Tropical montane cloud forest, particularly in Mexico, have high tree diversity because of their tropical and boreal components; however, many species are threatened by human activities (González-Espinosa et al. 2011). Furthermore, climate change projections for 2080 estimate that 68 % of current Mexican tropical montane cloud forests could be lost, with high impacts for many endemic species (Ponce-Reyes et al. 2012). One of the endangered species is a Mexican beech, Fagus grandifolia Ehrh. subsp. mexicana (Martínez) A.E. Murray, which is notable because it has been reported in only 11 localities (1,400-2,000 m asl), all of them in the Sierra Madre Oriental. The total area of beech forest in the country is ca. 200 ha (Rodríguez-Ramírez et al. 2013), with evidence of considerable perturbation from firewood extraction and sheep grazing.
Several studies have been carried out in Mexican beech forest, examining distribution (Williams-Linera et al. 2003, Rodríguez-Ramírez et al. 2013), population structure (Ortiz-Quijano et al. 2016), plant diversity (Gutiérrez-Lozano et al. 2017), population genetics (Rowden et al. 2004), seed germination, seedling survival and growth (Álvarez-Aquino & Williams-Linera 2002). These studies highlight the urgent need for beech forest conservation and restoration. To achieve these goals, it is necessary to obtain basic information about factors that affect natural regeneration, such as patterns and processes in seed and seedling banks. In general, late successional tree species such as Fagus grandifolia Ehrh., F. orientalis Lipsky and F. sylvatica L., do not form seed bank (Houle 1994, Olano et al. 2002, Schmidt et al. 2009, Esmailzadeh et al. 2011). Nevertheless, these types of tree species have high mortality rates in their seedling and sapling stages (from competition or herbivory), and those that survive make up the shade tolerant seedling bank (Gonzalez et al. 2008, Bedoya-Patiño et al. 2010). Seed and seedling banks have an essential role in ecological succession because they are sources of germplasm for regenerating the structure and function of forest ecosystems (Antos et al. 2005, Thompson 2000). Thus, in practice, both seed and seedling banks are useful for restoration projects (Martínez-Ramos & García-Orth 2007, Walker et al. 2007).
Species composition in forests can be explained by the relationships among the pools of species of each structural components (i.e., seed bank, seedling bank, and tree canopy), through an internal dynamic. Therefore, the process takes place within the community through feedback loops (Li et al. 2010), where many species are shared among forest components. However, the fragmentation process and human perturbation have affected the internal dynamic of the forest, inducing changes in the species composition. Recently, the effect of fragmentation in a rainforest on community composition was evaluated in seedling, sapling and tree components reporting lower seedling richness (number of genera per plot) in the smallest and most isolated fragments (Stride et al. 2018). Possibly, with a posterior increase in the abundance of species related to the disturbance (Rutledge 2003). Thus, a greater diversity of more light-requiring forest species occurred in small gaps than in sites without disturbance in a beech forest (Degen et al. 2005). The above could be going through because it facilitates the entry of seeds, mainly herbs, from nearby disturbed sites such as pasturelands and agricultural areas, active or abandoned. On the other hand, the diversity of seedling is higher under canopy with greater tree-layer diversity in a beech-oak forest, while that in Fagus sylvatica forest the herb-layer was principally dominated by juvenile beech (Dölle et al. 2017). Thus, it would be expected that in conserved forests the relationship in biodiversity among structural components would be higher than in those disturbed by human activities within anthropized landscapes. Mexican beech forests fragments usually are small, surrounded by agricultural and livestock areas. In addition, they suffer disturbance due to logging, grazing, and in some cases, the beech´s seeds were collected for human consumption. The above, could generate a negative effect on the dynamics within the forest, affecting for example the abundance of beech seedling. The objective of this study was to analyze the relationship among seed bank, seedling and tree canopy in a beech forest, considering their alfa and beta diversity.
Materials and methods
Study area. Field sampling was carried out in a beech forest located in the south-central zone of the Sierra Madre Oriental (20° 36' - 20° 18' N and 98° 03' - 98° 20' W; 2,150 m asl, Figure 1). Livestock and agriculture are the two main land uses in the area (INEGI 2009). The climate is humid with temperatures between 9 and 16 °C, and 1,200 mm of annual rainfall. The forest is on regosol soil type and a 40º slope. In the site, the beeches are between 100 to 171 years old (Ortiz-Quijano et al. 2016). The forest was considered as beech forest because F. grandiflora subsp. mexicana was the most important species with 53.5 % of tree canopy abundances. Other canopy tree species were Magnolia schiedeana Schlecht ., Quercus delgadoana S. Valencia, Nixon & L.M. Kelly, Symplocos limoncillo Bonpl., and Ostrya virginiana K. Koch (Rodríguez-Ramírez et al. 2013). Abundant shrub species are Eugenia capuli Schltdl., Ocotea klotzschiana Hemsl., Symplocos coccinea Bonpl., and Miconia glaberrima Naudin (Ortiz-Quijano et al. 2016).
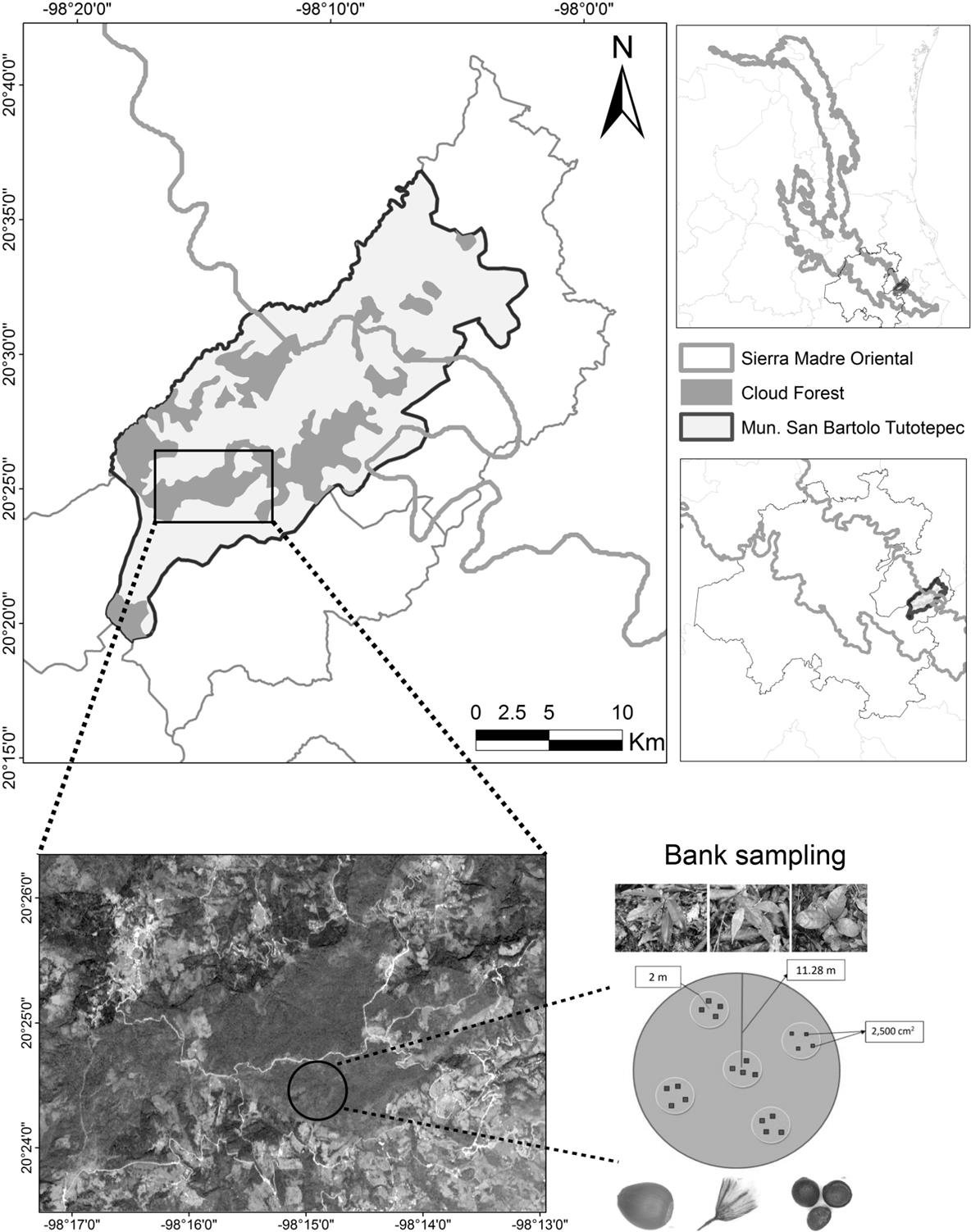
Figure 1 Location of the beech forest of San Bartolo Tutotepec, Hidalgo, Mexico. In the lower part, the satellite image and the sampling design were included.
Sampling design. Four circular plots de 11.28 m diameter (based on the National Forestry Inventory) (CONAFOR 2012; Figure 1) in the beech forest were established. Tree cover was measured in each plot using a densitometer. We recorded all tree species with a diameter at breast height ≥ 10 cm. For seed bank sampling, another five circular subplots (two meters in diameter) were established in each plot (Figure 1). Two soil samples (50 × 50 cm and 5 cm deep) were collected from each subplot. Seeds in each sample were hand-picked, and grouped by color, shape and testa type (Van der Valk et al. 1992). After this, in order to register the very small seeds, soil samples were placed in greenhouse conditions (August 2015 - January 2016) to promote germination and seedling emergence. Determination of species was realized using seedling and expert support.
In each subplot, the naturally established seedlings were recorded and marked in two 50 × 50 cm areas. All seedling species were identified using taxonomic keys and comparing them with live plants previously determinated into the forest. Plant survival was recorded from February 2015 to January 2016. Abundance was estimated as the total number of seeds or seedling by species.
Data analysis. Biodiversity was estimated using abundance values to calculated species richness as Shannon index modified by Jost (2006) with the exponential order 1. Inventory completeness was calculated using species abundances to obtain the percentage of total individuals in the community that were represented in the sample (Chao & Jost 2012). Rank-abundance graphs were also constructed to evenness analyzes.
In order to evaluate relationship among structural components (seed bank - seedling bank, seed bank - tree canopy and seedling bank - tree canopy), we first calculated Bray-Curtis dissimilarity indexes between pairs of samples using species abundances data. The Bray-Curtis index is widely used to generate distance matrices in vegetation ordination studies (Gotelli & Ellison 2004) and is very robust measure when sampling fractions are equal (Chao et al. 2006), as is shown here with the sample coverage (see results).
Then a permutational multivariate analysis of variance (PERMANOVA) was performed to compare differences between indices of each pair of structural components. PERMANOVA was realized with the 'vegan' package in R using the “adonis" function (Oksanen et al. 2017, R Core Team 2017).
Also, canopy cover was correlated with seed and seedling abundance using Spearman rank correlation analyses. In addition, we classified species according to their successional affinities, life forms, and dispersal types. Successional affinities were pioneer species (shade intolerant), secondary species (with fast growth and light shade tolerant), secondary-late species (shade tolerant with seed germination under canopy), and late or climax species (highly shade tolerant) (Rozza et al. 2007).
Results
Seed bank. A total 1,388 seeds were recorded from 32 species, 29 genera, 16 families, and seven undetermined morph-species; this inventory was 99 % of completeness according to the sample coverage. Asteraceae was the most abundant family, with 44.2 % of the total number of seeds. The seed bank was low evenness (Figure 2A). Four species had high abundance values, mainly Coreopsis sp., an early secondary species. In general, herbs were most abundant (44 %), followed by shrubs (36 %), trees (11 %), and vines (8 %) (Table 1). The most representative successional affinities were pioneer species (43 %) and early secondary species (36 %), while only 2.5 % were late successional species. Anemochory (46 %) and zoochory (43 %) were the most representative dispersal types. Two species, Cotula aff. australis and Poa annua L., were alien species (Table 1). On the other hand, the canopy cover was not correlated with seed abundance (r s = −0.80, p = 0.083).

Figure 2 Rank-abundance curves of the species present in the seed bank (A) and seedling bank (B). Black circles indicate pioneer species, black squares early secondary species, white circles intermediate secondary species, gray diamonds late-successional species; black triangles represent species found in early and early secondary affinities, white squares indicate intermediate and secondary-late affinities. The crosses represent species of both initial and late secondary affinities. Black diamonds indicate morph-species. Numbers in the figures represent the species (see Tabla1) and M= morphospecies.
Table 1 Species registered in Mexicana beech forest. Structural components: SB = seed bank, SeB = seedling bank, TC = tree canopy. Life forms: T= Tree, Sh=Shrub, HA= Annual herbaceous, HAB= Annual or biennial herbaceous, HP= Perennial herbaceous, L= liana. Successional affinity: Pi= Pioneer, SI= Initial secondary, ST= Intermediate Secondary, LS= Late successional. Distribution: N= native, I= invasive. Dispersal type: A= Anemochory, B= Barochory, H= Hydrochory, Z= Zoochory, M= Anthropochory.
| No. | Family/Species | Structural component | Lifeform | Successional affinity | Dispersion syndrome | Density (ind/m2) |
|---|---|---|---|---|---|---|
|
1 |
Acanthaceae Thunbergia fragrans |
SB |
HP |
SI |
H, M |
0.06 |
|
2 |
Adoxaceae Viburnum sp. |
SB |
T, Sh |
SI, ST |
Z |
0.06 |
|
3 |
Anacardiaceae Rhus sp. |
SB |
T, Sh |
SI, ST |
Z |
0.08 |
|
4 |
Apocynaceae Thevetia sp. |
SB |
T, Sh |
ST |
Z |
0.14 |
|
5 |
Araceae Syngonium podophyllum |
SB |
L |
Pi |
Z |
0.02 |
|
6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 |
Asteraceae Ageratina sp. Baccharis conferta Chromolaena aff. collina Coreopsis sp. Cotula aff. australis Erigeron karvinskianus Gamochaeta americana Mikania micrantha Piqueria trinervia Pseudognaphalium jaliscense Pseudognaphalium ehrenbergianum Pseudelephantopus spicatus Roldana albonervia Simsia amplexicaulis Sinclairia deppeana Verbesina microptera |
SB SB SB SB SB SB SB SB SB SB SB SB SB SB SB SB |
Sh Sh Sh Sh HA HP HA Be HP HP HP HA Sh HA Sh HA |
SI Pi Pi SI Pi Pi PI Pi Pi Pi-SI Pi-SI Pi SI-LS Pi Pi ST |
A A A A A A A A A A A A A Z A |
1.12 0.32 0.04 4.56 0.1 0.24 0.38 0.18 0.14 0.06 0.04 0.06 0.02 0.96 0.04 0.02 |
|
Clethraceae Clethra macrophylla |
TC |
T |
LS |
Z |
- |
|
|
22 |
Commelinaceae Commelina sp. |
SB |
HP |
SI |
A |
0.08 |
|
33 |
Ericaceae Vaccinium leucanthum |
SdB |
T |
LS |
Z |
0.1 |
| 34 23 35 36 |
Fagaceae Fagus grandifolia subsp. mexicana Quercus sp. Quercus conspersa Quercus corrugata Quercus delgadoana Quercus meavei |
SdB,
CT SB CT CT SdB, CT SdB, CT |
T T T T T T |
LS SI, ST LS LS ST - |
B,
Z B, Z B, Z B, Z - B, Z |
1 0.02 - - 1.1 1.1 |
|
37 38 39 40 |
Lauraceae Cinnamomum pachypodum Litsea glaucescens Nectandra salicifolia Ocotea klotzschiana |
SdB SdB SdB SdB, CT |
T-Sh Sh T T |
LS ST SI LS |
Z Z Z Z |
0.1 0.1 0.3 0.2 |
|
24 |
Linaceae Linum nelsonii |
SB |
HP, HA |
Pi |
Z |
0.02 |
|
41 |
Magnoliaceae Magnolia schiedeana |
SdB, CT |
T |
LS |
Z |
0.4 |
|
42 |
Melastomataceae Miconia glaberrima |
SdB |
Sh |
ST |
Z |
2.7 |
|
43 |
Myrtaceae Eugenia capuli |
SdB, CT |
T |
Pi-SI |
Z |
0.2 |
|
44 45 |
Pentaphylacaceae Cleyera theaeoides Ternstroemia sylvatica |
SdB SdB |
T T |
ST ST |
- Z |
1.1 0.1 |
|
25 |
Phytolaccaceae Phytolacca rivinoides |
SB |
Sh |
Pi |
Z |
1.36 |
|
26 |
Poaceae Poa annua |
SB |
HA |
Pi |
Z |
0.34 |
|
27 28 |
Polygalaceae Monnina sp. Monnina xalapensis |
SB SB |
Sh Sh |
SI SI |
Z Z |
0.08 0.02 |
|
46 47 |
Primulaceae Cybianthus sp. Gaultheria acuminata |
SdB SdB |
Sh Sh |
LS ST |
Z Z |
0.2 0.5 |
|
48 49 29 |
Rosaceae Prunus samydoides Rubus adenotrichos Rubus sp. |
SdB SdB SB |
T L Sh, Be |
LS ST SI, ST |
Z Z Z |
0.1 0.1 1.46 |
|
30 |
Rubiaceae Coccocypselum hirsutum var. hirsutum |
SB |
HP |
SI |
Z |
0.02 |
|
31 |
Solanaceae Solanum myriacanthum |
SB |
HA |
Pi |
Z |
0.24 |
|
32 |
Verbenaceae Verbenaceae sp1 |
SB |
- |
- |
- |
0.1 |
Seedling bank. Seedlings of 17 species, 16 genera, nine families, and two morph-species were recorded. Lauraceae (23.5 %) and Fagaceae (17.6 %) were the families with the most species. The inventory completeness was 91 %. Species diversity was 10.41 effective species, and the most abundant were M. glaberrima, Q. delgadoana, Cleyera theaeoides (Sw.) Choisy, and F. grandifolia subsp. mexicana. Rank-abundance curves showed higher evenness than in the seed bank (Figure 2 B). The majority of seedlings were of secondary-late and late species (83 %). Trees were the most abundant life form (72 %), followed by shrubby species (22 %). Zoochory was the most common dispersal type with 84.2 % of the total number of species (Table 1).
Some species included in the seedling bank also were important components of the canopy; namely F. grandifolia subsp. mexicana, E. capuli, M. scheideana, O. klotzschiana, Quercus delgadoana, and Q. meavei Valencia-A., Sabás & Soto. Canopy cover was not correlated with seedling abundance values (r s = −0.015, p = 0.93). Seedling survival was high in almost all species (> 80 %), with exception of the late secondary species O. klotzschiana and Vaccinium leucanthum Schltdl.
Tree canopy composition. Nine species and 1.6 true species were calculated in the tree canopy. Species with the highest abundance were F. grandifolia subsp. mexicana (53.5 %), Q. delgadoana (17.1 %), E. capuli (6.3 %), M. scheideana (5.5 %), O. klotzschiana (5.5 %), Q. meavei (3.5 %), Q, aff. conspersa (3.2 %), Clethra macrophylla M. Martens & Galeotti (2.5 %), and Q. corrugata Hook. (2.5 %) (Table 1). Canopy species were barely represented in the seed bank (one species) and seedling bank (six species), while only one species was shared with both banks.
Dissimilarity among structural components. Dissimilarity Bray Curtis indexes between seed bank and the seedling bank was 0.997 (S.D. = 0.005), between seed bank and tree canopy was 0.998 (S.D. = 0.005), and between the seedling bank and tree canopy was 0.792 (S.D. = 0.132). Consequently, species compositions among the three beech forest structural components were significantly different (Pseudo-F = 5.59, p < 0.001).
Discussion
Seed bank was dominated by herbs (pioneers), as has been reported in other beech forests (Williams-Linera 1993, Álvarez-Aquino et al. 2005, Ortiz-Arroña et al. 2008, Schmidt et al. 2009). This pattern could be the consequence of the reproductive strategies of herbs; small seeds, anemochorous seed dispersal, high seed dormancy, fast growth, and short life history (Dalling 2002). In a F. sylvatica forest, herbs with anemochorous seed dispersal dominated the seed bank (Schmidt et al. 2009). Gaps within forest facilitate the entrance of seeds with anemochorous dispersal (Degen et al. 2005), and human disturbance increases the formation of gaps. Moreover, the presence of highly perturbed sites nearby favors the entry of herb seeds into the forest (Álvarez-Buylla & Martínez-Ramos 1994, Ramírez-Marcial et al. 1992).
The seed bank is a source of germplasm for starting secondary succession following extraordinary events that provoke tree canopy loss (Lavorel 1999). Some authors (Quintana-Ascencio et al. 1996, Dougall & Dodd 1997, Bossuyt et al. 2002, Álvarez-Aquino et al. 2005) have reported that a poor seed bank with low richness could be an indicator of good conditions, similar to found in mature or climax forest. Usually pioneer herbs have low seed dormancy (less than two years); consequently, the seed bank has continuous seed turnover due to seed dispersion (Álvarez-Buylla & Martínez-Ramos 1990, Salazar et al. 2011, EPPO 2016). We found very few tree seeds; these are more prone to predation because they have significant nutrient contents (Dalling 2002). On other Mexican forest insects predated 29 % of beech seeds (Godínez-Ibarra et al. 2007).
Seedling bank was dominated by trees (secondary-late and late species). This composition is a good indicator of the potential for forest regeneration (Lavorel 1999, Brang 2001, Bedoya-Patiño et al. 2010). Similar results were reported in a F. crenata Blume forest with 20 species in its seedling bank (Hara 1987). We estimated a lower seedling density (one seedling per m2) compared to other Mexican beech forests with 3.2 seedlings per m2 (Álvarez-Aquino & Williams-Linera 2002). It is possible that direct perturbation consisting of seeds gathered for food by local inhabitants (Ortiz-Quijano et al. 2016) could be having an adverse effect on seedling recruitment. Furthermore, animals could eat early seedlings, for example, rodents consumed F. crenata seedlings (Abe et al. 2005).
Old records have shown that primary beech forests had high tree canopy diversity. Lutz (1930) reported 32 tree species in a forest in Pennsylvania at the beginning of the nineteenth century. However, only six tree species constituted 88 % of the stand, and F. grandifolia represented 30.8 % of the total abundance. In the beech forest studied, two species together account for 70.6 % of the tree abundance, F. grandifolia (53.5 %) and Q. delgadoana (17.1 %); although they coexist with 19 other canopy species. Thus, not all beech forest are monospecific, even in central Germany, there are a beech forests of F. sylvatica shares dominance with another three to six canopy tree species (Schmidt et al. 2009). Worldwide distribution of beech forest is restricted to specific ecological conditions, but its dominance may be a consequence of its capacity to create suitable environmental conditions (Pignatti et al. 2006). Many factors could explain the differences in tree canopy richness, such as the age of the stand, natural or anthropic perturbation, and climate conditions (Cao 1995, Cao & Peters 1997, Shen et al. 2015). In particular, human activities have a large impact; logging of the surrounding area and within the beech forest fragment, grazing, and beech seed extraction could change environmental conditions to favor other competitive species such as Q. delgadoana. The presence of species indicative of perturbation in the beech forest such as Baccharis conferta Kunth and Phytolacca rivinoides Kunth & C.D. Bouché, and the alien species Cotula aff. australis and P. annua, could be evidence of such changes (Díaz & Elcoro 2009, Martínez-Orea et al. 2013).
The relationships among the seed bank, seedling bank, and tree canopy in beech forest have been little explored. Most published studies have examined only one or two components (Houle 1994, Hopfensperger 2007, Esmailzadeh et al. 2011). Schmidt et al. (2009) reported a positive correlation between tree canopy richness and seed bank richness in temperate broad-leaved forests. However, this relationship was not found in the present study; rather, differences between seed bank and tree canopy were expected because different ecological succession processes are operating in different stages, with floristic turnover between stages (Grime 2006). Low floristic similarity among structural components has occurred in other beech forests (Olano et al. 2002, Schmidt et al. 2009). A meta-analysis using data from different types of forest showed a low floristic similarity (31%) between the seed bank and tree canopy, with only three studies with values lower than 10 % (Hopfensperger 2007). On the other hand, significant differences between a seedling bank and tree canopy are uncommon, considering that the seedling bank is a source for canopy renewal. High seedling survival suggests that shade tolerant plants can stay alive for several years (Gonzalez et al. 2008, Bedoya-Patiño et al. 2010); beech is an excellent example of a shade tolerant plant species (Nagel et al. 2010, Kuninaga et al. 2015). It has been reported that 92 % of F. grandifolia subsp. mexicana seedlings survive for at least 18 months (Álvarez-Aquino & Williams-Linera 2002). However, mortality increases significantly when the seedlings become juvenile trees, so this is a critical step for species regeneration (Collet et al. 2011, Kuninaga et al. 2015).
Gap dynamics are a key factor in beech forest regeneration because light reaches the forest floor and allows Fagus seedling growth (Peters & Platt 1996, Degen et al. 2005). In F. crenata forests, light also causes stem thickening in juvenile plants, and this could be a competitive advantage over other plant species (Abe et al. 2005, Kuninaga et al. 2015). F. sylvatica seedlings had greater success for gap regeneration than other canopy species such as Acer campestre L., A. platanoides L., Carpinus betulus L., or Fraxinus excelsior L. (Collet et al. 2008). However, beech species could be replaced by other canopy species; for example, Abies, Acer, and Betula, that can take the place of F. sylvatica and F. crenata (Peters & Platt 1996, Waltert et al. 2002, Abe et al. 2005, Nagel et al. 2010). Greater physiological plasticity has been reported in Q. robur seedlings than in those of F. sylvatica, with a better photosynthetic response to light (Valladares et al. 2002), which could be interpreted as a competitive advantage in gaps. This may explain, at least partly, that beech regeneration was negatively correlated with canopy openness (Barna & Bosela 2015). Besides, the beech regeneration was negatively correlated with canopy openness. For above is possibly that the forest studied could be a beech-oak forest (F. grandiflora subsp. mexicana and Q. delgadoana) with particular process to coexistence, as other similar European forest (Dölle et al. 2017). On the other hand, if human perturbation continues, together with climate change, Q. delgadoana could replace F. grandifolia subsp., mexicana, because the oak species was the second most important canopy tree, with the highest seedling density and is the dominant species in the adjacent oak forest. Replacement patterns of beech by others dominant species has been reported in other forest (Poulson & Platt 1996, Forrester & Runkle 2000, Peñuelas et al. 2007).











 nueva página del texto (beta)
nueva página del texto (beta)



