilent Technologies). Each sample was analyzed in triplicate.
The phenolic composition of each extract was assessed in an Ultra-Performance Liquid Chromatograph (UPLC) coupled to a Diode Array Detector (DAD) and a Quadrupole Time-of-Flight (Q-ToF) mass spectrometer (MS) with an electrospray ionization (ESI) interphase (Vion IMS, Waters Co). The sample solutions were filtered (0.45 µm) and directly injected into a BEH Acquity C18 column (2.1 × 100 mm, 1.7 mm) at 35 ºC. For the chromatographic separation, water with 0.1% formic acid (A) and acetonitrile (B) were used as mobile phase at a flow of 0.5 mL/min. The gradient conditions were 0 % B/0 min, 15 % B/2.5 min, 21 % B/10 min, 90 % B/12 min, 95 % B/13 min, 0 % B/15 min and 0 % B/17 min. The absorbance was measured at 214, 280, 320 and 360 nm. The following commercial standards were used for quantification: chlorogenic acid, caffeic acid, coumaric acid, hydroxybenzoic acid, apigenin, genistein, quercetin, rutin and naringenin. Results were expressed as µg/g. The following MS conditions were used: capillary voltage, 2.0 kV; cone voltage, 40 eV; low collision energy, 6 V; high collision energy, 15-45 V; source temperature, 120 ºC; cone gas flow, 50 L/h; desolvation gas, N2 at 450 ºC and 800 L/h. Data acquisition was carried out at negative ionization mode (ESI-) within a 100-1,200 Da mass range. Leucine-enkephalin solution (50 pg/mL) was used for lock mass correction at 10 µL/min. Identification was carried out by analysis of the exact mass of the pseudomolecular ion (mass error < 5 ppm), isotope distribution, and fragmentation pattern.
Statistical analysis. The results were reported as mean ± standard deviations. Significant differences among the collection site and plant organs were determined by variance analysis and Fisher´s least significant difference (LSD) tests (P < 0.05) using the Statgraphics® Centurion XVI statistical software (StatPoint Technologies Inc., Bedford, MA, USA, 2010).
Results
Total phenolic compounds. Figure 2A represents the TPC of LME and FME obtained from the plants H. longipes collected in Conca, SJB and Beltran. The TPC values varied widely from 17.59 to 98.1 mg GAE/g, observing the highest values in LME (98.1 mg GAE/g) and FME (78.17 mg GAE/g) obtained from the plants of Beltran. The statistical analysis showed significant differences (P < 0.05) between the collection sites and the plant organs, being the extracts coming from Beltran leaves and flowers, the ones that showed the highest TPC, followed to the extracts coming from plants from the Conca and SJB regions. The obtained values were found in the reported range (17.6-188 mg GAE/g) in leaves and flowers extracts from other species of Tenacetum L. (Arituluk et al. 2016), Schizogyne sericea (L.f) DC (Caprioli et al. 2017) and Tithonia diversifolia Hemsl. (Pretti et al. 2018) that belong to the Asteraceae family.
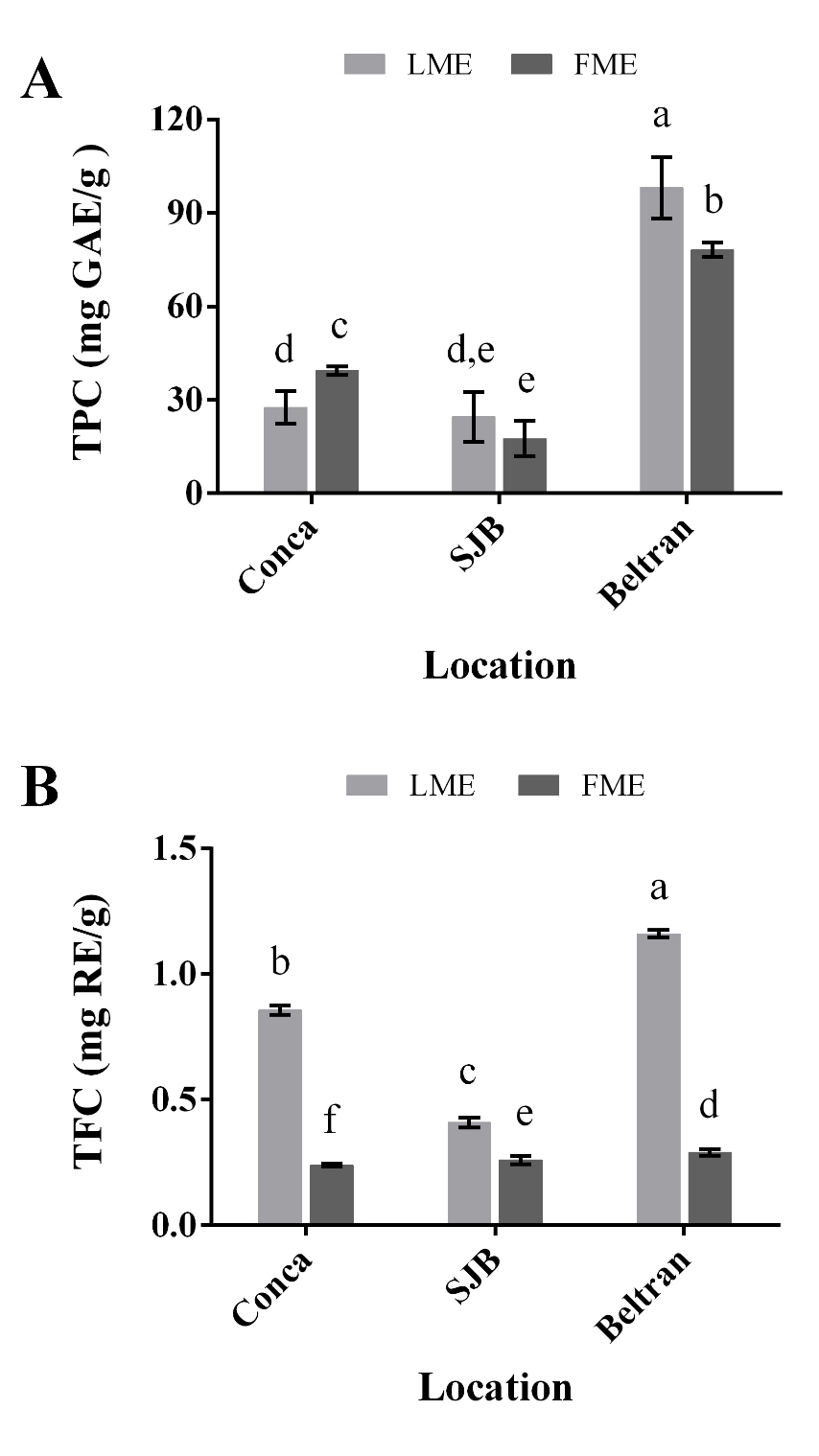
Figure 2 Phenol compounds content of methanolic extracts from leaves (LME) and flowers (FME) of Heliopsis longipes. (A) Total polyphenol content (TPC, mg GAE/g). (B) Total flavonoid content (TFC, mg RE/g). Results are plotted as mean ± standard deviation. Different letters indicate significant differences (P < 0.05). GAE (Gallic acid equivalents); RE (Rutin equivalents).
Figure 2B represents the TFC of LME and FME from the different collection sites. The TFC values were found between 0.24-1.16 mg RE/g. It was observed that in the three locations, the LME presented a major flavonoid concentration in comparison to the FME. Furthermore, the TFC was significantly different (P < 0.05) in all the extracts, with a similar behavior observed for polyphenols, which is, between the collection sites, the extracts coming from leaves and flowers of the plants of Beltran, are those that contain the highest flavonoid concentration. These results are similar to the reported (0.02-1.27 mg RE/g) in leaves and flowers of Vainilla planifolia Jacks. ex Andrews from the family Orchidaceae (Andrade-Andrade et al. 2018), as well as some species from the family Asteraceae (Jamei & Anvari 2018).
On the other hand, the presence of condensed tannins was only observed in LME coming from plants from Conca and Beltran, with values of 0.64 ± 0.05 mg CE/g and 1.09 ± 0.07 mg CE/g, respectively. In LME of SJB and FME from the three locations, tannins were not detected. In this case, as for polyphenols and flavonoids, the LME coming from Beltran showed the highest CTC. Furthermore, the CTC values, were found in the reported range (0.02-5 mg EC/g) for leaves and flowers extracts of Cirsium arvense L. (Hossain et al. 2016) and T. diversifolia (Pretti et al. 2018) from the family Asteraceae, as in extracts of V. planifolia (Andrade-Andrade et al. 2018).
Therefore, the results indicate that under the methanol extraction conditions, the leaves and flowers of H. longipes from Beltran are those that represent the highest total content of phenolic compounds (polyphenols, flavonoids and tannins).
AOx of LME and FME. As shown in Figure 3, all the extracts showed AOx, with values ranging from 32.98 to 48.0 % DRSA. Besides, all the extracts obtained from leaves showed a higher DRSA, obtaining the major percentage in LME of Beltran. Similarly, the FME from Beltran showed a DRSA significantly higher (P < 0.05) than the found for the extracts coming from Conca and SJB. These results were similar to the reported in methanolic and ethanolic extracts of other Asteraceae genera as Tenacetum L. (Arituluk et al. 2016) and Cirsium L. (Hossain et al. 2016), as well as in Asphodelus microcarpus (Mayouf et al. 2019) and Beta bulgaris (Edziri et al. 2019) belonging to Liliaceae and Amaranthaceae, respectively. Respect to the trolox equivalent antioxidant capacity (TEAC-DPPH) of the extracts, values from 368.75 to 545.62 µmol TE/g were obtained (Table 3). These TEAC-DPPH values were higher than those reported for ethanolic and aqueous extracts obtained from aerial parts of S. sericea (121-283 µmol TE/g) (Caprioli et al. 2017). It is worth mentioning that a TEAC value higher corresponds to a greater capacity of radical removal.
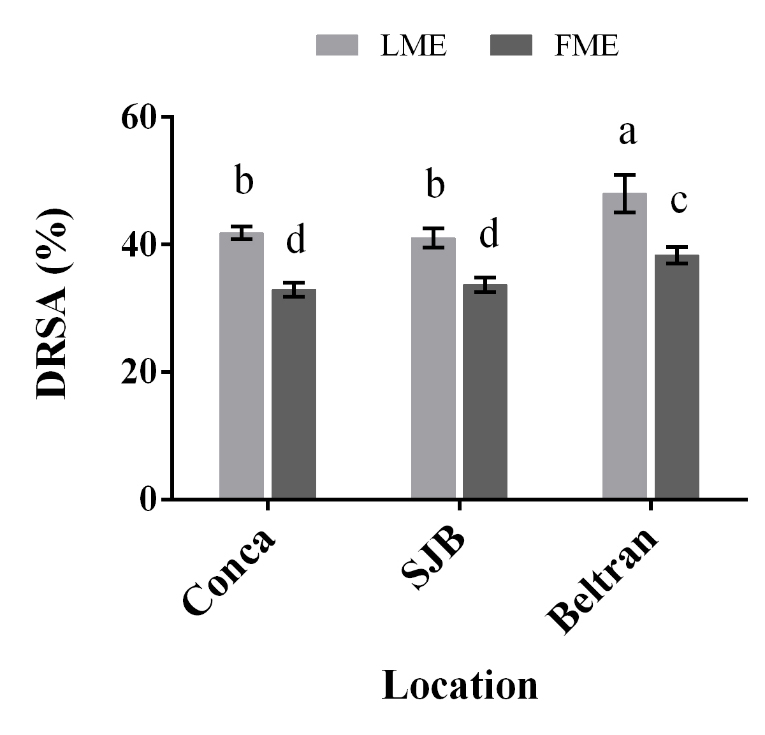
Figure 3 DPPH• radical scavenging activity (DRSA, %) of methanolic extracts from leaves (LME) and flowers (FME) of Heliopsis longipes. Results are plotted as mean ± standard deviation. Different letters indicate significant differences (P < 0.05).
Figure 4 shows that all the extracts, as for leaves and flowers exhibited a higher ARSA, reaching values between 88-89 %. Therefore, the values of the trolox equivalent antioxidant capacity (TEAC-ABTS) were between 735.83 and 744.79 µmol TE/g (Table 3). These results indicate that LME and FME obtained from the different locations, presented a strong ARSA, which were comparable to the reported for extracts obtained from the aerial part of S. sericea (Caprioli et al. 2017). In general, the methanolic extracts from both leaves and flowers showed free radicals scavenging capacity (DPPH• y ABTS•+), which indicates that the phenolic compounds present in the extracts confers an antioxidant effect.
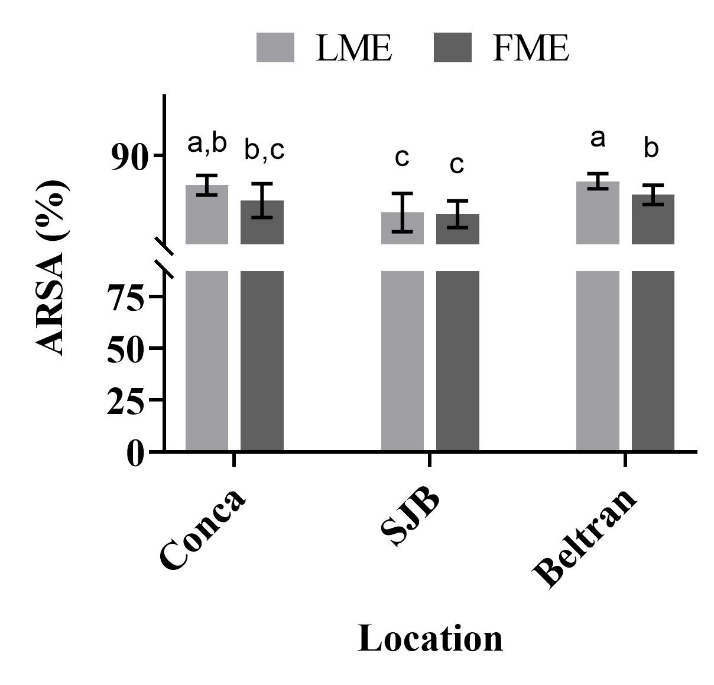
Figure 4 ABTS•+ radical scavenging activity (ARSA, %) of methanolic extracts from leaves (LME) and flowers (FME) of Heliopsis longipes. Results are plotted as mean ± standard deviation. Different letters indicate significant differences (P < 0.05).
Furthermore, the antioxidant potential of the extracts was determined by their EC50 value, which is a parameter of high significance since it establishes the necessary concentration of an antioxidant compound to achieve 50 % inhibition of the radical activity. In other words, a lower EC50 value indicates a higher antioxidant capacity of the extract. The values of EC50 that were obtained in LME and FME ranges between 741.89 and 1343.55 µg/mL for DPPH assay and between 609.76 and 3389.13 µg/mL for ABTS assay (Table 3). These EC50 values were similar to those previously found by Pretti et al. (2018) when evaluating the antioxidant activity of ethanolic extracts of leaves from T. diversifolia (Asteraceae), but does not coincide with the findings of Edziri et al. (2019) and Mayouf et al. (2019) in methanolic extracts of B. vulgaris (Amaranthaceae) and A. microcarpus (Liliaceae), respectively. As seen in Table 3, the extracts from the Beltran region had the lowest EC50 values, noticing the same behaviour for both radicals (DPPH• and ABTS•+). Nevertheless, the EC50 of the reference compound trolox was significantly lower than those obtained in LME and FME of Beltran, therefore, it requires a higher concentration of extracts H. longipes to achieve 50 % elimination of free radicals.
Table 3 TEAC and EC50 values of methanolic extracts from leaves and flowers of Heliopsis longipes.
| Location | Methanolic extract | DPPH assay | ABTS assay | ||
|---|---|---|---|---|---|
| TEAC (µmol TE/g) | EC50 (µg/mL) | TEAC (µmol TE/g) | EC50 (µg/mL) | ||
| Conca | LME | 480.55 ± 10.31b | 1,343.55 ± 94.01a | 743.71 ± 2.68a,b | 3,389.13 ± 237.15a |
| SJB | 472.26 ± 16.19b | 969.20 ± 49.3b | 736.28 ± 5.21c | 1,304.57 ± 17.71b | |
| Beltran | 545.62 ± 31.10a | 754.58 ± 12.15c,d | 744.79 ± 2.05a | 609.76 ± 9.82d | |
| Conca | FME | 386.75 ± 11.76d | 1,303.21 ± 59.78a | 739.50 ± 4.59b,c | 1,112.83 ± 46.17b |
| SJB | 394.52 ± 12.53d | 848.22 ± 31.59c | 735.83 ± 3.63c | 884.31 ± 66.37c | |
| Beltran | 443.77 ± 13.55c | 741.89 ± 30.78d | 741.11 ± 2.61b | 847.29 ± 38.87c | |
| Reference compound | |||||
| Trolox | 145.38 ± 5.01e | 110.11 ± 3.79e | |||
Values are shown as mean ± standard deviation.
Means with different superscripts, in the same column, are significantly different (P < 0.05).
TEAC (Trolox equivalent antioxidant capacity); EC50 (sample concentration needed to scavenge 50% of DPPH• and ABTS•+ radicals’ activity); SJB (San Juan Buenaventura); LME (Leaves methanolic extract); FME (Flowers methanolic extract).
Characterization by UPLC. In the chromatographic profiles (UPLC) of LME and FME of H. longipes collected in three different regions of the Sierra Gorda, a total of nine phenolic compounds were identified: hydroxybenzoic, chlorogenic, caffeic and coumaric acids, as well as rutin, quercetin, apigenin, genistein and naringenin (supplementary material 1). The type of phenolic compound and its concentration in the extracts are shown in Table 4. In general, the main compounds identified in LME were hydroxybenzoic acid and chlorogenic acid, highlighting that rutin (120.86 ± 13.29 µg/g) and genistein (24.45 ± 3.01 µg/g) existed only in the extracts of Beltran and Conca, respectively. On the other hand, in FME the presence of all the identified compounds was observed, with the exception of rutin in the SJB extracts. Thus, for each extract a particular phenolic composition was obtained, chlorogenic acid is the only compound that exists in all the extracts and the most abundant in Beltran's LME. Similar phenolic compositions have been described by Hossain et al. (2016) and Caprioli et al. (2017) in extracts obtained from C. arvense and S. sericea, species that belong to the same family of H. longipes.
Table 4 Phenolic profile of methanolic extracts from leaves and flowers of Heliopsis longipes.
| No. | Compound | Retention time (min) | Methanolic extrac | |||||
|---|---|---|---|---|---|---|---|---|
| LME (µg/g) | FME (µg/g) | |||||||
| Conca | SJB | Beltran | Conca | SJB | Beltran | |||
| Phenolic acids | ||||||||
| 1 | Hydroxybenzoic acid | 2.40 | 1,141.91 ± 191.90 | 410.76 ± 45.18 | nd | 333.23 ± 36.66 | 1,469.01 ± 118.37 | 313.97 ± 43.55 |
| 2 | Chlorogenic acid | 2.60 | 139.34 ± 14.63 | 127.82 ± 14.06 | 503.41 ± 55.37 | 400.92 ± 51.31 | 433.00 ± 33.84 | 177.46 ± 35.49 |
| 3 | Caffeic acid | 4.28 | nd | n.d | nd | 1,764.37 ± 194.08 | 2,923.90 ± 550.77 | 97.59 ± 19.41 |
| 4 | Coumaric acid | 4.80 | nd | n.d | nd | 36.75 ± 4.04 | 58.81 ± 9.78 | 49.87 ± 9.97 |
| Flavonoids | ||||||||
| 5 | Rutin | 4.64 | nd | nd | 120.86 ± 13.29 | 1,354.64 ± 149.01 | nd | 462.54 ± 72.18 |
| 6 | Apigenin | 7.37 | nd | nd | nd | 143.59 ± 15.79 | 103.18 ± 1.79 | 129.09 ± 12.97 |
| 7 | Genistein | 7.78 | 24.45 ± 3.01 | nd | nd | 371.08 ± 40.82 | 307.91 ± 1.27 | 363.52 ± 35.36 |
| 8 | Quercetin | 8.97 | nd | nd | nd | 44.31 ± 4.87 | 71.60 ± 1.75 | 47.61 ± 7.14 |
| 9 | Naringenin | 10.95 | nd | nd | nd | 42.66 ± 4.69 | 30.57 ± 3.47 | 87.86 ± 4.39 |
SJB (San Juan Buenaventura), nd = not detected
Discussion
In the present study, similarities were found in the phenolic compounds content in the extracts (LME and FME) from H. longipes with other traditional medicinal plants found in the same botanical family (Asteraceae), as in species from other families such as Amaranthaceae, Liliaceae and Orchidaceae . The last demonstrated that the maceration (assisted by ultrasound) using methanol, is an efficient method in the phenolic compounds extraction, just like it has been documented in the scientific literature (Jovanović et al. 2017, Rodríguez-Méndez et al. 2018, Edziri et al. 2019, Mayouf et al. 2019).
Diverse studies have reported that the phytochemical composition of the plants is influenced for numerous factors as the soil, altitude, environmental conditions of growth, stress (biotic and abiotic), phenological stage, among others (Pretti et al. 2018, Quiroz-Sodi et al. 2018, Li et al. 2020). In this study, it was demonstrated that the phenolic compounds content (TPC, TFC and CTC) in the methanolic extracts of H. longipes varies considerably among the collection sites (Conca, SJB and Beltran) and the organ (leaf and flower) of the plant. Based on the results, the greatest content of polyphenols, flavonoids and tannins was observed in LME and FME from Beltran, location found in a different municipality, at a higher altitude and with different climatic conditions, respect to the other locations (Conca and SJB). In this sense, the plants H. longipes from Beltran are exposed to a higher ultraviolet (UV) radiation, lower mean temperature and minor accumulated precipitation, environmental factors that may generate stress in the plants and favor the production of secondary metabolites. Manach et al. (2004) point that the phenolic compounds are secondary metabolites mainly involved in the adaptive response of the plants to the UV radiation. Meanwhile, Vázquez-Hernández et al. (2019) describe that the UV radiation, temperature and hydric stress are factors representing an inducer effect on the secondary metabolites of the plants. Additionally, previous studies performed in traditional medicinal plants have reported positive correlations between the decrease in temperature and altitude increase with a major production of phenolic compounds (Sampaio et al. 2011, Pretti et al. 2018). Therefore, the differences in the environmental factors (altitude, climatic classification and temperature) among the locations of H. longipes significantly influenced in the phenolic compounds biosynthesis. It has been documented that some metabolic and physiologic processes of the plants are related with the chemical composition of the soil, nutrient availability and salinity conditions (Tapia et al. 2019). The soil in the Beltran location highlighted for its high content of calcium, phosphorus and magnesium, essential compounds in the growth and development regulation of the aerial part of the plant (Li et al. 2019). Likewise, the soil in this location presented a higher sodium content, which functions as osmotic agent that favors the efficient water absorption (Song et al. 2020). Additionally, the soil in this location is loam type, different to Conca and SJB that is clayey type. Tapia et al. (2019) report that the loam soils favor the water retention in the prolonged periods, facilitating to the plants the nutrient and water absorption, allowing in this manner the good development of this. On the other side, the soil in these locations showed a pH mildly alkaline and low electric conductivity, classifying them as soils with imperceptible effects of salinity. Zhao et al. (2020) describe that high salinity conditions in the soil may cause effects in the growth of the plant due to an unbalance in the nutrient absorption. In this study, the loam type soil with high presence of calcium, phosphorus, magnesium and potassium suggests a positive influence on the phenolic compounds metabolism in H. longipes, mainly polyphenols and flavonoids.
As previously mentioned, the LME from H. longipes (from the three locations) presented a higher content of phenolic compounds, being the flavonoids and tannins, the compounds that differ the most. These metabolites are usually transported from the roots to the stems and leaves, via xylem or phloem, and store in reproductive structures, such as flowers, so that they can be found in different parts of the plant (Lin et al. 2018). In this context, flavonoids confer color to the leaves and flowers, although they tend to focus mainly in leaves, while the tannins accumulate mainly in the root, leaves, fruits and seeds (Manach et al. 2004). Studies performed have demonstrated that climatic factors (type of soil, temperature, solar exposition and precipitation) may affect the presence and accumulation of phenolic compounds in the plant organs (Li et al. 2020). The concentration of flavonoids is affected directly for the sun exposition, due to its biosynthesis is coupled to the photosynthesis. It should also be remembered that the polyphenols and flavonoids have an antioxidant function that protects the plant from the presence of free radicals (Dias et al. 2016). On the other hand, both the polyphenols and tannins may contribute in the tissue protection when they are exposed to pathogens, predators and UV radiation, as to favor the tissue healing (Brillouet et al. 2013). It is known that the phenolic phytochemicals perform important functions during growth, development and reproduction of the plant (González-Aguilar et al. 2014), in this way before the presence of different types of abiotic stress, its biosynthesis and accumulation is altered in the different plant organs (Vázquez-Hernández et al. 2019).
Assays that evaluate the free radicals reduction have been widely used as a first valid and important parameter of potential antioxidant compounds (Sarmadi et al. 2011, Brahmi et al. 2012). In our study, LME and FME from H. longipes presented AOx through the reduction of DPPH• and ABTS•+ radicals, being the electrons transference the mechanism by which the phenolic compounds present in the extracts neutralize the free radicals. Neha et al. (2019) describe that free radicals are continuously produced through biochemical reactions, which occur as a part of aerobic cellular metabolism and are associated with the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). An excessive amount of these radicals leads to oxidative damage of lipids, proteins and DNA, causing oxidative stress. In the human body, oxidative stress plays an important role by being the initiator of chronic degenerative and neurodegenerative diseases along with some types of cancer (Sindhi et al. 2013, Neha et al. 2019). In this sense, an effective antioxidant complex can remove free radicals and ROS at different cell sites, limiting the harmful effects on cells and tissues (Adegbola et al. 2020). Therefore, the phenolic compounds existing in the extracts of H. longipes could contribute towards the protection against oxidative damage triggered by overproduction of free radicals.
According to the results, the DRSA may associate the total phenolic content of the extracts, instead ARSA suggests being influenced to a greater extent for the chemical composition of the phenolic compounds, indicating that the molecular characteristics of the polyphenols, flavonoids and tannins present in the extracts of the different locations contribute in the anti-radical capacity. LME and FME of Beltran exhibited the greatest radical DPPH• and ABTS•+ scavenging effect, showing the lowest EC50 values, which suggests that it may be related to the higher content of phenolic compounds (TPC, TFC and CTC) in the extracts, mainly with TPC. Pretti et al. (2018) found high correlations between the total content of phenolic compounds (polyphenols, flavonoids and tannins) with the reducing capacity of DPPH and ABTS of extracts from T. diversifolia (species belonging to the same family of H. longipes). On the other hand, in extracts obtained from other botanical families (Amaranthaceae and Liliaceae), the anti-radical effect was correlated mainly with the high content of flavonoids (Edziri et al. 2019, Mayouf et al. 2019).
The phenolic compounds possess biological activity and have been related to the prevention of cardiovascular disease, some types of cancer and the oxidative damage that suffers the organism (Souyoul et al. 2018). These properties of the phenolic compounds have been attributed to the antioxidant effect exerted in direct manner as free radical scavengers (Lin et al. 2018). González-Aguilar et al. (2014) mention that the AOx of the phenolic compounds depend on their structure, particularly the hydroxylation degree of the aromatic rings and the position of the hydroxyl groups. Other studies have also proposed that exists a correlation between AOx and the polyphenols, flavonoids and tannins content in the different parts of the plants (Caprioli et al. 2017, Pretti et al. 2018, Mayouf et al. 2019).
According to the UPLC analysis, in LME the main classes of identified phenolic compounds were hydroxybenzoic acid and chlorogenic acid, which were existing in the extracts from the three locations; in addition to flavonols (rutin) and isoflavones (genistein) existing only in the extracts of Beltran and Conca, respectively. On the other hand, in FME of all localities, in addition to the compounds identified in LME, there was also the presence of other phenolic acids (caffeic and coumaric), apigenin (flavone), naringenin (flavanone) and quercetin (flavonol). The differences between LME and FME can be attributed both to the type of organ and the growth conditions of H. longipes, as described above. Both phenolic acids and flavonoids represent secondary metabolites that intervene in defense mechanisms of plants (Manach 2004). In this sense, rutin and genistein could be characteristic flavonoids of Beltran and Conca leaves, respectively. Andrade-Andrade et al. (2018) mentioned that this type of flavonoids helps to fight oxidative stress and act as a growth regulator. On the other hand, the flowers presented greater diversity of metabolites, which may suggest that in H. longipes this organ has high metabolic activity due to the growth conditions. In addition, flowers contain flavonoid pigments in their structure. The phenolic acids and flavonoids identified in LME and FME have been previously reported as bioactive metabolites responsible for the reduction of the level of harmful free radicals and consequently for a high antioxidant effect (García-Mier et al. 2015). In particular, chlorogenic acid has proven to be a powerful antioxidant, since it inhibits carcinogenesis and has protective effects against oxidative stress (Caprioli et al. 2017). For their part, caffeic acid, apigenin, and quercetin have been shown to have anticarcinogenic and protective effects against photoaging of the skin, due to their antioxidant properties (Lorencini et al. 2014). Therefore, in the present study, the phenolic composition existing in the extracts (LME and FME) of H. longipes is the one that provided its AOx.
So far, and to the best of our knowledge, this is the first study focused in the obtainment of antioxidant phenolic extracts of leaves and flowers of H. longipes. The obtaining of the extracts provides added value to the chilcuague plant for the use of foliage (specifically leaves and flowers), so that currently is discarded and with no significant use. It is necessary to perform more studies related to the extraction process optimization, characterization and purification of phenolic substances and evaluation in vivo of their antioxidant potential for its possible application as ingredients in functional foods, cosmetics products and phytomedicines.
Supplementary material
Supplementary material for this article can be accessed here: https://doi.org/10.17129/botsci.2671.











 nueva página del texto (beta)
nueva página del texto (beta)



