Freshwater ecosystems are subject of severe impacts by human activities like industrial, agricultural or urban discharges. This promotes habitat loss, threatening the biodiversity of these ecosystems (Ojeda 2011). Biodiversity loss in fresh-water ecosystems is even greater than in the most disturbed terrestrial ecosystems (Sala et al. 2000, Dudgeon et al. 2006, Vörösmarty et al. 2010). This is even worse in tropical regions, where most of biodiversity is located (Mittermeier et al. 1998, Bawa et al. 2004). In the face of the loss of thousands of threatened plant species it is fundamental to promote assertive conservation programs. This need has been recognized by the 10-year Global Strategy for Plant Conservation, which established the goal of preserve at least 75 % of threatened plant species worldwide through seed-based in ex situ collections (Sharrock 2012). Thus, generating information of seed storage and seed germination is helpful to design effective strategies for germplasm conservation such as ex situ seed banks.
Podostemaceae family is the largest group of strictly aquatic angiosperms, which inhabit oligotrophic rivers in tropical and subtropical regions of the world. Podostemad life cycle is closely associated with the seasonality of the rivers they inhabit. During the rainy season when the water level is high, vegetative structures grow submerged and anchored to bedrock; later, when the water level decreases during the dry season, reproductive structures emerge (Figure 1A). Then flowering and pollination occurs, and fruits and seeds are formed; later, these seeds will reestablish the population during the next rainy season (Castillo et al. 2013). Podostemaceae seeds bear a mucilaginous seed coat, derived from the outer integument of the ovule (Philbrick & Novelo 1997). When seeds are released from the capsule, the cells of the outer integument are dry and collapsed. These cells hydrate rapidly when moistened after the first rain of monsoon, expand and become mucilaginous and sticky. This mucilage is a common feature in Podostemaceae that is involved in plant fixation to the substrate (Philbrick & Novelo 1997. Reyes-Ortega et al. 2009). Unlike most aquatic plants, sexual reproduction is the main way of reproduction of podostemads. Because of this, seed germination is a fundamental process in Podostemaceae, as it drives their distribution, survival, and abundance (Philbrick & Novelo 1995, 1997, Luna et al. 2012). Moreover, approximately 33 % of neotropical species of the family are single-river or two-river endemics (Cook & Rutishauser 2007, Philbrick et al. 2010, Katayama et al. 2016). This makes podostemads extremely susceptible to local extinction caused by habitat loss as a result of dams construction or water pollution through runoff of nutrients used in crop fields (Philbrick et al. 2010, Masahiro & Lansdown 2012).

Figure 1 A) Characteristics of the river where podostemads fruits were collected. B) Marathrum foeniculaceum in vegetative phase. C) Fruits of M. foeniculaceum. D) Noveloa coulteriana in vegetative phase. E) Fruits of N. coulteriana (marked with red arrows).
Five genera of podostemads are distributed in México: Marathrum, Podostemum, Tristicha, Noveloa and Vanroyenella, containing seven species of which M. plumosa, N. longifolia and N. coulteriana are endemic to Mexico (Novelo & Philbrick 1997, Novelo et al. 2009, Tippery et al. 2011). N. coulteriana is considered a protected species by Mexican laws (SEMARNAT 2010). Considering that about 70 % of the river systems in Mexico show some degree of perturbance (Quiroz et al. 1997, Pérez et al. 2010, Vörösmarty et al. 2010), it is of high importance to generate information about podostemads seed storage and seed germination behavior. This knowledge will be extremely valuable to design strategies for germplasm conservation strategies like ex situ seed banks.
Seed germination is regulated by different external factors like light quality or temperature. Seed response to light is strongly regulated by the phytochrome (Pr/Pfr), which is a red (R) and far red light (FR) photoreceptor in plants (Sawada et al. 2008, Kami et al. 2010). Absorption of red light (±660 nm) converts the inactive form of the phytochrome Pr to its active form Pfr (promoting or inhibiting germination), whereas the absorption of far red light (±730 nm) converts Pfr back to Pf. According to their response to light, seeds can be classified as positive photoblastic if light activates germination or negative photoblastic if light hinders germination. If germination is not related with light, seeds are considered non-photoblastic. Temperature can also influence the endogenous chemical mechanisms of germination (e.g., ABA/GA hormone balance, phytochrome activity), modifying germination rate and/or germination lag time (Baskin & Baskin 2014). Germination of a given species occurs in a temperature range related to the habitat they live in (i.e., thermic window) and the maturation stage of the seed embryo. Seeds whose germination is regulated by temperature are considered thermoblastic (Orozco-Segovia & Sánchez-Coronado 2013). Furthermore, storage time can affect seed viability; according to their tolerance to dryness, seeds can be classified as recalcitrant if they do not survive drying and/or freezing and germinate immediately after been released, or as orthodox when they can resist drying and/or freezing and remain viable in a seed bank (Hong & Ellis 1996, Bewley et al. 2013).
Previous studies in Mexican Podostemaceae have found that (i) light quality, (ii) temperature, (iii) storage time influence their germination (Philbrick & Novelo 1994, Reyes-Ortega et al. 2009, Castillo et al. 2017). But there is not information for any podostemad species about how seed origin (rivers), and their location, affect seed germination. Recently, Castillo et al. (2017) pointed that light incidence at the rivers affect germination rates for both M. foeniculaceum and N. coulteriana.
Here we studied seed germination response of N. coulteriana and M. foeniculaceum, two species of Mexican Podostemaceae, collected from four collection sites in Puerto Vallarta, Mexico (Figure 2). Seeds were exposed to different light qualities (white, red, far-red and darkness) and temperature treatments (constant 25 °C and alternate 15-25 °C). We also explored the influence of storage time (1-17 years) on accumulated seed germination percentage and final seed germination percentage of both species. We hypothesize that seed germination decreases with storage time and that temperature, light quality and collection site affects germination of both studied species.
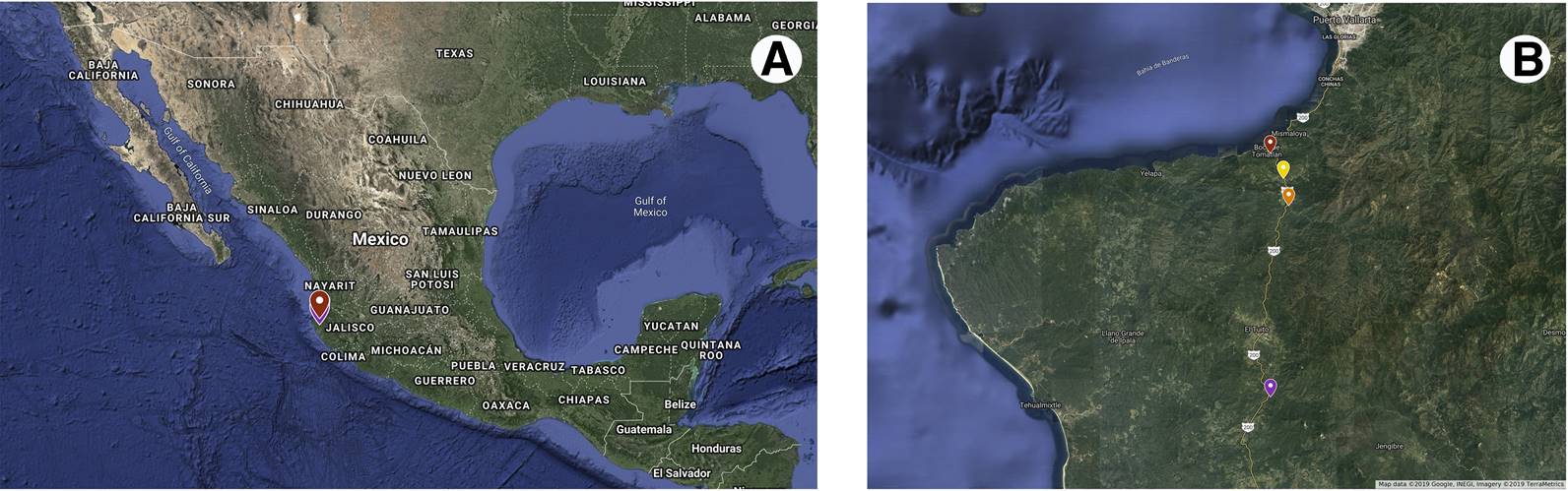
Figure 2 A) Map of Mexican Republic pointing out Jalisco state. B) Approximation indicating the collection sites of fruits of N. coulteriana and M. foeniculaceum included in this study: Horcones River, Las Juntas del Tuito River (Las Juntas), Las Juntas y Los Veranos river, Boca de Tomatlán river.
Materials and methods
Study system. Marathrum foeniculaceum. Annual or perennial plant; characterized by repeatedly pinnately divided leaves to 80 cm, hair-like to flatten; roots 0.2-1 mm wide; stems to 20 cm; flowers actinomorphic with pink tepals; capsules 4-6 ×1.7-2.5 mm, seeds 0-1,500 per capsule, 0.24-0.45 × 0.12-0.27 mm; distributed from central Mexico to Colombia (Novelo et al. 2009, Garcia-Posada & Muñoz-López 2011, Tippery et al. 2011) (Figures 1B-C). Noveloa coulteriana. Annual or perennial plant; divided leaves 2-10 cm long; roots prostrated and flattened 15 cm long; capsules 1.6-2.8 × 0.8-1.2 mm, each valve 3-ribbed, 7-73 seeds per capsule, 0.17-0.34 × 0.11-0.25 mm. N. coulteriana is a species endemic to Mexico, which is distributed along the Pacific slope from Sonora to Guerrero and in Baja California Sur (Novelo & Philbrick 1997) (Figures 1D-E).
Plant material. Fruits of M. foeniculaceum and N. coulteriana were collected in non-consecutively yr from 1996 to 2013 (see Appendix 1) in different collection sites of the rivers Horcones and Arroyo del Rincon, both rivers located in Puerto Vallarta, México (Figure 1). Complete and not predated fruits (> 20) of each studied species were collected from randomly selected rocks along the river course at each collection site (Figure 1). Since fruits of both species are naturally dry and dehiscent, fruits were stored in paper bags at room temperature (20±2 °C) until the beginning of germination experiments.
Seed germination experiment. From each collection site and species (Appendix 1), we extracted seeds of 10 randomly chosen fruits to obtain a heterogeneous sample. With a stereoscopic microscope, 30 seeds were sowed in Petri dishes, by triplicate, with absorbent paper and commercial bottle water low in salts to resemble the oligotrophic condition of the rivers. Petri dishes with water was sealed with parafilm an not was open until the end of the experiment in order to maintain humidity constant. Seed germination experiment was carried out during April 2014. Seeds were subjected to a combination of constant (25 °C) and alternate (15/25 °C) temperature with white light (WL), red light (RL) (R: RL = 4.44), far-red light (FRL) (R: RL = 0.05) and darkness (D). For RL and FRL treatments, acrylic Plexiglas boxes with different filters were used to do so, for darkness treatment Petri dishes were wrapped with aluminum foil. All boxes were placed in a controlled environment chamber Biotronette 84 (Labline Instruments, USA) with photoperiod 16/8 day and night, relative humidity at the inside of the chamber ~40 %. Germinated seeds in WL were counted daily with a Zeiss 475262 stereoscopic microscope until germination was depleted. For the other treatments, only the final number of germinated seeds was counted when seed under WL treatment reached asymptote to avoid the interruption of the treatments. We estimated accumulated germination percentage (AGP) for the WL treatment only, and final germination percentage (FGP) for all treatments. The criterion of seed germination was the emergence of the radical pole through the seed coat. We grouped seeds of one and two years (Group I) and seeds of six and seven years (Group II) based on observations made in a preliminary experiment where germination between seeds did not showed significant differences (data not shown).
Statistical Analyses. We used a Cox regression model to study the effect of storage time (Group I vs. Group II), temperature treatments and differences between species upon the time seed germination (AGP) takes to happen in WL treatment. In addition, to study the effect of storage time, light quality and temperature treatments in FGP we performed a quasi-binomial GLM with a logit-link function.
We also explored the effect of light quality. temperature treatments and collection site in seed germination. To do so, we performed a quasi-binomial GLM with a logit-link function with FGP as response variable. To avoid aging effect, we performed this analysis exclusively with data of Group I. Analyses were carried out using R statistical software v. 2.13.0 (R Core Team 2011).
Results
Storage time. Seeds older than 9 yr did not germinate in any case. In WL, seeds from Group I, of both species, began to germinate between days two and three. The highest AGP in N. coulteriana was at sixth day at constant temperature and at twelfth day in alternate temperature (Figure 3A); in M. foeniculaceum the highest AGP was at day five at constant temperature and by day six for seeds germinated at alternating temperature (Figure 3C). In group II, N. coulteriana at constant temperature began germination at day eight and reached the highest AGP at day fourteen, and do not germinate in alternate temperature (Figure 4A); M. foeniculaceum started germination between days six and eight at constant and alternate temperature, respectively (Figure 4C), and the highest AGP at day nine in constant temperature, and at day thirteen at alternate temperature. There was a significant effect of the storage time on AGP (see Table 1). The time needed to reach the highest AGP is slower in Group II compared to Group I (Figures 3A, C and 4A, C).

Figure 3 Accumulated germination percentage (AGP) by time intervals and final germination percentage for Group I of N. coulteriana (A, B) and M. foeniculaceum (C, D), exposed to different temperature and light quality treatments: WL = white light, RL = red light. Error bars represent SD.
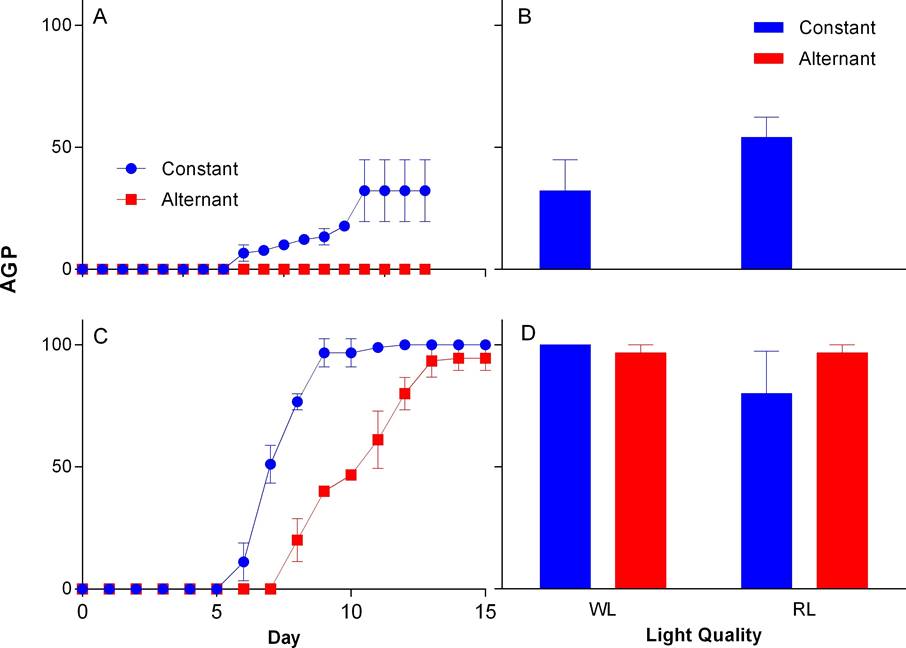
Figure 4 Accumulated germination percentage (AGP) by time intervals and final germination percentage for Group II of N. coulteriana (A, B) and M. foeniculaceum (C, D), exposed to different temperature and light quality treatments: WL = white light, RL = red light. Error bars represent SD.
Table 1 COX regression that shows the effect of species, temperature treatment and storage time in the accumulated germination percentage of N. coulteriana and M. foeniculaceum.
| Variable | χ2 | p | Risk ratio | 95 % lower-upper limits |
|---|---|---|---|---|
| Species | 2.8407 | 0.0919 | 1.8366 | 0.8722-2.9021 |
| Temperature | 0.2053 | 0.6504 | 2.2731 | 1.0794-3.5849 |
| Storage time | 4.4196 | 0.0355 | 1.1432 | 0.5431-1.8063 |
The FGP achieved by both species in Group I in WL was ≥ 90 %. In RL, seeds of M. foeniculaceum remained with high FGP ≥ 99% in both temperature treatments, while seeds of N. coulteriana decreased significantly (χ2 = 15.1188, p = 0.0001) to about 85 % germination at both temperature treatments compared with WL treatment (Figures 3B, D). In Group II, when seed were exposed to WL we found that the FGP for M. foeniculaceum was ≥ 96 % in both temperature treatment, unlike N. coulteriana which FGP reduces significantly (χ2 = 4.4196, p = 0.0355) at constant temperature (≥ 35 %) when compared to seeds of Group I and do not respond at alternate temperature. Under the RL treatment, M. foeniculaceum showed high FGP 90-98 % while N. coulteriana decreased its FGP to 54 % with constant temperature, meaning that the effect of alternating temperature reduced significantly (χ2 = 3.9411, p = 0.0471) germination of Group II of N. coulteriana (Figures 4B, D).
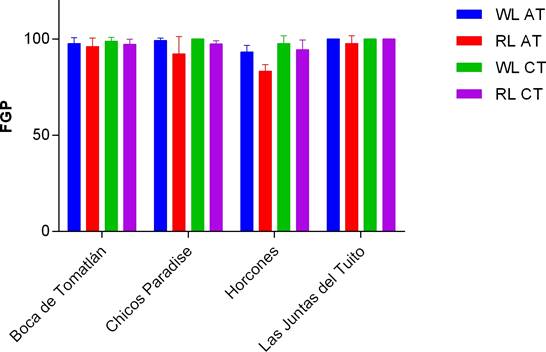
Collection site. We found that RL treatment in Group I reduced seed germination of N. coulteriana collected from Las Juntas del Tuito, having 71 % of germination compared to the 90 % of the seed germinated under the WL treatment (Figure 5). While seeds of M. foeniculaceum from Las Juntas del Tuito, keep germination percentages ≥ 90 % in both light treatments (Figure 6). For both species, seeds originated from Horcones river had percentages ≥ 95 % in both light treatments. There is a significant difference between seed germination of N. coulteriana from different rivers, when applying different light treatments (χ2 = 11.6231, p = 0.0007). In M. foeniculaceum there are not differences that could be attributed to collection site, keeping percentages ≥ 90 % in both light treatments. No significant difference was registered in the effect of temperature treatments on the germination capacity for any species or population (χ2 = 2.5327, p = 0.1115), nor in the interaction with light quality (χ2 = 0.306, p = 0.5801).
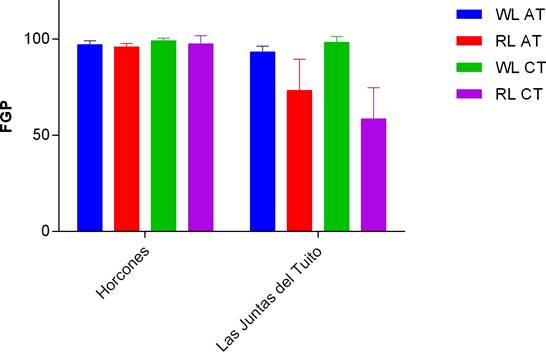
Figure 5 Final germination percentage for Group I of N. coulteriana seeds from distinct populations exposed to different temperature treatments (AT = alternant temperature; CT = constant temperature) and the light quality (WL = white light; RL = red light). Error bars represent SD.
Discussion
As far as we known in Mexico, and all over the world, Podostemaceae seed banking is null. There are only small collections of seeds belonging to researchers working with the family, and optimal long storage conditions for most of Podostemaceae species are still unknown. It’s important to generate information about podostemads seed storage and seed germination for its importance for the species reproduction and population maintenance (Philbrick & Novelo 1995). Here, in agreement with previous investigations, we found that seeds of N. coulteriana and M. foeniculaceum are positive photoblastic and have no latency (Philbrick & Novelo 1994, Reyes-Ortega 2009, Luna et al. 2012, Castillo et al. 2013).
Seeds of the Group I showed high germination percentages (> 90 %) regardless of temperature and light quality treatments. These levels are interpreted as a strategy that evolved in Podostemaceae in response to the difficulty of finding a suitable microhabitat for seedling establishment in the stressful environments that these species inhabit (Castillo et al. 2017). Moreover, storage time affects N. coulteriana to a larger extent when compared to M. foeniculaceum. Storing reduced germination of both species after 6 yr, however, this effect is more pronounced in N. coulteriana than in M. foeniculaceum, where storage time only influences the beginning of germination and the time it takes to reach the maximal germination percentage (AGP). This effect is greater that the reported by Reyes-Ortega (2009) who found that seeds of M. rubrum and M. schiedeanum (now classified as M. foeniculaceum) reduces its FGP to 87 % and 2 % respectively after 3 yr of storage. In the case of N. coulteriana, aging decrease FGP ≤ 54 % and even loss germination response in alternant temperature, this decay has also been reported for seeds of six months of storage by Castillo (2013). Delayed speed germination could be attributed to the time needed to repair the structures and restart metabolism due there’ve been deteriorating for longer (Waterworth et al. 2015). Seeds lose its viability when stored ≥ 9 yr.
We also found that N. coulteriana is more susceptible to aging, light quality and temperature treatments than M. foeniculaceum. WL result to be the optimal for reaching the highest FGP (≥ 90 % and ≤ 35 % respectively) for both species, while RL only decrease significantly FGP in Group I and II (85 % and 54 % respectively) of N. coulteriana, reducing FGP to 0 % when alternating temperature is applied to seeds of Group II. Constant temperature is also the optimal to reach high FGP unlike alternating temperature that reduces significantly FGP of N. coulteriana, having a significantly effect on those exposed to RL. Results point that light quality have a greater effect on seed germination of N. coulteriana seeds than in M. foeniculaceum. In this study we discard N. coulteriana as recalcitrant (Castillo et al. 2013) and propose that seeds of both species are orthodox in agreement with other authors (Philbrick & Novelo 1994, Reyes-Ortega 2009). We consider that the low metabolic activity, the absence of latency and mucilage formed in the seed upon contact with water, are characteristics that together reflect dynamics of seeds in their aquatic environment where germination and seed dispersal will be determined by the water level, which constantly changes over the years. Another feature that allows us to classify those seeds as orthodox is their ability to be stored at very low temperatures (-12 °C) without causing cell damage (Philbrick 1984, Berjak & Pammenter 2010) since it has been seen that may remain stored at very low temperatures without loss of ability for at least 2 months (Philbrick 1984).
There are limited and empirical information in Mexico about pollution of rivers where podostemads grow, some species can tolerate a degree of nutrient loading, but it is not known long-term impacts of these nutrients have on populations (Quiroz et al. 1997). Here we analyzed germination response collected from different rivers. We found that N. coulteriana population of Las Juntas del Tuito showed a diminution in his FGP under the RL treatment. Although we do not know what the cause of this behavior in the population of Las Juntas del Tuito might be, we suggest that this may be caused by the exposure of the mother plants to the shadow caused by the canopy covering the river at the location, influencing seed germination trough maternal effects (Castillo et al. 2017, Wulff 2017).
Altogether, ours results indicate that N. coulteriana is more susceptible to variation in storage conditions (e.g., storage time, light quality and temperature) compared to M. foeniculaceum. Moreover, we found that collection site is another factor that can affect seed germination after storing in Podostemaceae. These highlights the importance of testing germination conditions of Podostemaceae at species level as well as the collection site election in order to find optimal storage options to preserve germplasm. Even though N. coulteriana and M. foeniculaceum can potentially form ex situ seed banks up to 8 yr of age, seeds older than 9 yr did not germinate in any case.
These results point that long-term conventional ex situ seed germination storage is not a viable strategy to conserve germplasm of Podostemaceae. Ex situ plant conservation depends on the species, the methods employed and the desired storage time (Li & Pritchard 2009), so further studies should explore different mechanisms of maintenance as seed desiccation tolerance and storage at sub-zero conditions. Such studies need to explore the viability of seed banking using cryopreservation techniques that can help us to protect Podostemaceae from habitat loss and potential species extinction. Besides knowing the optimal storage conditions and time for seeds of podostemads, it will be also very important to know the capacity of the plants to stablish in their environment after being stored for several years in ex situ seed banks.











 nueva página del texto (beta)
nueva página del texto (beta)