Wetlands are ecosystems with shallow water or saturated soils that possess unique flora and fauna adapted to these unusual environmental conditions. They share common features with both aquatic and terrestrial ecosystems. Wetlands are distinguished from terrestrial systems because soil is often anaerobic. Wetlands vegetation is dominated by trees, shrubs, grasses, and other large plants, in contrast to aquatic systems (van der Valk 2012). It is estimated a global wetland area of 135-1,782 × 106 ha in the Neotropics (Ramsar Convention Secretariat 2010). High biodiversity and productivity are found in wetlands, providing diverse ecosystem services including fishing, fruits, carbon sequestration, nutrient cycling, and water decontamination, among others. Mexican neotropical region harbors several freshwater wetlands which are flooded during the rainy season.
In nature, most plants are colonized by different groups of fungi; the fungal diversity in the roots of a single plant species growing in a single sampling location has been reported to cover all phyla (Vandenkoornhuyse et al. 2002). The most commonly found fungal groups inhabiting plant roots comprise arbuscular mycorrhizal fungi (AMF) and dark septate endophytes (DSE). AMF [Phylum Mucoromycota, Subphylum Glomeromycotina (Spatafora et al. 2016)] associate with the roots of more than 80 % of vascular plants under a mutualistic interaction denominated arbuscular mycorrhiza (AM) (Smith & Read 2008). AMF facilitate the assimilation of less mobile nutrients, such as phosphorus (P), through the development of an extended network of hyphae in the soil (Parniske 2008). DSE are mainly Ascomycota, which are capable of colonizing the roots of nearly all plant species (Jumpponen & Trappe 1998). Knowledge on the roles that the plant root-DSE association plays continues to be very limited. The roles that have been suggested include involvement in enhancing plant survival and performance during periods of stress (Newsham 2011, Mandyam & Jumpponen 2015). DSE interact with their host plant and/or other endophytes, synthesizing bioactive metabolites, which may play important ecological roles, and may lead to biotechnological applications (Kusari et al. 2012). In a tripartite plant-DSE-AMF interaction, a synergistic relationship has been hypothesized between both fungal groups in relation to P availability and uptake by plants: DSE increase the P pool in the rhizosphere, and AMF are responsible for P transfer to the host (Della Monica et al. 2015).
AMF and DSE exhibit aerobic metabolism and have been reported to co-exist inside the roots of submerged plants in temperate wetlands (Fuchs & Haselwandter 2004, Sraj-Krzic et al. 2006, Kohout et al. 2012). However, little information is available on the diversity and co-colonization of these two fungal groups and the role that they play in neotropical wetlands, where the water column exhibits a reduction, or even the absence of dissolved oxygen. Particularly in Mexico, there is little information on soil fungi (Sarukhán et al. 2015).
Based on the species endemism of vascular plants and terrestrial vertebrates, the southern half of the country corresponds to the Mesoamerican biodiversity hotspot, which is threatened by habitat destruction and land-use change (Myers et al. 2000). Considering the role of plants as umbrella species, these areas also function as hotspots for other, less well-studied organisms such as the soil fungi associated with them (Stork & Habel 2014). Up to 2012, 95 known species of AMF had been reported in Mexico, considered as a high AMF diversity reservoir linked with plant and ecosystem diversity (Montaño et al. 2012). Scarce information exists on the diversity and roles of DSE in neotropical ecosystems (Heredia-Acuña et al. 2014). The aim of the present work was to describe plant root interactions with AMF and DSE in a neotropical freshwater wetland characterized by its hypoxic or anoxic conditions and belonging to a biodiversity hotspot region.
Materials and methods
Description of the study area. This work was conducted in the seasonally flooded La Mixtequilla neotropical wetland in Veracruz state, Eastern Mexico. La Mixtequilla is located in the Sistema Lagunar Alvarado, which was designated as the Ramsar Site 1355 (Matthews 2013). Blanco River traverses this wetland. The climate is warm, Aw2(i’)w” type, with an average annual temperature of 25.9 °C; in the coldest month, the temperature reaches 22.6 °C, while in the warmest months, it rises to 28.3 °C. Average annual rainfall is 1,531.8 mm, with rain beginning in June and ending in October. Generally, the dry season runs from January to June (Viccon-Pale et al. 2016), with the wetland flooded during the remainder of the year. This region has been negatively impacted by industrial and domestic pollution, by land-use change, as well as by livestock, agriculture, and fishing (Rivera-Becerril et al. 2008, Cejudo-Espinosa et al. 2009, Moreno-Casasola et al. 2009).
Field work. For soil analysis, two sampling stations were established at the eastern and western banks of the Blanco River: Don Rufino (18° 31´ 57.9" N, 95° 57´ 35.7" W; 2 m ASL), and El Llanete (18° 31´ 40.2" N, 95° 56´ 45.9" W; 2 m ASL), respectively. Both sites are employed as livestock grasslands, and they exhibit differences in plant communities, particularly, in the abundance of some plant species. During the dry season, five plots (1 m2 each) were randomly established at each pastureland (El Llanete and Don Rufino). Three soil samples from each plot were taken from the top 20 cm of soil using a spade. Throughout the whole year, considering their high abundance, representative plants from these five plots at each station were collected (roots, shoots, flowers) for identification. Root fungal colonization was estimated once a year in each plant species shown in table 4. In addition, it was carried out repeated sampling for a year in order to monitor fungal colonization in Cyperus articulatus from Don Rufino, and in Mimosa pigra from El Llanete, because these were the dominant species. An aliquot of each root system from the top 20 cm of soil was stored at 4 °C to quantify fungal colonization.
Table 1 Physical and chemical properties in soils of the pasturelands Don Rufino and El Llanete at La Mixtequilla wetland (mean ± SE; n = 15, except for sand, silt, and clay, where n = 5).
| Don Rufino | El Llanete | |
|---|---|---|
| Physical properties | ||
| Bulk density (g cm−3) | 1.2 ± 0.01 | 1.2 ± 0.02 |
| Particle density (g cm−3) | 2.2 ± 0.04* | 2.3 ± 0.01* |
| Porosity (%) | 46.1 ± 0.89* | 49.1 ± 0.77* |
| Sand (%) | 15.5 ± 2.10 | 11.5 ± 0.82 |
| Silt (%) | 12.1 ± 1.76 | 28.7 ± 11.70 |
| Clay (%) | 72.4 ± 0.93 | 59.8 ± 12.13 |
| Texture | Clay | Clay |
| Color (dry) | 2.5Y 4/1 | 2.5Y 4/1 |
| Color (wet) | Gley1 3/N | Gley1 3/N |
| Chemical properties | ||
| pH | 6.5 ± 0.04 | 6.3 ± 0.15 |
| Organic matter (%) | 6.2 ± 0.48 | 6.9 ± 0.49 |
| Organic carbon (%) | 3.6 ± 0.28 | 4.0 ± 0.28 |
| Total nitrogen (%) | 0.3 ± 0.02 | 0.3 ± 0.02 |
| Available phosphorus (mg kg−1) | 9.9 ± 0.45* | 17.0 ± 2.68* |
| Cation exchange capacity (meq 100 g−1) | 40.3 ± 0.51 | 39.1 ± 0.72 |
| Ca2+ (meq100 g−1) | 40.0 ± 1.13 | 37.0 ± 1.56 |
| Na+ (meq 100 g−1) | 6.3 ± 0.31* | 11.1 ± 1.20* |
| Mg2+ (meq 100 g−1) | 3.7 ± 0.64* | 7.4 ± 1.53* |
| K+ (meq 100 g−1) | 2.6 ± 0.19* | 3.5 ± 0.31* |
Asterisks indicate significant differences (p <0.05) between both pasturelands, following a Student’s t-test.
Table 2 Water column properties during the flood season of the Don Rufino (DR) and El Llanete (EL) pasturelands at La Mixtequilla wetland (mean ± SE; n = 252, except for orthophosphate concentration, where n = 4).
| Temperature (°C) | O2 concentration (mg L-1) | Orthophosphate concentration (mg L-1) | ||||
|---|---|---|---|---|---|---|
| Sampling date (month/year) | DR | EL | DR | EL | DR | EL |
| 09/11 | 34.5 ± 1.7 | 30.1 ± 0.4 | 2.6 ± 0.7 | 0.5 ± 0.1 | 0.312 ± 0.22 | 0.196 ± 0.09 |
| 10/11 | 29.9 ± 1.5 | 27.5 ± 0.5 | 2.3 ± 0.4 | 4.3 ± 0.2 | 0.145 ± 0.01 | 0.028 ± 0.02 |
| 11/11 | 28.1 ± 1.3 | 28.0 ± 0.5 | 3.3 ± 0.4 | 2.4 ± 0.5 | 0.033 ± 0.06 | 0.050 ± 0.02 |
| 12/11 | 27.7 ± 1.0 | 24.7 ± 0.3 | 3.0 ± 0.7 | 1.7 ± 0.3 | 0.104 ± 0.04 | 0.335 ± 0.28 |
| 01/12 | 24.9 ± 1.5 | 21.4 ± 0.2 | 3.3 ± 0.6 | 1.2 ± 0.4 | 0.002 ± 0.004 | 0.044 ± 0.04 |
| 02/12 | 21.7 ± 1.8 | 25.3 ± 1.4 | 5.5 ± 1.1 | 0.7 ± 0.3 | 0.117 ± 0.06 | 0.042 ± 0.03 |
| 04/12 | — | — | — | — | — | — |
| 06/12 | — | — | — | — | — | — |
| 07/12 | 30.9 ± 2.3 | 30.6 ± 1.2 | 1.2 ± 0.5 | 1.5 ± 0.7 | 0.179 ± 0.09 | 0.108 ± 0.01 |
| 09/12 | 30.6 ± 1.1 | 29.7 ± 1.0 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.163 ± 0.01 | 0.133 ± 0.03 |
-, Absence of water column during the dry season.
Table 3 Representative plant species in two pasturelands at La Mixtequilla wetland.
| Family | Plant species | Observations |
|---|---|---|
| Don Rufino pastureland | ||
| POLYPODIOPHYTA | ||
| Salvianiaceae | Salvinia minima Baker | Aquatic fern, native |
| MAGNOLIOPHYTA | ||
| LILIOPSIDA | ||
| Alismataceae | Echinodorus paniculatus Micheli |
Herbaceous, native |
| Cyperaceae | Cyperus articulatus L. | Perennial, herbaceous, native |
| Poaceae | Echinochloa crus-pavonis (Kunth) Schult. | Perennial, herbaceous, native |
| Hymenachne amplexicaulis (Rudge) Nees | Perennial, herbaceous, native | |
| Paspalum repens P.J. Bergius | Perennial, herbaceous, native | |
| Pontederiaceae | Eichhornia crassipes (Mart.) Solms | Aquatic, herbaceous, exotic |
| MAGNOLIOPSIDA | ||
| Bignoniaceae | Crescentia cujete L. | Tree, native |
| Boraginaceae | Heliotropium indicum L. | Herbaceous, native |
| H. macrostachyum (DC.) Hemsl. | Herbaceous, native | |
| Euphorbiaceae | Chamaesyce prostrata (Aiton) Small | Herbaceous, native |
| Fabaceae | Lonchocarpus hondurensis Benth. | Tree, native |
| Pithecellobium dulce (Roxb.) Benth. | Tree, native | |
| Mimosa pigra L. | Shrub, native | |
| Neptunia natans W. Treob. | Perennial, herbaceous, native | |
| Maranthaceae | Thalia geniculata L. | Aquatic, herbaceous, native |
| Solanaceae | Solanum rostratum Dunal | Invasive, herbaceous, native |
| Verbenaceae | Phyla nodiflora (L.) Greene | Herbaceous, native |
| El Llanete pastureland | ||
| POLYPODIOPHYTA | ||
| Marsileaceae | Marsilea crotophora D.M. Johnson | Aquatic fern, native |
| Salvinia minima Baker | Aquatic fern, native | |
| MAGNOLIOPHYTA | ||
| LILIOPSIDA | ||
| Cyperaceae | Cladium jamaicense Crantz | Perennial, herbaceous, native |
| Cyperus articulatus L. | Perennial, herbaceous, native | |
| Fimbristylis spadicea (L.) Vahl | Perennial, herbaceous, native | |
| Nymphaceae | Nymphaea ampla DC. | Perennial, herbaceous, native |
| Poaceae | Paspalum fasciculatum Willd. ex Flüggé | Perennial, herbaceous, native |
| Paspalum sp. | Perennial, herbaceous | |
| MAGNOLIOPSIDA | ||
| Acanthaceae | Ruellia geminiflora Kunth | Herbaceous, native |
| R. paniculata L. | Herbaceous, native | |
| Alismataceae | Echinodorus paniculatus Micheli |
Herbaceous, native |
| Arecaceae | Sabal mexicana Mart. | Arborescent, native |
| Asteraceae | Erigeron aff. heteromorphus | Herbaceous, native |
| Euphorbiaceae | Euphorbia marginata Pursh | Herbaceous, exotic |
| Fabaceae | Crotalaria pallida Aiton | Herbaceous, native |
| Mimosa pigra L. | Shrub, native | |
| Neptunia plena (L.) Benth. | Seedling, native | |
| Sesbania herbacea (Mill.) McVaugh | Herbaceous, native | |
| Lythraceae | Cuphea spp. | Herbaceous, native |
| Maranthaceae | Thalia geniculata L. | Seedling, native |
| Onagraceae | Ludwigia helminthorrhiza (Mart.) H. Hara | Prostrate, herbaceous, native |
| L. leptocarpa (Nutt.) H. Hara | Aquatic, herbaceous, native | |
| Solanaceae | Solanum lanceifolium Jacq. | Shrub, native |
All of the plant species follow the nomenclature of vascular flora by MEXU Herbarium (http://www.ib.unam.mx/botanica/herbario/), and W3 Tropicos Missouri Botanical Garden (http://www.tropicos.org)
Table 4 Root colonization by arbuscular mycorrhizal fungi and dark septate endophytes in plants established at two pasturelands of La Mixtequilla wetland (mean ± SE; n = 3). Evaluation was carried out once in a year.
| Arbuscular mycorrhizal fungi | Dark septate endophytes | ||||
|---|---|---|---|---|---|
| Plant species | M (%) | A (%) | V | C (%) | MS (%) |
| Don Rufino pastureland | |||||
| Echinochloa cruspavonis b | 7.7 ± 4.7 | 0.1 ± 0.1 | + | 3.9 ± 2.2 | 0.5 ± 0.3 |
| Echinodorus paniculatus b | 0 | 0 | - | 0 | 0 |
| El Llanete pastureland | |||||
| Sesbania herbaceaa (flowering period) | 29.9 ± 4.0* | 2.6 ± 1.0 | + | 4.5 ± 1.9* | 0.6 ± 0.4 |
| Sesbania herbaceaa (fructification period) | 53.4 ± 1.5* | 3.5 ± 1.2 | + | 8.7 ± 4.1* | 0.6 ± 0.4 |
| Ruellia geminiflora a | 17.4 ± 7.5 | 1.7 ± 1.0 | + | 7.1 ± 2.9 | 0.2 ± 0.2 |
| Fimbristylis spadicea a | 3.8 ± 2.8 | 0 | + | 16.0 ± 4.3 | 0.6 ± 0.1 |
| Thalia geniculata b | 5.3 ± 1.8 | 0 | + | 4.5 ± 1.5 | 0.3 ± 0.2 |
M, intensity of mycorrhizal colonization in the root system; A, abundance of arbuscules in the root system; V, vesicles (+, present; -, absent); C, intensity of colonization by DSE in the root system; MS, abundance of microsclerotia in the root system; a, unflooded plants; b, flooded plants; asterisks indicate significant differences (p < 0.05) between M % and C % in S. herbacea, following a Student’s t-test
For water quality evaluation, two sampling stations were also established at each pastureland, Don Rufino, and El Llanete. During the rainy season, both livestock grasslands were flooded. Water depth was determined with a wooden ruler. Temperature and dissolved oxygen concentration in the water column were measured with a Hydrolab DS5 multi-parameter instrument (Hach Company, Loveland, CO, USA) one day per month, from September 2011 to September 2012, each hour (36 recordings during 6 min) from 11:00 to 17:00 h. Additionally, a water sample was collected every two hours from 11:00 to 17:00 h in plastic bottles and stored at 4 °C for orthophosphate (PO4 3-) estimation.
Soil and water chemical analyses. Soil samples (1.5 kg) were dried at room temperature and sieved through a 2.0 mm-diameter sieve. Bulk and particle densities were measured using a pycnometer with the paraffin-coated clod method and the undisturbed soil core method, respectively (USDA 2004). Soil porosity was calculated according to USDA (2011). Soil texture was determined following the Gee & Bauder method (1986), and soil color, according to the Munsell notation. Soil pH was measured in water with a glass electrode potentiometer (Orion STR 3) at a 1:2.5 ratio (w/v). Soil organic matter (SOM) content was determined employing the wet combustion method (USDA 2004); organic carbon (OC) and total nitrogen (TN) were calculated from these results as follows: OC = SOM/1.724, TN = SOM (0.05). The concentration of available P in soils was determined using the USDA method (2004). Cation-exchange capacity (CEC) was calculated by saturation with ammonium acetate and analysis with EDTA (Jackson 1982), the content of exchangeable cations Ca2+ and Mg2+ by the Versenate method (USDA 2004), and Na+ and K+, by flame photometry (Corning 400). Orthophosphate (PO4 3-) concentration in water was determined following the 4500-P E ascorbic acid method (APHA-WEF 2005).
Root colonization measurements. Identification of plant species was conducted according to López-Ríos & Rosas-López (1988) and to Calderón de Rzedowski & Rzedowski (2001). To estimate fungal colonization, roots from three individual of each plant species were cleared with 10 % KOH and stained with 0.05 % trypan blue (Phillips & Hayman 1970) in lactoglycerol. From each root system, 30, 1-cm root segments were placed on a slide with glycerol and observed under a light microscope. The ocurrence of Paris (absence of intercellular hyphae, presence of intracellular hyphal coils) and Arum (intercellular hyphal growth, terminal arbuscules on intracellular hyphal branches) morphotypes of AM was qualitatively recorded (Dickson et al. 2007). Levels of AMF colonization were calculated considering the intensity of mycorrhizal colonization (M %: hyphae, vesicles, and arbuscules) and the abundance of arbuscules (A %) in the root system (Trouvelot et al. 1986). This methodology was also applied for estimating root colonization by DSE; the same 30, 1-cm root segments that had been previously clarified and stained were evaluated for estimating intensity of colonization (C %: septate hyphae and microsclerotia) and abundance of microsclerotia (MS %) in the root system.
Molecular identification of arbuscular mycorrhizal fungi. A composited sample of flooded M. pigra roots containing arbuscules from El Llanete was washed with tap water, disinfected, and rinsed with sterilized water. Extraction of genomic DNA was carried out following the DNAzol Reagent kit protocol. DNA was resuspended in ultrapure water; its concentration was estimated in a NanoDrop 2000c spectrophotometer (Thermo Scientific, Wilmington, DE, USA). A nested-PCR protocol was applied for amplifying ribosomal sequences of AMF (Krüger et al. 2009). The first PCR mix (25 μl total volume) was prepared as follows: buffer 1X 1.5 mM MgCl2; 0.2 mM of each deoxynucleotide triphosphates (dNTP); 0.2 μM of each primer (SSUmAf1-2 mix, LSUmAr1-4); 1 U Platinum Taq polymerase (Invitrogen, Brazil), and 10 ng DNA as template. PCR reactions were performed in a MultiGene Thermal Cycler (Model TC9600-G; Edison, NJ, USA) with the following parameters: initial denaturation at 94 ºC/2 min; 32 cycles of denaturation at 94 ºC/30 s; annealing at 50 ºC/30 s; elongation at 72 ºC/2 min, and a final elongation at 72 ºC/10 min. Amplified fragments were used after a 1:10 dilution in ultrapure water. The second PCR mix (25 μl total volume) was prepared using primer mixes SSUmCf1-3 and LSUmBr1-5. The same PCR conditions were employed, except that the annealing temperature was reduced to 48 °C, and that Taq polymerase (2 U) and MgCl2 (3 mM) were doubled. Products were visualized by electrophoresis and stained with ethidium bromide. Amplification bands of ~ 1.5 kb for SSUmCf/LSUmBr were cut from the gel and DNA was extracted with the QIAquick Gel Extraction kit (Qiagen, Oregon, USA). PCR bands were ligated to pGEM-T vector (Promega, Madison, WI, USA) and used to transform competent Escherichia coli JM-109 cells (Promega). Plasmid minipreps were conducted (QIAprep Spin Miniprep Kit), and the clones were sequenced with the aid of an ABI Prism 3100 Automated Sequencer (LANGEBIO, CINVESTAV-Irapuato, Mexico). Sequencing was unidirectional and conducted using the T7 primer. The Glomeromycotina origin of the sequences was confirmed by BLAST-N comparison with sequences deposited in GenBank (NCBI) and Glomeromycotina dedicated database MaarjAM (Öpik et al. 2010). Multiple alignment was performed using MUSCLE (Edgar 2004), and the alignment was manually optimized with Jalview software (Waterhouse et al. 2009). The evolutionary history was inferred by using the Maximum Likelihood method based on the Kimura 2-parameter model (Kimura 1980). The tree with the highest log likelihood (-3,947.04) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 54 nucleotide sequences. All positions with less than 95 % site coverage were eliminated. That is, fewer than 5 % alignment gaps, missing data, and ambiguous bases were allowed at any position. There was a total of 453 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016).
Statistical analyses. Data were checked for normal distribution with the Shapiro-Wilk test. One-way analysis of variance (ANOVA) at a significance level of 0.05 was performed on fungal colonization variables. Whenever there were significant differences, Tukey tests were conducted to determine differences among homogeneous groups of means (p < 0.05). Comparisons for each soil property between both pasturelands, as well as comparisons of AMF/DSE colonization in plant species, were subjected to a Student’s t-test. Pearson correlations were carried out between AMF and DSE colonization in M. pigra and C. articulatus, as well as between properties of water and fungal colonization. These analyses were computed using Origin 8.6 software.
Results
Soil and water column properties in pasturelands. Concerning soil properties, bulk density was similar in both pasturelands under study; particle density and porosity were significantly higher (p < 0.05) in El Llanete than in Don Rufino (Table 1). Soils from both pasturelands were dominated by clay particles (60 and 72 %), with a clay texture; no significant differences were observed between pasturelands when comparing concentrations of sand, silt, and clay in soils. Both soils were dark gray (2.5Y 4/1) when dry, and very dark gray (Gley1 3/N) when wet. Among chemical properties, there were no statistically significant differences between pasturelands on the pH, organic matter content, organic carbon, total nitrogen and CEC. The CEC complex was dominated by Ca2+, which did not show significant differences between pasturelands; Na+, Mg2+, and K+ were significantly higher (p < 0.05) at El Llanete. The available P in soil, measured as orthophosphate, was significantly higher (p < 0.05) at El Llanete than at Don Rufino by nearly two-fold (Table 1).
During the flood season, the water column was shallow in both pasturelands (25.1 ± 10.5 cm). The highest water temperatures were recorded in September at Don Rufino (34.5 ºC) and July at El Llanete (30.6 ºC); lowest temperatures were registered during winter at both sites (Table 2). Higher levels of dissolved oxygen were found during February at Don Rufino (5.5 mg L-1) and in October at El Llanete (4.3 mg L-1). Orthophosphate in water was higher in September at Don Rufino (0.3 mg L-1) and in December at El Llanete (0.3 mg L-1).
Floristic composition. In both pasturelands, the plant community known as “popal” was predominant with majority of herbaceous native species from Mexico (Table 3). At the Don Rufino pastureland, 18 plant species belonging to 12 families were identified; some of these, such as Thalia geniculata, Cyperus articulatus, and Mimosa pigra, are adapted to both flood and dry conditions; others (Salvinia minima and Eichhornia crassipes) exhibit aquatic habits. During the flood season, the most abundant plant species were C. articulatus, Echinodorus paniculatus, and T. geniculata, while during the dry season, the latter predominated. In the El Llanete pastureland, 23 plant species belonging to 14 families were identified; some of these, such as T. geniculata and M. pigra, tolerate both flood and dry seasons; others (Marsilea crotophora, S. minima, and Nymphaea ampla) demonstrate aquatic habits. During the whole year, including flood and dry seasons, the most abundant plant species was M. pigra, followed by T. geniculata.
Fungal colonization of roots. As a first insight into the status of AMF and DSE interactions in the wetland, six plant species from both pasturelands were sampled during the flood or dry seasons and analyzed for the presence of these fungi (Table 4). Echinodorus paniculatus did not exhibit any fungal colonization, while five plant species were co-colonized by both fungal groups at different levels. Concerning AMF, S. herbacea during fructification showed the highest percentage of root colonization (M = 53 %), as well as the highest abundance of arbuscules in the whole root system (A = 3.5 %). Vesicles were present in all plant species, with S. herbacea exhibiting the highest abundance. Arum and Paris-type morphologies of AM were visualized in all plant roots, but the former predominated. Fimbristylis spadicea demonstrated highest intensity of DSE root colonization (C = 16 %), while in the remaining plants, this was ≤ 9 %. The abundance of microsclerotia in the whole root system (MS) was ≤ 1 % in all cases. In S. herbacea, the percentage of root colonization by AMF (M) was significantly higher (p < 0.05) than that of DSE (C). Finally, S. herbacea in fructification attained highest levels of root colonization by both groups of fungi (M + C = 62 %).
Repeated monitoring of fungal colonization in the roots of two plant species. In C. articulatus roots, AMF colonization was present at each sampling time (Figure 1). Only hyphae and vesicles were detected, and arbuscules were absent; Paris-type morphology predominated, as judged by presence of intracellular hyphal coils and absence of intercellular hyphae. The highest intensity of root colonization (M = 13.5 %) was observed in July, but without significant differences among the sampling months. DSE colonization was also present throughout the year. The percentage of root colonization (C) was < 10 % in flooded plants, increasing during the dry season, demonstrating the significant, highest peak (p < 0.05) in July 2012 (C = 25 %) when the second flood period started. Highest abundance of microsclerotia in the whole root system (MS) was also observed in July 2012 (MS = 4 %), but without differences among sampling months. During the dry season, the percentage of root colonization by DSE was always higher than that of AMF, particularly in April, with significant differences (p < 0.05). In July 2012, C. articulatus attained highest levels of colonization by both groups of fungi in the whole root system (M + C = 38.2 %). A Pearson correlation (r) considering all data of intensity of root colonization by AMF (M) and DSE (C) in C. articulatus, showed a significant correlation (r = 0.62; p < 0.05) between both fungal groups.
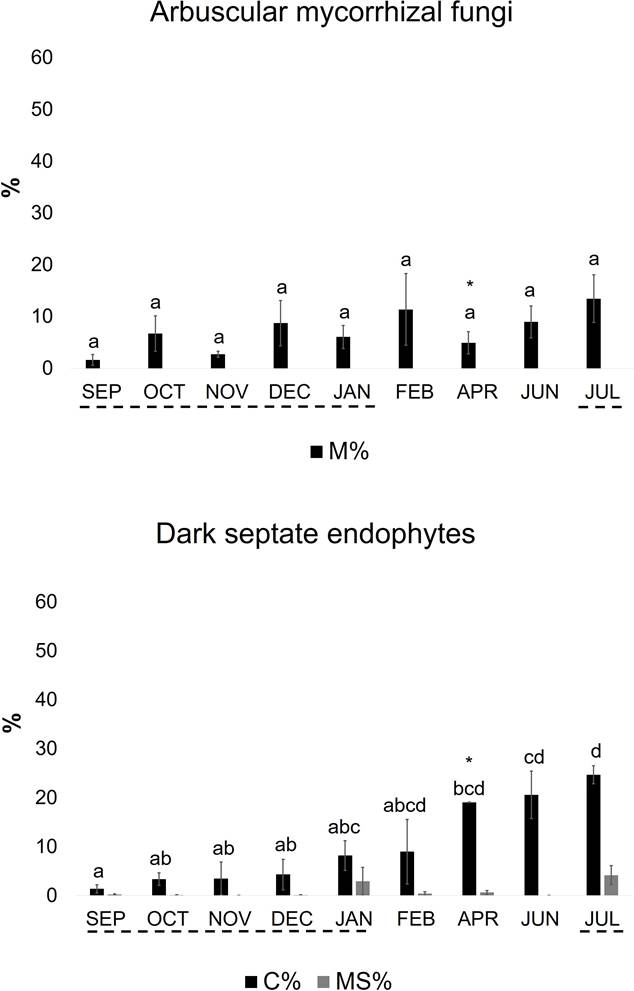
Figure 1 Root colonization by arbuscular mycorrhizal fungi and dark septate endophytes in Cyperus articulatus established in the pastureland Don Rufino at La Mixtequilla wetland (mean ± SE; n = 3). Evaluation was carried out nine times in a year. M, intensity of mycorrhizal colonization in the root system; C, intensity of colonization by DSE in the root system; MS, abundance of microsclerotia in the root system; broken lines indicate plants under flooding conditions; asterisks indicate significant differences (p < 0.05) between M % and C % (Student’s t-test); different letter on the bars indicate differences among homogeneous groups of means (p < 0.05), following an ANOVA.
AMF and DSE colonized M. pigra roots during the whole annual cycle (Figure 2). Concerning AMF, intensity of root colonization (M) was significantly highest (p < 0.05) in February 2012 during the flooded season (61 %). The highest abundance of arbuscules in the whole root system (A) was observed under dry conditions. During nearly all the year, few vesicles were present in the roots; Arum and Paris-type morphologies were present. Concerning DSE, the highest percentage of root colonization (C) was observed in April (11 %), without differences among sampling months; abundance of microsclerotia in the whole root system (MS) was < 1 %. In February 2012, M. pigra attained highest levels of colonization by both groups of fungi in the whole root system (M + C = 62 %). At 6 sampling times, colonization by AMF was significantly higher (p < 0.05) than that of DSE. There was no correlation between intensity of root colonization by AMF (M) and DSE (C) in M. pigra, following a Pearson test.
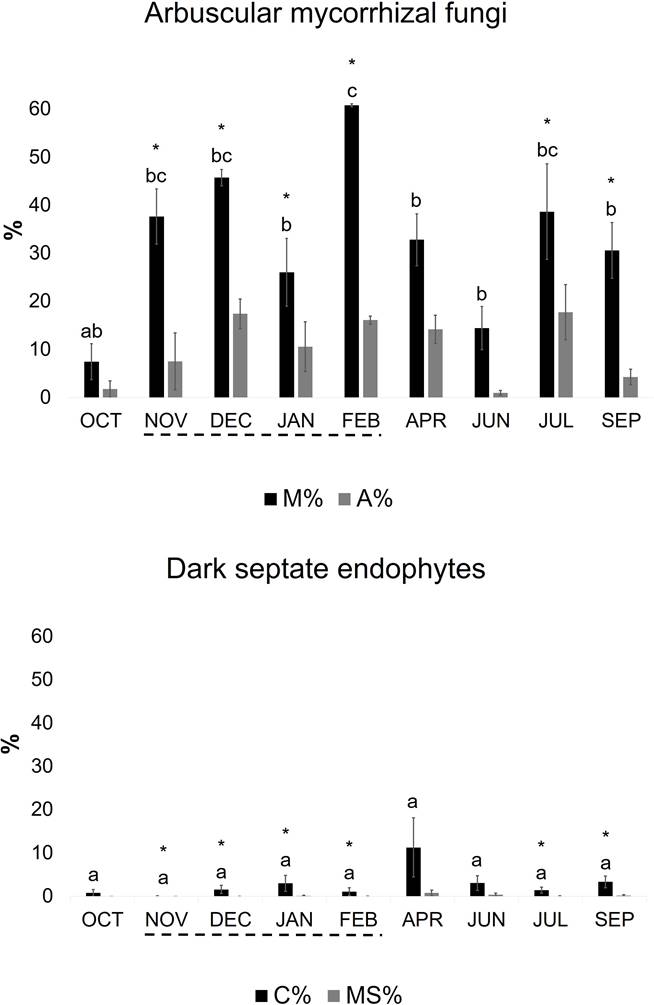
Figure 2 Root colonization by arbuscular mycorrhizal fungi and dark septate endophytes in Mimosa pigra established in the pastureland El Llanete at La Mixtequilla wetland (mean ± SE; n = 3). Evaluation was carried out nine times in a year. M, intensity of mycorrhizal colonization in the root system; A, abundance of arbuscules in the root system; C, intensity of colonization by DSE in the root system; MS, abundance of microsclerotia in the root system; broken lines indicate plants under flooding conditions; asterisks indicate significant differences (p < 0.05) between M % and C % (Student’s t-test); different letter on the bars indicate differences among homogeneous groups of means (p < 0.05), following an ANOVA.
Relations between fungal root colonization and water column properties. Significant correlations (p < 0.05) were observed between fungal colonization parameters and water column properties (Table 5). In C. articulatus established in Don Rufino pastureland, a negative correlation between M or C and temperature was observed. In addition, higher concentrations of dissolved oxygen in the water column favored root colonization by AMF (M) and DSE (C). A negative correlation between C and the concentration of orthophosphates was also identified. In M. pigra established in El Llanete, negative correlations were identified between C or MS and temperature; dissolved oxygen was negatively correlated with M and A. Finally, higher orthophosphate concentrations in the water column increased the abundance of arbuscules (A) in the whole root system.
Table 5 Pearson correlation (r) between water column properties and fungal colonization in Cyperus articulatus and Mimosa pigra established at La Mixtequilla wetland.
| Arbuscular mycorrhizal fungi | Dark septate endophytes | |||
|---|---|---|---|---|
| Plant species | M (r) | A (r) | C (r) | MS (r) |
| Cyperus articulatus | ||||
| Temperature (°C) | -0.790* | - | -0.951* | -0.359 |
| O2 concentration (mg L-1) | 0.641* | - | 0.785* | 0.106 |
| PO43— concentration (mg L-1) | -0.304 | - | -0.607* | -0.491 |
| Mimosa pigra | ||||
| Temperature (°C) | -0.121 | -0.473 | -0.977* | -0.844* |
| O2 concentration (mg L-1) | -0.817* | -0.864* | -0.469 | -0.486 |
| PO4 3— concentration (mg L-1) | 0.320 | 0.616* | 0.147 | -0.306 |
M, intensity of mycorrhizal colonization in the root system; A, abundance of arbuscules in the root system; C, intensity of colonization by DSE in the root system; MS, abundance of microsclerotia in the root system. Asterisks indicate significant correlations (p < 0.05)
Molecular identification of AMF in Mimosa pigra roots. Because M. pigra was highly colonized by Glomeromycotina, identification of AMF inside roots containing arbuscules was addressed by a molecular approach targeting fungal rDNA sequences (SSU-ITS-LSU) (Krüger et al. 2009). The size of the final amplified fragments was ~ 1.5 kb, corresponding to that expected for Glomeromycotina species. These amplicons were employed to construct a SSU-ITS-LSU rDNA library. Fifty recombinant clones of E. coli were randomly selected and subcultured; isolated plasmid DNA was digested with EcoRI enzyme in order to corroborate the exact size of inserts; 38 positive samples were sequenced, but only 30 of these exhibited good sequence quality. No chimeric amplicon sequences were detected, and all of the sequences obtained showed a high degree of similarity to Glomeromycotina SSU-ITS-LSU rDNA sequences. All the 30 sequences were submitted to the GenBank database at NCBI (accession numbers from MK418498 to MK418527). Phylogenetic analyses revealed four monophyletic ribotypes (Figure 3); three of these belonged to the Order Glomerales and one, to Archaeosporales. Concerning Glomerales, 11 of the sequences were grouped with Rhizophagus irregularis/Rh. proliferum, and seven sequences showed affinity with Rhizophagus diaphanum; a group of two sequences were related to Glomus. Estimates of evolutionary divergence between representative sequences of clades observed in the tree confirmed these ribotypes of Glomerales (Table 6). Finally, a group of ten sequences were included in the Order Archaeosporales and were closely related with Archaeospora.
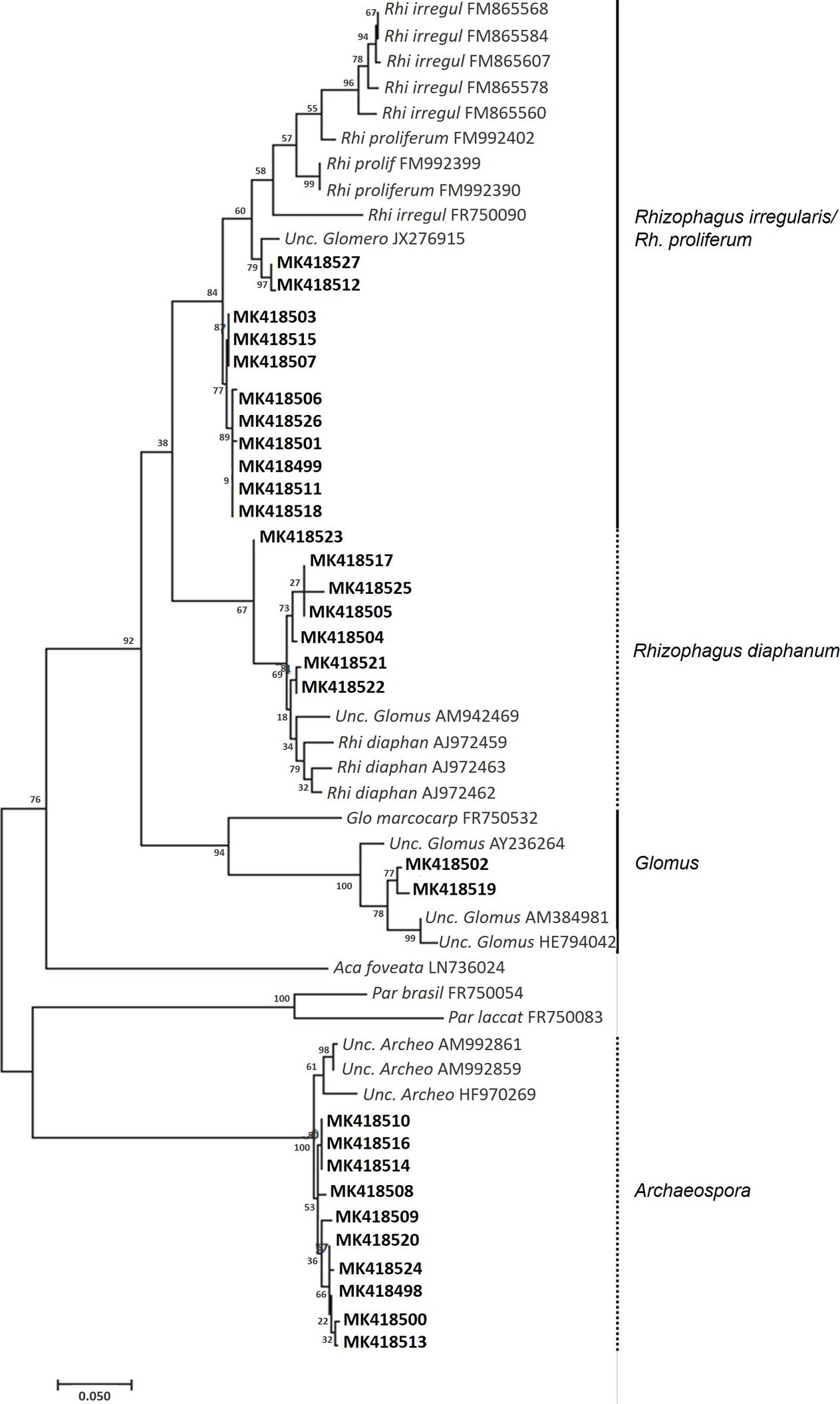
Figure 3 Molecular phylogenetic analysis by Maximum Likelihood method representing SSU-ITS-LSU rDNA sequences of Glomeromycotina fungi colonizing Mimosa pigra flooded roots at La Mixtequilla wetland. Four identified ribotypes are indicated.
Table 6 Estimates of evolutionary divergence between representative ribotypes observed in the phylogenetic tree representing SSU-ITS-LSU rDNA sequences of Glomerales colonizing Mimosa pigra flooded roots. The number of base substitutions per site between sequences are shown. Analyses were conducted using the Maximum Composite Likelihood model and it involved five nucleotides sequences. All positions containing gaps and missing data were eliminated. There was a total of 517 positions in the final dataset. Best hits were following NCBI or MaarjAM databases.
| Sequence | Distance | Max identity (%) | Best hit | Propose name |
|---|---|---|---|---|
| MK418527/1-555 | 96.3 | AY744275 | Rhizophagus | |
| MK418515/1-561 | 0.038 | 99 | HG969372 | Rhizophagus |
| MK418522/1-551 | 0.211 0.19 | 97 | AJ972463 | Rhizophagus |
| MK418523/1-551 | 0.183 0.163 0.028 | 97 | AM972463 | Rhizophagus |
| MK418519/1-566 | 0.303 0.291 0.288 0.275 | 94 | AM384981 | Glomus |
Discussion
Soil and water properties in pasturelands. Soils from both pasturelands in the La Mixtequilla wetland showed typical characteristics of Gleysols, such as clay texture and saturation with groundwater for long periods, which develop a typical gleyic color pattern and favor a prolonged O2-deficit phenomenon (Driessen et al. 2001). The significantly higher concentrations of available P, Na+, Mg2+, and K+ at El Llanete in comparison with the Don Rufino pastureland could be attributed to the different strategies of soil management utilized at these sites (agricultural and/or livestock breeding), as well as to biological activity. Driessen et al. (2001) state that Gleysols in depressions or lowlands possess higher organic matter, CEC, P, and K+ than adjacent upland soils; at El Llanete, the flooding season is normally longer than at Don Rufino (Rivera-Becerril et al. 2008), which can influence a higher transport of nutrients from uplands via the Blanco River. River nutrient contribution into the floodplain constitutes an elemental process for floodplain production (Junk et al. 1989). Fertility is also favored by fine soil texture and slow rate of organic matter decomposition (Driessen et al. 2001). Flatlands, temperate to warm temperatures, proliferation of plant cover, and its contribution to high levels of autochthonous organic matter, comprise the causes for very low levels of dissolved oxygen in the water columns of both pasturelands. In general, moisture, temperature seasonality, soil fertility, and/or substrate availability control wetland microbial processes (Gutknecht et al. 2006).
Floristic composition and fungal colonization in roots. Gleysols are covered with swamp vegetation and are normally used for extensive grazing (Driessen et al. 2001) of livestock, as occurs at La Mixtequilla. The “popal” plant community is normally present in the neotropical region of the Atlantic coast of Mexico (Rzedowski 1986). In both pasturelands, most plant species belonged to Magnoliopsida, and were herbaceous, consistently with a report from other neotropical wetland (Moreno-Casasola et al. 2009). Three exotic plant species (E. crassipes, E. marginata, and C. pallida) were detected; the free-floating E. crassipes is known as one of the worst macrophyte invaders (Thomaz et al. 2015); in addition to its presence in Don Rufino pastureland, large areas of the Blanco River were covered by this plant species. M. pigra, native to the tropics, is a well-recognized invasive plant in Southeast Asia and Australia (Lonsdale 1993). At La Mixtequilla, M. pigra is adapted to both flooded and dry conditions throughout the year; local people normally attempt to eliminate it in order to avoid the perturbation of agricultural practices, livestock breeding, and fishing.
Seven plant species (C. articulatus, E. cruspavonis, M. pigra, F. spadicea, R. geminiflora, S. herbacea, and T. geniculata) showed a differential co-colonization between AMF and DSE in roots, either under dry or flooded conditions, as reported for other wetlands (Fuchs & Haselwandter 2004, Weishampel & Bedford 2006, de Marins et al. 2009, Sudová et al. 2011). Flooded M. pigra showed the highest abundance of arbuscules, indicating that this symbiosis is functional under flooded conditions at low levels of dissolved O2 (see later). Weishampel & Bedford (2006) consider that Magnoliopsida exhibit more affinity for AMF than DSE, as observed in three plant species analyzed in this work (M. pigra, R. geminiflora and S. herbacea).
Repeated monitoring of fungal colonization in roots from two plant species. Cyperus articulatus, a native plant from Mexico, is well adapted to tropical wetlands, where is used as forage (Olivares et al. 2002), as observed at the Don Rufino pastureland. This plant species exhibited low levels (up to 13.5 %) of AMF colonization, but only hyphae and vesicles were present in roots. Members of Cyperaceae typically show low mycotrophy; their mycorrhizal status could be influenced strongly by environmental conditions such as flooding/dry periods (Muthukumar et al. 2004). The lack of arbuscules has been previously reported in cultivated C. rotundus (Muthukumar et al. 1997) or established in a tropical ecosystem (Muthukumar & Udaiyan 2002). In contrast, high levels of DSE root colonization indicate that these could play a more relevant role in this sedge. Low root colonization by DSE during the flooded season (<10 %), in comparison with the higher levels observed during the beginning/ending of the dry period (19-25%), could suggest that DSE are sensitive to low oxygen concentrations in the water column. In April, during the dry season, a four-fold reduction in colonization by AMF, in contrast with increased colonization by DSE, is probably a consequence of fungal competition for resources during the grazing time (García et al. 2012), as was observed for the grass species Bouteloua gracilis (Medina-Roldán et al. 2008). In this study, a significant correlation was observed between root colonization by AMF and DSE during the annual cycle in C. articulatus. As previously reported for some plant species (Ruotsalainen et al. 2002), this positive correlation might indicate a synergism between both groups of fungi.
The native plant from the tropics, M. pigra, is present in different ecosystems. Members of Fabaceae are highly mycotrophic, mainly when environmental conditions threaten plant development (Azcón 2000). Considering that M. pigra demonstrated high levels of root colonization by AMF in the freshwater wetland under study (M = 7-61 %; A = up to 17.7 %), it can be postulated that AMF could facilitate the spread of this plant species, as suggested for Ambrosia artemisiifolia growing in disturbed sites (Fumanal et al. 2006). In addition, M. pigra also interacts with nitrogen-fixing bacteria harboring in root nodules (Parker et al. 2007). These bacteria, together with AMF, might render this plant species highly competitive for colonizing stressful environments conferring a better nutritional status. AMF colonization in some Mimosa species is required for enhancing root nodulation (Lammel et al. 2015).
In this wetland, it was observed that properties prevailing in the water column influence fungal root colonization. High temperatures tended to reduce fungal colonization by AMF and DSE in both plants (M and C in C. articulatus, and C and MS in M. pigra), and DSE appeared to be more susceptible than AMF to high temperatures. Within this context, plant-root colonization by AMF was reduced when the temperature exceeded 30 °C; elevated temperatures might increase plant night respiration resulting in diminished photosynthate for root-inhabiting fungi (Zhu et al. 2011). High concentrations of dissolved O2 in the water column favored higher M and C in C. articulatus; the negative correlation between dissolved O2 in the water column and M and A in M. pigra could suggest a particular root physiology for supplying dissolved O2. A floating growth habit and aerenchyma are root adaptations described in M. pigra living in a flooded environment in a neotropical ecosystem (James et al. 2001). Finally, the negative correlation in C. articulatus between C and the orthophosphate concentration could indicate that DSE are not involved in P nutrition in this plant. In contrast, concentrations of orthophosphates in the water column at El Llanete, which correlated positively with A in M. pigra, pointed out the role of AMF in transferring P from soils and/or the column water to the plant, even under suboxic conditions.
Molecular identification of AMF in Mimosa pigra roots. This is the first time, to the best of our knowledge, in which an AMF community of flooded roots from the shrub M. pigra established in a freshwater wetland was molecularly identified. Arbuscules confirmed visually the presence of AMF inside roots; molecular analysis allowed to identify the whole fungal community, constituted by members of Rhizophagus, and Glomus (Order Glomerales), as well as by Archaeospora (Order Archaeosporales). All identified AMF are reported to produce arbuscules; observed vesicles inside roots could belong to Rhizophagus and Glomus since members of Archaeospora do not produce them (Oehl et al. 2011). Glomerales, the largest Order of Glomeromycotina, predominated inside M. pigra roots; Glomerales are considered as generalist AMF (Davison et al. 2011). Consistently with results presented here, AMF spores extracted from the rhizosphere soil of colonized native and exotic Fabaceae showed the dominance of Glomerales, but Archaeosporales and Diversisporales were also present (Tibbett et al. 2008). In a recent meta-analysis, Ramírez-Viga et al. (2018) concluded that AMF improve plant performance in wetlands through higher nutrient acquisition, photosynthetic activity, biomass generation, and mitigation of abiotic stress. However, understanding the functionality of AMF in M. pigra plants is a future task.
The Neotropics harbor several freshwater intermittent wetlands. Nearly one-hundred known AMF species have been reported in Mexico (Montaño et al. 2012, Chimal-Sánchez et al. 2016, Fabián et al. 2018), considered a high AMF diversity reservoir linked with plant and ecosystem diversity. The Mesoamerican biodiversity hotspot (Myers et al. 2000), where La Mixtequilla wetland is located, certainly harbors a still unknown diversity of AMF that is threatened by habitat destruction and land-use change.











 nueva página del texto (beta)
nueva página del texto (beta)


