Seed banks are defined as the mature viable seed stocks present on the soil’s surface, or buried in the soil, duff or litter (Simpson et al. 1989). Germination is the most important process in the initial composition of plant communities (Thompson & Fenner 2005), essential to their establishment and permanence through time (McGee & Feller 1993, Bueno & Baruch 2011, Pensado-Fernández et al. 2014). Seed banks can be classified into transient and persistent, according to the time the seeds remain viable (< 1 year, or ≥ 1 year, respectively) (De Souza-Maia et al. 2006). Pine-dominated plant communities are known to fail to form seed banks given that, once dispersed, their seeds remain viable for less than a year, due to biotic and abiotic factors (Carrillo-Anzurez et al. 2009). However, the presence of persistent seed banks has been reported for Pinus pinaster Aiton and Pinus halepensis Mill. (Trabaud et al. 1997, Ferrandis et al. 2000). Pinus patula Schltdl. & Cham. seeds may remain stored up to 10 years at room temperature (Aparicio-Rentería et al. 2014), which indicates that knowledge about the ability of pine species to form bank seeds is yet to be obtained.
The altitudinal gradient is of great importance in the formation of seed banks, as it influences the production of female strobili, as well as the quantity and availability of seeds (Sáenz-Romero et al. 2006, Zelikova et al. 2008). Tree populations growing at the extreme altitudinal limits (lower and upper) of their natural distribution range produce seeds in smaller quantity than their counterparts at medium altitudes (Quiroga & Premoli 2013). At the lower altitudinal limit, a smaller quantity of seeds is associated to the periodic exposition of trees to extreme climatic events such as droughts and high temperatures (Mátyás et al. 2010), whereas at the upper limit it is associated to frost (low temperatures) (Sáenz-Romero et al. 2006). Environmental resources and conditions at intermediate altitudinal areas may favor a higher concurrence of predators and greater seed displacement (Sánchez-Cordero 2001). López-Toledo et al. (2017) found that one of the intermediate altitude populations of Pinus pseudostrobus Lindl. presented a larger number of seeds with embryo than those at a lower altitude.
Granivore guilds play an important role in the persistence of seed banks. Their activity as predators and seed removers has a spatial effect, the magnitude of which varies depending on the group to which they belong (i.e., birds, insects or small mammals) (Hulme 1998). In order to evaluate seed removal by different groups of granivores, several studies have resorted to exclusion treatments, consisting in applying a mesh of different opening sizes according to the size of the granivores in question (Campos et al. 2007, Álvarez-Aquino et al. 2014), or a repellent, in the case of insects (Matamoros-Juárez & Gaitán-Martínez 2017, Bordones et al. 2018). In temperate forests, birds, insects and rodents are efficient removers of pine seeds with different foraging strategies (Hulme & Kollmann 2005, Flores-Peredo et al. 2011, Vander-Wall & Beck 2012). In the majority of forest ecosystems, rodents are the main seed removers (Hulme & Benkman 2002), and in pine forests they may remove up to 99 % of the seeds that have fallen to the ground (Vander-Wall 2008), which indicates the important role they play in regeneration dynamics (Vander-Wall 2008, Lobo et al. 2009). However, other granivores, such as birds and insects, also play an important role, as has been reported for Pinus patula, P. pseudostrobus, P. teocote Cham. & Schltdl. and P. montezumae Lamb. in the central part of the state of Veracruz, Mexico (Flores-Peredo et al. 2011, Flores-Peredo & Bolivar-Cimé 2016).
Although differences in seed removal rates are usually associated to seasonal changes (rainy versus dry seasons), the altitudinal gradient also influences the production, availability and quality of seeds for food (Fleury et al. 2014, Wang et al. 2014, López-Toledo et al. 2017). Seed production impacts the reproductive periods of granivore animals (Ofori et al. 2015). For example, the size of rodent communities changes seasonally due to changes in weather conditions, which determine both ecosystem productivity and the phenology of seed production (Wang et al. 2009, Cortes-Flores et al. 2011). This is found among pine communities with mast seeding, the number of years varying from species to species (Perry 2009). In Pinus hartwegii Lindl., for example, seeding years occur every six or seven years (Musálem-Santiago & Solís-Pérez 2000). Pinus hartwegii develops at the altitudinal limits of mountainous areas in Mexico and Central America (up to 4,300 m a.s.l.), and in Veracruz it is present in the Cofre de Perote and Pico de Orizaba national parks (Narave & Taylor 1997). Due to its biological characteristics and geographic location at the altitudinal limit of montane forests, P. hartwegii is at risk of losing its habitat as a result of global warming (Farjon et al. 1997). Moreover, it is not known whether it forms transient or persistent seed banks, and whether its granivore seed removal dynamics is associated to an altitudinal gradient. In this study we pose the following questions: 1) Do Pinus hartwegii seeds remain viable and have the possibility to form transient or persistent seed banks? 2) If that is the case, does its capacity to do so vary along an altitudinal gradient? 3) Does seed removal vary seasonally along an altitudinal gradient and according to the group of granivores (birds, rodents and insects)? The hypotheses are: a) At a high altitude, Pinus hartwegii will show potential to produce transient and probably persistent seed banks, as low temperatures help maintain seed metabolism low; and b) Seed removal will vary with altitude, season, and group of granivores, probably being greater at medium altitudes, where food availability for granivores is higher.
Materials and methods
Study area. This study was undertaken at the Cofre de Perote or Nauhcampatepetl National Park, located in the southern portion of the Sierra Madre Oriental, at its confluence with the eastern end of the Transversal Neovolcanic Axis, in the central-western region of the state of Veracruz, Mexico. The four altitudinal tiers selected were: Site 1 (altitude: 3,400 m; latitude: 19° 31’ 13.33’’ N; longitude: 97° 09’ 49.65’’ W); Site 2 (altitude: 3,600 m; latitude: 19° 30’ 52.15’’ N; longitude: 97° 09’ 51.31’’ W); Site 3 (altitude: 3,800 m; latitude: 19° 30’19.42’’ N; longitude: 97° 09’31.37’’ W), and Site 4 (altitude: 4,000 m; latitude: 19° 29’ 44.47’’ N; longitude: 97° 09’ 09.27’’ W). Climates are semi cold subhumid with long cool summers [Cb’(w2)], and cold subhumid ETH (García 2004). Mean annual rainfall is 1,500-1,800 mm, and the predominant soil is ochric andosol. Plant communities are represented by pure P. hartwegii forests in high altitudes, while at low altitudes associations of P. hartwegii, P. montezumae, and Abies religiosa (Kunth) Schltdl. & Cham. are found. The undergrowth is composed of species such as Vaccinium geminiflorum Kunth, Baccharis conferta Kunth and Juniperus monticola Martínez, and the herb stratum includes the following species: Alchemilla procumbens Rose, Agrostis tolucensis Kunth, Calamagrostis schiedeana Steud., Calamagrostis rigens Fr., Cirsium jorullense Spreng., Draba jorullensis Kunth, Echeveria secunda Booth ex Lindl., Eryngium proteiflorum Delaroche, Erysimum macradenium J. Gay, Festuca tolucensis Kunth, Gnaphalium liebmannii Sch. Bip. ex Klatt., Lupinus montanus Kunth., Muhlenbergia macroura Hitch., Ottoa oenanthoides Kunth, Oxylobus arbutifolius A. Gray, Penstemon gentianoides Poir, Sedum obcordatum R.T. Clausen, and Senecio roseus Sch. Bip. (Narave 1985, Vázquez-Ramírez 2014). As for animal communities, there are 14 species of amphibians, 25 species of reptiles, 89 species of birds, and 51 species of mammals (Morales-Mávil et al. 2007).
Seed bank. In order to obtain seeds, mature cones were collected from 10 healthy trees at each altitudinal tier. The persistence of seeds on the ground was evaluated at each tier, an experiment was conducted consisting of three treatments with 25 seeds and three repetitions (i.e., 900 seeds in total). Treatments were: a) seeds placed on the soil’s surface, b) seeds planted 5 cm deep into the soil, and c) seeds planted 10 cm deep. Before being planted, seeds were wrapped in a 2 mm-opening plastic insect mesh measuring 10 × 10 cm, so as to secure them and prevent mixing with other seeds of the same species. A year later, seeds were retrieved, and their viability was evaluated by the tetrazolium chloride test at 1 %, to determine the type of seed bank (transient or probably persistent) (Corral-Aguirre & Sánchez-Velásquez 2006). Seeds considered viable were those in which at least 75 % of their embryos took on a red-violet dye, and the rest were considered unviable (Kolotelo et al. 2001, Flores-Peredo et al. 2011).
Seed removal. During July-September 2015 (rainy season) and January-March 2016 (dry season), four random granivore exclusion treatments with five repetitions each were conducted at each altitudinal tier (Arias-Le Claire 2001, Côté et al. 2003, Flores-Peredo et al. 2011). Treatments availed of meshes with different opening sizes to separate birds, rodents, and insects, and evaluate their respective contributions to seed removal (Hulme 1998, Hulme & Kollmann 2005, Álvarez-Aquino et al. 2014, Magalhaes et al. 2018). The experimental unit was a plastic Petri box with 20 seeds of P. hartwegii (n = 20). The treatments were: 1) exclusion of rodents and birds (access to insects only) by means of a cage measuring 16 × 16 × 9 cm, formed with a 0.5 cm-opening mesh; 2) exclusion of birds and insects (access to rodents only) with a cage measuring 16 × 16 × 9 cm, formed with a 2 cm opening mesh, around which a garlic-based repellent was sprayed to keep insects out, its effectiveness previously tested with ants and consigned in the literature (Karunamoorthi & Hailu 2014, Matamoros-Juárez & Gaitán-Martínez 2017, Bordones et al. 2018); 3) exclusion of rodents and insects (access to birds only) placing the seeds at the upper side of the cage, far from rodents, and using garlic-based repellent to avoid insects, and 4) control with free access (no exclusions). The cages were fixed to the ground by means of four 10 cm steel nails.
Statistical analysis. In order to understand how seed banks are formed, a variance analysis with the general lineal model (GLM) of SAS (SAS 2015) was conducted along the altitudinal gradient, considering a nested pattern in which the depth of seed planting (0, 5 and 10 cm) was nested in each tier (3,400, 3,600, 3,800 and 4,000 m a.s.l.) and the response variable was the quantity of viable seeds (natural logarithm [ln]). Mean comparisons were conducted using the Bonferroni adjustments (Zar 1999). Seed removal (by rodents, birds, and insects), altitude (3,400, 3,600, 3,800 and 4,000 m a.s.l.), and seasons (rainy, dry) were evaluated by means of a GLM procedure with Poisson distribution (McCullagh & Nelder 1989, Crawley 2007, Agresti 2015), using as dependent variable the number of seeds removed (count data) because did not assume normality and as independent variables the exclusion, altitude, and season treatments. This is because the Poisson distribution is applied to discrete phenomena of nature, that is, phenomena that imply counts during a defined period of time or in a certain area (Guerriero 2012). For the evaluation of differences between treatments we used the Multcomp package, Version 1.3-5 (Bretz et al. 2011) in R language (R Development Core Team 2016).
Results
A year after the seeds were planted at different depths (at the surface, and 5 and 10 cm deep into the soil), viable seeds were found, which proved the formation of transient and probably persistent seed banks during that period. The number of viable P. hartwegii seeds varied significantly with depth (P < 0.0001) and altitude (P = 0.0110). In average, approximately three viable seeds were retrieved at the surface and at a depth of 5 cm, and approximately one viable seed was retrieved at a depth of 10 cm (Figure 1A). The average number of seeds found at the surface (0 cm) and at a depth of 5 cm was similar in all the altitudinal tiers (approximately three seeds); the smallest number (approximately one seed) was found at a depth of 10 cm at the altitudinal tiers 3,400, 3,600 and 3,800 m a.s.l., and the largest average number of viable seeds (approximately six seeds) was found at a depth of 10 cm at the altitudinal tier of 4,000 m a.s.l. (Figure 1B).
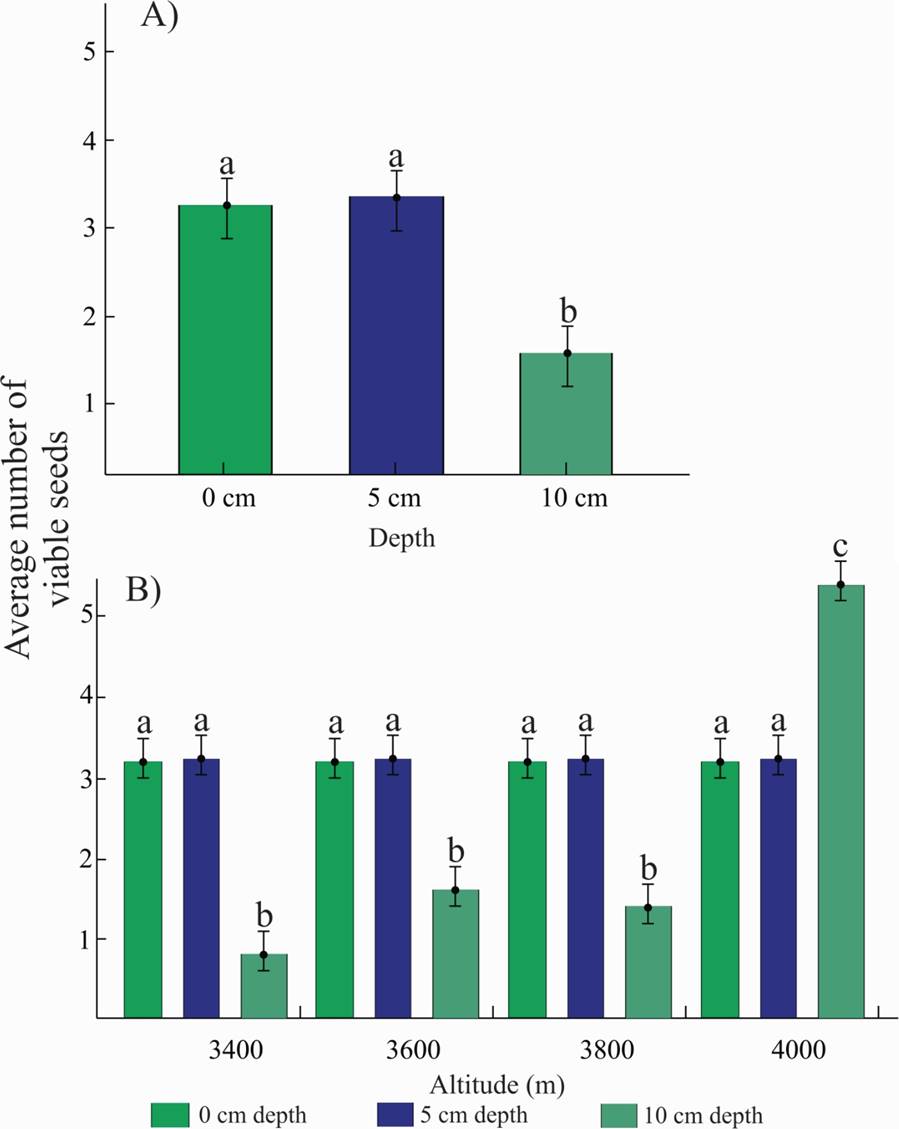
Figure 1 A. Average number of viable P. hartwegii seeds retrieved at different depths along an altitudinal gradient. B. Average number of viable P. hartwegii seeds retrieved at different depths along an altitudinal gradient at the Cofre de Perote National Park, Veracruz, Mexico. Dissimilar letters indicate significant differences (P < 0.05).
The season-altitude interaction had a significant effect on the removal of P. hartwegii seeds (F = 13.61, df = 3, P < 0.001). The greatest removal of seeds was registered at 4,000 m a.s.l. during both the dry season (11.79 ± 0.75) and the rainy season (8.40 ± 0.75). Conversely, at 3,800 m a.s.l., the removal of seeds was greater during the dry season (5.76 ± 0.75) (Figure 2A). Altitude and the exclusion treatments also had a significant effect on the removal of P. hartwegii seeds (F = 2.24, df = 9, P = 0.017). At 3,800 m a.s.l., more seeds were removed by rodents, birds, and at the open treatment (4.91 ± 1.05, 5.11 ± 1.05, 5.54 ± 1.05), whereas at 4,000 m a.s.l., rodents were the main seed removers (13.12 ± 1.05) (Figure 2B).
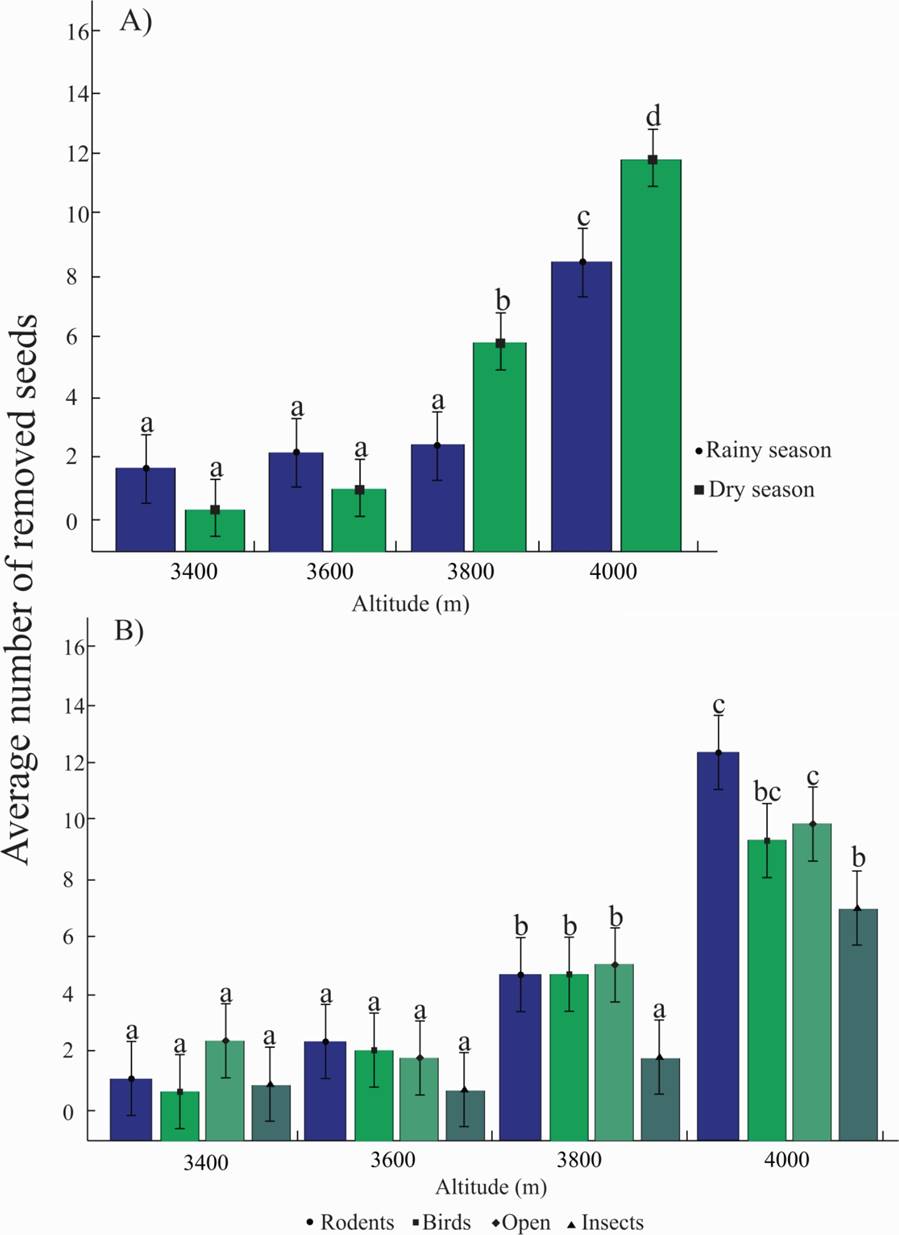
Figure 2 A. Average number of removed P. hartwegii seeds in two evaluation seasons along an altitudinal gradient. B. Average number of P. hartwegii seeds removed through an exclusion treatment along an altitudinal gradient at the Cofre de Perote National Park, Veracruz, Mexico. Dissimilar letters indicate significant differences (P < 0.05).
Discussion
The percentage of viable P. hartwegii seeds found after a year of lying at the soil’s surface or planted into it indicates the ability of the species to form transient and probably persistent seed banks. Along with Pinus pinaster Aiton, Pinus halepensis Mill. (Ferrandis et al. 2000, Trabaud et al. 1997) and other pine species from eastern Canada (Thomas & Wein 1985), Washington, USA (Pratt et al. 1984), and Idaho, USA (Kramer & Johnson 1987), P. hartwegii belongs to the list of pines with potential to form transient and probably persistent seed banks. The fact that the number of viable seeds found in the soil increased with depth suggests that the micro-environmental stability found at the soil’s deeper layers favor seed development, as seeds are exposed to less stress than those lying on the soil’s surface (Cavieres & Arroyo 2001, Fenner & Thompson 2005). This has also been reported for Pinus ponderosa (Pratt et al. 1984) and other conifers (Archibold 1989). In the case of P. hartwegii, its response has to do also with its life history (Campanhã-Bechara et al. 2013). It has been suggested that in high montane environments, low temperatures can be associated to low embryonic metabolic rates, which may favor seed longevity (Murdoch & Ellis 2000) and, therefore, the formation of transient and probably persistent seed banks (Cavieres & Arroyo 2001). This may help explain the larger number of viable seeds found at the deepest level of the highest altitudinal tier, where frost and low temperatures are frequent.
The interactions between season and altitude had a significant effect on seed removal. Seed removal was greater at 4,000 m a.s.l., during both seasons, as opposed to other studies where higher seed removal was greater at medium altitudes (e.g., Sánchez-Cordero 2001). During the dry season (February-March), a temperature rise occurs, which favors cone aperture and seed dispersal. This results in a higher availability of pine seeds on the ground (Perry 2009), which generates a movement of granivores towards the areas with higher availability of resources, a process called denso-dependency (Hulme & Kollmann 2005, Flores-Peredo et al. 2011).
On the other hand, the greatest seed removal at 4,000 m a.s.l. during the rainy season (particularly that carried out by several species of rodents) suggests a synchronicity between food availability, reproductive activity, and the presence of juveniles in large numbers (Ceballos & Oliva 2005). In pine forests of the state of Veracruz, Mexico, for example, a greater richness of species of granivore rodents during the rainy season has been documented, although they were more abundant during the dry season (Flores-Peredo & Vázquez-Domínguez 2016). This suggests that seasonal variations in the availability of food influence the movements of granivores among plant communities (Hulme & Benkman 2002, Rautenbach et al. 2014, Ofori et al. 2015) and altitudinal gradients (Shuai et al. 2017).
The exclusion treatments carried out showed that rodents and birds were the most important seed removal agents at 4,000 m a.s.l. Coinciding with this study is a report from the San Juan del Monte Ecological Reserve in Veracruz, Mexico, where the natural vegetation is pine-oak forest, and where rodents and birds were reported as the main seed removers for four pine species, the former acting at night and the latter during the day (Flores-Peredo et al. 2011). Although the lesser removal of seeds found in altitudinal tiers between 3,500 and 3,600 m confirms the meaningful effect of altitude, it may also be due to the lower density of P. hartwegii in a more diverse forest (Murrieta-Hernández et al. 2014). In a situation in which seeds from diverse plants are abundant, P. hartwegii seeds may not be the preferred food resource to granivores. Conversely, the greater removal registered at 4,000 m a.s.l., where P. hartwegii is the only tree species, suggests that granivores feed on its seeds because little is available of other resources.
In order to understand the dynamics of the establishment and permanence of endangered plant species, and of the animal groups associated to their regeneration, it is essential to evaluate the presence of transient and probably persistent seed banks, and the effect of the different seed-removing granivores. The results suggest that even though the percentage of viable seeds after 1 year was low (from 12 to 24 %) Pinus hartwegii has the potential to form a seed bank (transient and probably persistent). The most important seed removal agents were rodents and birds and removal varies according to altitude and season.











 nueva página del texto (beta)
nueva página del texto (beta)



