Salvia circinata Cav. (syn. Salvia amarissima Ortega) is an endemic herbaceous plant widely distributed in Mexico (Martínez-Gordillo et al. 2013). In Santiago Huauclilla, Oaxaca, a Mexican region where traditional medicine using plants is extensively common, this plant is known as “bretónica”, and according to the citizens it is frequently used as an infusion for its analgesic and anti-inflammatory properties mainly to alleviate gastrointestinal illness that includes diarrhea and stomachache (Nambo 2015) and for the treatment of ulcers and diabetes (Castro et al. 2014, Flores-Bocanegra et al. 2017).
Phytochemical studies of S. circinata have reported the presence of diterpenoids such as amarissinins A-E (Bautista et al. 2016, Fragoso-Serrano et al. 2019) and teotihuacanin (Bautista et al. 2015, Fragoso-Serrano et al. 2019), and glucoside diterpenoids as amarisolides A-F (Maldonado et al. 1996, Flores-Bocanegra et al. 2017, Fragoso-Serrano et al. 2019). Flavonoids like pedalitin (Maldonado et al. 1996), apigenin-7-O-β-D-glucoside, the flavone 2-(3,4-dimethoxyphenyl)-5,6-dihydroxy-7-methoxy-4H-chromen-4-one, and new biflavone (Flores-Bocanegra et al. 2017).
Pharmacological studies have reported the cytotoxic effect of teotihuacanin isolated from S. amarissima as potent compound with multidrug resistance (MDR) modulatory activity in the vinblastine-resistant MCF-7 cancer cell line (Bautista et al. 2015). Cytotoxicity of the amarissinins has been also reported against five human cancer cell lines, as well as MDR modulatory activity in a breast cancer cell line (MCF-7) resistant to vinblastine (Bautista et al. 2016). In addition, the in vivo antihyperglycemic activity and the α-glucosidase in vitro inhibitory effects have been reported for the extract of S. circinata aerial parts and its flavonoids and clerodane diterpene glucosides (Flores-Bocanegra et al. 2017). However, scientific studies supporting the efficacy and security of the use of this plant for abdominal pain are lacking, so in this study we explore the acute toxicity in mice following the OECD (2001) and the pharmacological evidence of the antinociceptive activity of S. circinata and bioactive compounds evaluating extracts from different polarity in an abdominal pain model.
Material and methods
Plant material. Salvia circinata aerial parts were collected in Santiago Huauclilla, Oaxaca, in July 2014. This region is located at the parallels 17° 25’ and 17° 34’ latitude north and meridians 96° 56’ and 97° 08’ longitude west, and at altitude between 1,200 and 2,700 m (INEGI 2010). A voucher specimen (Number 16360) was identified by Dra. Martha J. Martínez Gordillo and deposited in the IMSS Herbarium of CDMX, Mexico.
Preparation of the extracts. Organic extracts were obtained by maceration of S. circinata, dry and ground aerial parts at room temperature, three successive extractions each 24 h were done using solvents (2.5 L) in increased polarity (hexane, ethyl acetate, and methanol analytical grade purchased in Tecsiquim, SA de CV, Mexico). Solvent excess was completely retired by evaporation in a rotoevaporator RII (Büchi Labortechnik AG, Switzerland) to obtain a final yield of the crude extracts (Figure 1). To identify chemical compounds involved in the pharmacological activity of S. circinata, samples of the crude extracts (3 mg) were subjected to a high-performance liquid chromatographic (HPLC-DAD) analysis. Since the hexane extract was obtained in a less yield than the ethyl acetate and methanol extracts (Figure 1), only these two were fractionated to isolate individual pure compounds (Figure 1).

Figure 1 Diagram showing chromatographical fractionation of the S. circinata extracts to isolate pure compounds.
Aqueous extract of S. circinata dried aerial parts was obtained by pulverizing 50 g of plant material and boiled in 500 mL of distilled water for 10 min. Afterwards, the liquid was filtered at room temperature and then frozen in liquid nitrogen to be lyophilized (HETO FD3, Heto-Holten A/S, Denmark) to obtain a total yield of 10.6 g.
High performance liquid chromatography (HPLC-DAD). Bioactive constituents of S. circinata were determined and quantified using a HPLC apparatus Agilent Technologies, series 1100 equipped with a diode array detector. A sample of each extract (3 mg) was dissolved in methanol (1 mL, HPLC grade from J.T.Baker, USA) and filtered into Acrodisc® syringe filters Nylon membrane, diameter 25 mm, pore size 0.45 μm to inject 15 µL of each solution.
For identification and quantification of terpenes, a Zorbax Eclipse XDB-C8 column (125 × 4.0 mm diameter and a 5 µm particle size) was used with a mobile phase of acetonitrile (MeCN, HPLC grade from J.T. Baker, USA)/water, 80:20 with a flow rate of 1 mL/min and temperature at 40 ºC. Equipment was calibrated at a wavelength of 215 and 220 nm in a running time of 21 min. Standard curves of calibration were built with five concentrations from 0.037 to 1.29 μg of standard terpenoids and amarisolide (99 % purity, determined by HPLC-DAD) obtained from S. circinata in this study. Other standards showing purity from 90 to 99 % were ursolic acid, oleanolic acid, α- and β-amyrin, and β-sitosterol purchased at Sigma-Aldrich (St. Louis Mo. USA). Interpolation was done with the ChemStation program, Agilent Tech version B.02.01.
For identification and quantification of phenolic acids, a Nucleosil 100 A column (125 × 4.0 mm of diameter and a 5 µm particle size). Mobile phase has a flow rate of 1 mL/min with a gradient of water at pH 2.5 using trifluoroacetic acid/MeCN: 0-10 min, 85 % water: 15 % MeCN; 10-20 min, 65 % water: 35 % MeCN; 20-23 min, 65 % water: 35 % MeCN at 30 ºC. The wavelength was fixed at 280 nm in a running time of 23 min. Standards of phenolic acids were chlorogenic, caffeic, ferulic, gallic, syringic, and vainillinic (purity from 90 to 99 %, Sigma-Aldrich, St. Louis Mo. USA).
For flavonoids identification and quantification, a Hypersil ODS C18 column (125 × 4 mm diameter and at 5 µm particle size) was used with a mobile phase flow rate 1 mL/min. The gradient consisted in water at pH 2.5 and trifluoroacetic acid (Sigma-Aldrich, St. Louis Mo. USA)/MeCN 0-10 min, 85 % water: 15 % MeCN; 20 min, 65 % water: 35 % MeCN; 25 min, 65 % water: 35 % MeCN at 30 ºC. Equipment was calibrated for detection at a wavelength of 254, 316, and 365 nm with a running time of 25 min. Standards of flavonoids like kaempferol, quercetin, rutin, luteolin, naringin, naringenin, phloretin and phlorizin were purchased by Sigma-Aldrich (Purity from 90 to 99 %, St. Louis Mo. USA). Pedalitin (80 % purity, determined by HPLC-DAD) was isolated and purified from the methanol extract of this study.
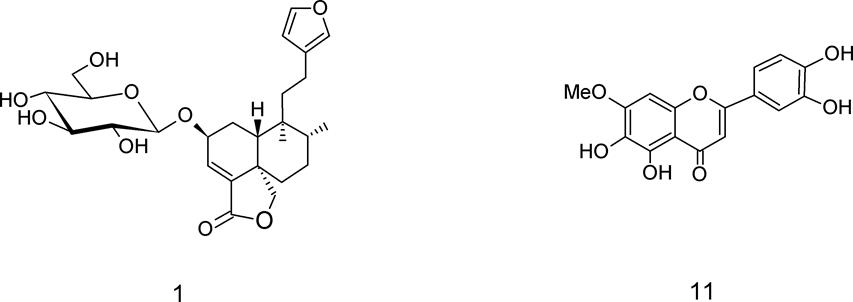
Fractionation of the organic extracts. The ethyl acetate (17.2 g) and methanol (31.7 g) extracts were partitioned using a chromatographic column packed with silica gel (Macherey-Nagel). The elution program started with hexane, to be continued with ethyl acetate, and finally methanol. The ethyl acetate fraction was separated on silica gel column chromatography in a proportion of 1:15, extract-eluent. The elution started with hexane followed by using a gradient of increasing polarity of hexane-ethyl acetate (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9), ethyl acetate (100 %), ethyl acetate-methanol mixture (9:1, 8:2, 7:3), and finally methanol. A total of 58 subfractions (100 mL each) were collected and then grouped by similarity according to their profiles acquired by thin layer chromatography (TLC) (Figure 1). From subfractions 51-56 eluted with ethyl acetate-methanol 8:2 and 7:3 was obtained compound 1, which was crystallized from methanol-acetone and analyzed trough ESIMS, 1H and 13C NMR. Identification of 1 was determined by comparision of its spectroscopic data with those described for amarisolide A, which were the same. Allowed purifying by crystallization a pure compound (m.p. 206 ºC, Fisher Johns equipment) that was analyzed by ESIMS in positive mode with a Cap LC coupled MicromassVR Q-ToF Ultima ESI system (Waters Corp., Milford, MA), as well as 1H NMR and 13C NMR analysis (Bruker, Avance DPX400). The NMR signals matched with that previous reported for this compound preliminary isolated from S. amarissima (Maldonado et al. 1996, Flores-Bocanegra et al. 2017).
Amarisolide A (1, yield 108 mg): white powder, mp 206 °C; 1H NMR (400 MHz, CD3OD) δ = 7.40 (t, J = 1.4 Hz, 1H, H-15), 7.36 (s, 1H, H-16), 7.01 (d, J = 6.5 Hz, 1H, H-3), 6.25 (dd, J = 1.4, 0.8 Hz, 1H, H-14), 4.62 (m, 1H, H-2), 4.56 (d, J = 7.8 Hz, 1H, H-1’), 4.41 (d, J = 8.0 Hz, 1H, H-19a), 4.07 (dd, J = 8.0, 2.0 Hz, 1H, H-19b), 3.95 (dd, J = 11.0, 4.2 Hz, 1H, H-6a’), 3.78 (dd, J = 11.3, 3.2 Hz, 1H, H-6b’), 3.50 (t, J = 8.5 Hz, 1H, H-3’), 3.43 (t, J = 9.0 Hz, 1H, H-4’), 3.35 (m, 1H, H-5’), 3.25 (m, 1H, H-2’), 2.68 (m, 1H, H-12a), 2.45 (d, J = 13.0 Hz, 1H, H-10), 2.37 (td, J = 13.8, 4.8 Hz, 1H, H-12b), 2.09 (d, J = 13.0 Hz, 1H, H-1β), 1.94 (d, J = 11.5 Hz, 1H, H-6α), 1.87 (m, 1H, H-8), 1.70-1.60 (m, 2H, H- 7β and H-11a), 1.54-1.50 (m, 2H, H-7α and H-11b), 1.35 (t, J = 12.0 Hz, 1H, H-6β), 1.31 (m, 1H, H-1α), 0.85 (s, H3-17), 0.65 (s, H3-20); 13C NMR (100 MHz, CD3OD) δC 172.2 (C-18), 142.5 (C-15), 147.2 (C-4), 139.5 (C-16), 132.6 (C-3), 126.4 (C-13), 112.8 (C-14), 103.5 (C-1’), 78.6 (C-3’), 77.1 (C-5’), 76.1 (C-2’), 74.2 (C-19), 72.9 (C-2), 70.9 (C-4’), 63.3 (C-6’), 47.5 (C-5), 40.2 (C-10), 39.5 (C-9), 38.9 (C-11), 38.2 (C-8), 36.1 (C-6), 29.2 (C-1), 28.2 (C-7), 19.1 (C-12), 16.9 (C-17), 18.6 (C-20); ESIMS: m/z 493 [M + H]+, 475 [M + H - H2O]+ (C26H36O9).
Constituents from ethyl acetate fraction obtained from the methanol extract by partition; were separated by column chromatography eluted with mixtures hexane-acetone (5:5, 4:6, 3:7, 2:8), acetone, and acetone-methanol 8:2. A total of 18 subfractions (100 mL) were obtained and grouped in 4 pools (Figure 1). Fraction eluted with hexane-acetone (3:7) gave a pale-yellow solid (m.p. 300 °C), and was analyzed by 1H and 13C NMR, and ESIMS. This compound was identified as pedalitin by comparison of their spectroscopic data with literature values (Maldonado et al. 1996, Flores-Bocanegra et al. 2017).
Pedalitin (11, yield 20 mg): pale yellow powder, mp 300 °C; 1H NMR (400 MHz, DMSO-d6), δ = 7.40 (m, 2H, H-2´ and H-6´), 6.91(d, J = 8.0 Hz, 1H, H-3’), 6.80 (s, 1H, H-3), 6.60 (s, 1H, H-8), 3.99 (s, 3H, -OCH3). 13C NMR (100 MHz, DMSO-d6) δ = 183.3 (C-4), 165.5 (C-2), 154.9 (C-7), 152.0 (C-9), 151.2 (C-5), 150.2 (C-4’), 146.5 (C-3’), 131.0 (C-6), 123.0 (C-1’), 119.9 (C-6’), 116.4 (C-5’), 113.9 (C-2’), 106.2 (C-10), 103.5 (C-3), 91.5 (C-8), 56.1 (-OCH3). ESIMS: m/z 317 [M + H]+ .
Animals. Male mice CD1 (25-30 g) available from “Facultad de Medicina, Universidad Nacional Autónoma de México” were used in this study. The animals were kept at constant room temperature (22 ± 1 °C) and maintained in a 12 h light/dark cycle. The animals were fed ad libitum with standard feed and water. Experiments were carried out in accordance with local (Project NC12.3280.0) and national (NOM-062-ZOO-1999) Ethical Committee Guidelines, as well as international (approved by the Institutional Animal Care and Use Committee based on US National Institutes of Health publication No. 85-23, revised 1985), regarding the care and use of animals for experimental procedures. For each treatment the animals were separated into groups of at least six mice. Each animal was tested once.
Drugs and reactives. Hexanic extract was resuspended in the vehicle [0.5 % tween 80 in saline solution (s.s.)]. Ethyl acetate, methanol, and aqueous extracts, as well as the reference drug Ketorolaco (SupraDol®), were dissolved in s.s. All treatments were administrated by intraperitoneal (i.p.) way in a volume of 10 mL/kg. Acetic acid (Baker) diluted at 1 % was used as nociceptive agent. Drugs were freshly prepared on the day of the experiments.
Acute toxicity. Mice receiving the acute administration of the methanol and aqueous extracts at a maximal dosage of 2,000 mg/kg i.p. allowed by OECD (2001) were observed for 14 days to register toxicological manifestations such as: loss of the consciousness, ataxia or respiratory depression, as well as possible death. At the end of the observation period of 14 days, surviving mice were euthanized to analyze macroscopically possible tissue alteration.
Nociceptive test (writhing). To build dose-response curves, all the extracts and pure bioactive compounds (1 and 11) were tested in independent groups at doses of 1, 10, 30, 100 and 300 mg/kg. The antinociceptive activity was evaluated 30 min after their administration. The test consisted in the induction of an exaggerated extension of the abdomen combined with the outstretching of the hind limbs as previously reported (Collier et al. 1968). This nociceptive behavior was induced after i.p. administration of 10 mL/kg of diluted acetic acid solution at 1 %. The number of writhes was immediately counted each 5 min for a total period of 30 min after the injection of the nociceptive agent (Viana et al. 2003).
Statistical analysis. The area under the curve (AUC) values were calculated from the respective temporal course curves obtained in the nociceptive behavior assays using the trapezoidal rule and they were considered as an expression of the nociceptive behavior in the writhing test. Data are expressed as the mean ± standard error of the mean (SEM) of 6 animals. The statistical analysis was performed using one-way ANOVA followed by Dunnett´s post hoc test. Graphpad Prism® version 6.0 for Windows (Graphpad Software, San Diego, CA, USA). A P < 0.05 was considered statistically significant.
Results
Phytochemical analysis. According to the phytochemical analysis using several chromatographic techniques, the presence of possible bioactive metabolites was obtained as follows:
Terpenoids.- S. circinata showed five terpenoids in the hexane and ethyl acetate extracts: amarisolide A (1) (Figure 2), ursolic acid (2), oleanolic acid (3), α-amyrin (4), and β- sitosterol (5) (Table 1); as well as in the methanol extract with exception of 4. The most abundant terpenoids in the hexane extract from major to lower were 2, 3, and 1; and in the ethyl acetate and methanol extracts was principal the terpenoid 1 (Table 1). In the case of the aqueous extract, the compounds identified were 1, 2 and 5, with the compound 1 as the most abundant (Table 1); all they were corroborated in the HPLC-DAD chromatograms (Figure 3).
Table 1 Analysis of terpenoids, phenolic acids and flavonoids obtained from Salvia circinata aerial parts
| Peak No. | Compound | Retention time (min) | Hexane (μg/mg) | Ethyl acetate (μg/mg) | Methanol (μg/mg) | Aqueous (μg/mg) |
|---|---|---|---|---|---|---|
|
1 2 3 4 5 |
Terpenoids Amarisolide A Ursolic acid Oleanolic acid α-amyrin β-sitosterol |
1.69 2.73 4.65 5.76 18.79 |
55.33 96.33 67.00 22.66 3.86 |
558.16 250.05 69.23 62.60 31.66 |
255.33 12.76 197.83 n.d. 40.30 |
182.60 7.35 n.d. n.d. 1.02 |
|
6 7 8 |
Phenolic acids Chlorogenic acid Caffeic acid Ferulic acid |
4.97 7.32 10.20 |
n.d. n.d. n.d. |
14.01 0.43 32.91 |
40.39 5.79 12.26 |
44.93 5.96 12.32 |
|
9 10 11 12 13 |
Flavonoids Rutin Phlorizin Pedalitin Quercetin Phloretin |
4.94 6.98 10.20 10.82 12.72 |
n.d. n.d. n.d. n.d. n.d. |
n.d. n.d. n.d. 23.33 8.24 |
18.33 27.28 134.06 n.d. n.d. |
21.43 10.39 5.16 n.d. n.d. |
n.d. = not detected.

Figure 3 HPLC-DAD chromatograms of the S. circinata showing terpenoids, phenolic acids, and/or flavonoids as identified compounds in the hexane (A), ethyl acetate (B, E, and H), methanol (C, F, and I), and aqueous (D, G, and J) extracts. Amarisolide A (1), ursolic acid (2), oleanolic acid (3), α-amyrin (4), β-sitosterol (5), chlorogenic acid (6), caffeic acid (7), ferulic acid (8), rutin (9), phlorizin (10), pedalitin (11), quercetin (12), phloretin (13).
Phenolic acids.- Phenolic acids were identified as follows: ferulic acid (8) was majority in the ethyl acetate extract that showed also caffeic acid (7) and chlorogenic acid (6) (Table 1 and Figure 3). These three compounds were identified again in the aqueous and methanol extracts, where 6 was the most abundant in both (Table 1 and Figure 3).
Flavonoids.- Quercetin (12) and phloretin (13) were identified in the ethyl acetate extract; whereas rutin (9), phlorizin (10), and pedalitin (11) (Figure 2) were obtained in the methanol and aqueous extracts. The compound 11 was the most abundant flavonoid in the methanol extract and compound 9 in the aqueous extract as corroborated by HPLC-DAD analysis (Figure 3).
Pharmacological analysis. Regarding to the pharmacological evaluation, all the treatments including organic extracts (Figure 4A-4F), aqueous (Figure 5A, B) and individual pure compounds (1 and 11, Figure 5C-5F), significantly decreased (P < 0.05) the number of writhes from a dosage of 1 mg/kg, except for the aqueous extract that produce its significant antinociceptive response after a dosage of 10 mg/kg (Figure 5A, B) in comparison to the group receiving vehicle. Antinociceptive response produced by extracts and the pure compounds resembled the effect of the reference drug ketorolac (1 mg/kg), the pharmacological response was dose-dependent in the evaluation with the medium polar (ethyl acetate) and polar extracts (methanol and aqueous) (Figures 4 and 5).
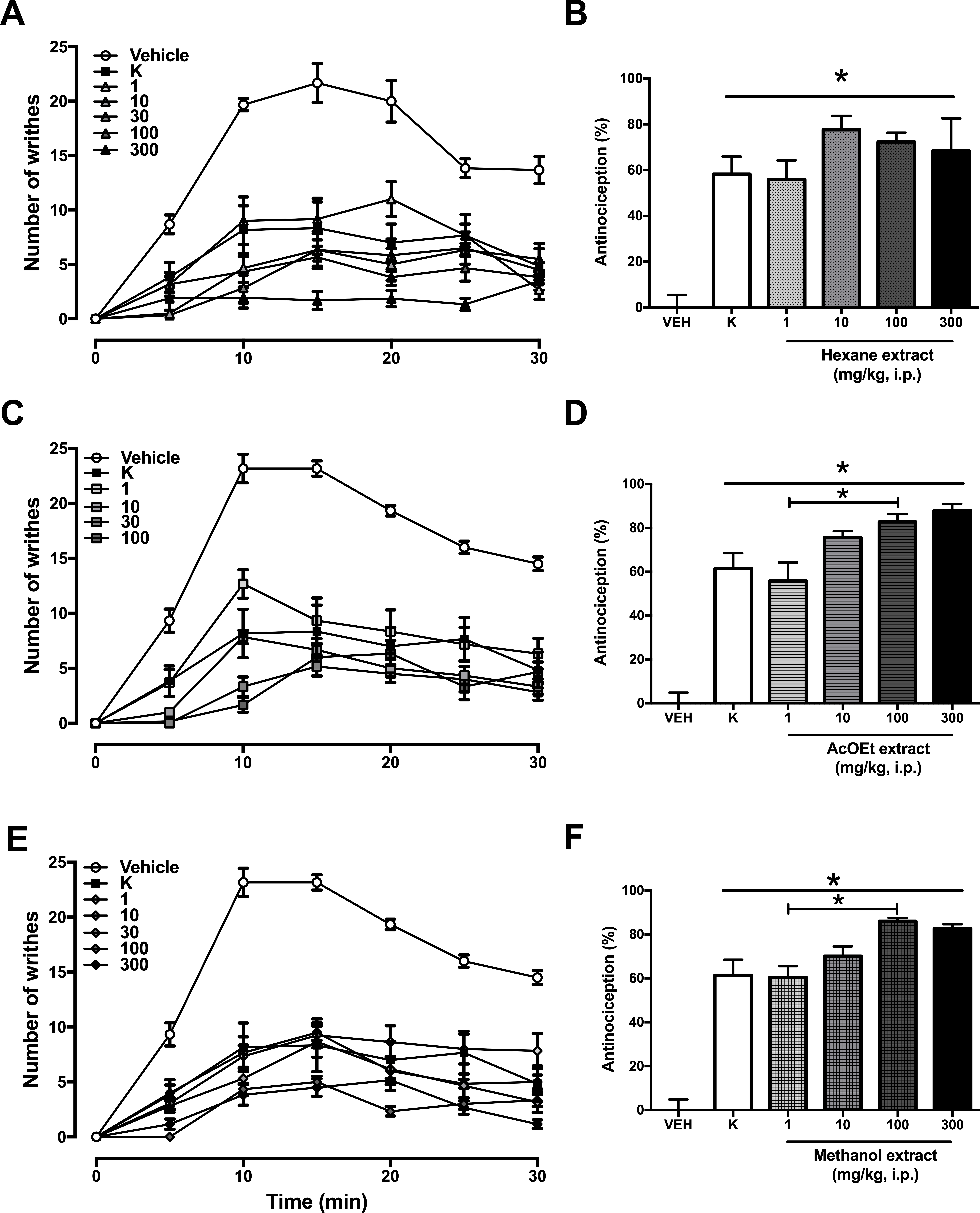
Figure 4 Temporal course curves (TCC) of the nociceptive activity induced by 1 % acetic acid in the presence of the vehicle, ketorolac (reference drug) and the crude organic extracts hexane (A), ethyl acetate (C), and methanol (E). The corresponding antinociceptive activity expressed as percentage from the TCC is indicated in the panels (B, D, and F, respectively). Each point and bars represent the mean ± standard error of 6 mice. One-way ANOVA followed by Dunnett´s test, *P < 0.05 was considered significant.
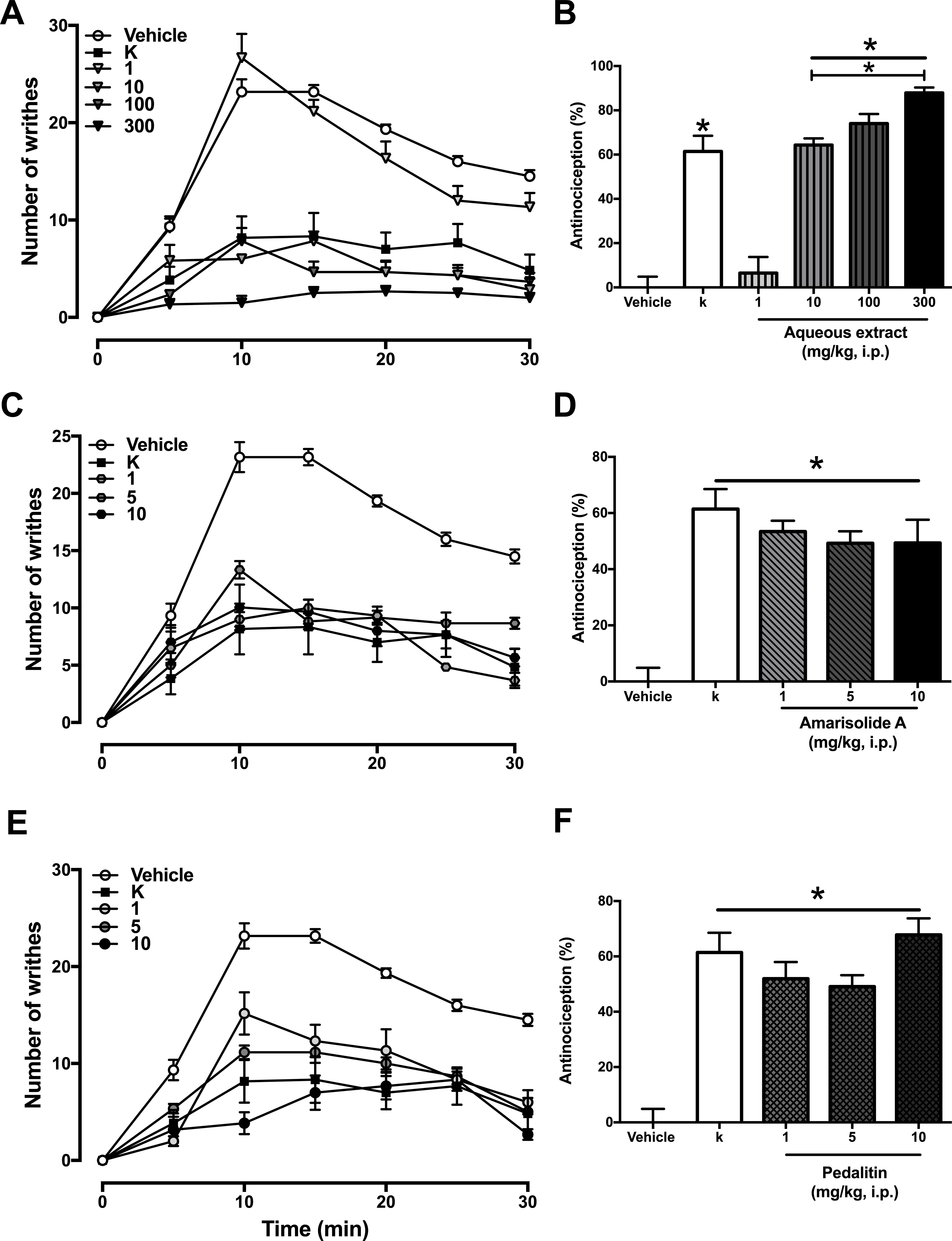
Figure 5 Temporal course curves (TCC) of the nociceptive activity induced by 1 % acetic acid in the presence of the vehicle, ketorolac (reference drug) and the aqueous (A) and individual pure compounds amarisolide A (C) and pedalitin (E). The corresponding antinociceptive activity in percentage obtained from the TCC is indicated in the panels (B, D, and F, respectively). Each point and bars represent the mean ± standard error of 6 mice. One-way ANOVA followed by Dunnett´s test, *P < 0.05 was considered significant.
Acute toxicity of the organic and aqueous extracts was calculated to be > 2,000 mg/kg. Mice did not show weight loss during the 14-days observation period and it was not observed macroscopic tissue injury in those surviving suggesting that low toxicity might be expected in the use of this species to alleviate abdominal pain.
Discussion
The present study demonstrates for the first time that organic and aqueous extracts, as well as some isolated and purified compounds from Salvia circinata, reduce nociception in mice. The three organic extracts (hexane, ethyl acetate and methanol) of S. circinata showed a similar pharmacological profile in the antinociceptive responses in mice treated with a range of doses in a logarithmic increase from 1 to 300 mg/kg showing a dose-dependent effect in case of the ethyl acetate and methanol extracts, but not with the hexane extract and the pure compounds amarisolide A and pedalitin.
Compounds 1, 2, 3 and 5 were identified in all the organic extracts, as well as in the aqueous extract, with an exception of oleanolic acid. While, α-amyrin was determined in the hexane and ethyl acetate extracts. In the case of amarisolide A, its presence was abundant mainly in the ethyl acetate and methanol extracts. Pharmacological antinociceptive activity for this terpenoids is lacking in literature; consequently, investigation about it is important to explore and describe. Recently, this terpenoid was isolated from aerial parts of S. circinata, and its antihyperglycemic activity was evaluated (Flores-Bocanegra et al. 2017). In the present study, the antinociceptive activity was tested using a model of abdominal pain in which it produced at least 50 % inhibition from a minimal dose of 1 mg/kg. The effect did not show increase by increasing doses and it was like that produced by different organic extracts even in those in which it was detected in greater abundance. It is possible that amarisolide A is one of the main bioactive metabolites responsible for the antinociceptive activity of this plant species. Nevertheless, there was detected other compounds that likely contribute to the final effect of the crude extracts. The mechanism of action of amarisolide A was not explored in this study; however, it was isolated from Salvia rubescens and reported as anti-inflammatory by inhibition of the elastase and myeloperoxidase enzymes (22 ± 4 % and 38 ± 10 %, respectively) at 100 μM in a model of murine inflammation (Rodriguez et al. 2005).
Other terpenoids identified in S. circinata were the ursolic and oleanolic acids; these compounds have been already reported in S. officinalis due to their antinociceptive activity, which was observed at the dose of 30 mg/kg, producing inhibition in the inflammatory phase of the formalin test, and antinociception in the mechanical allodynia induced by cinnamaldehyde possibly through TRPA1-receptors (Rodrigues et al. 2012). On the other hand, terpenoids both have been isolated as responsible bioactive metabolites of pharmacological antinociceptive effects of Rosmarinus officinalis (Martínez et al. 2012) and Agastache mexicana (Verano et al. 2013), showing dose-dependent effects with an ED50 = 1.6 mg/kg and 2.1 mg/kg, respectively. A participation of cGMP pathway and serotonergic neurotransmission through 5-HT1A receptors, as well as TRPV1 receptors were also considered in the antinociceptive responses of ursolic acid in the writhing and capsaicin tests in mice (Verano et al. 2013). In the case of oleanolic acid, its antinociceptive effects were mediated by an opioidergic and serotonergic, but not by adrenergic receptors, in glutamate-induced like pain (Park et al. 2013). Regarding β-sitosterol, its antinociceptive properties were responsible of the activity of Buddleja thyrsoides (Fialho et al. 2017) and Moringa oleifera at doses of 18, 25 and 35 mg/kg significantly attenuated hyperalgesia and tactile allodynia in a neurophatic pain model (Raafat & Hdaib 2017). Finally, α-amyrin obtained in the hexane and ethyl acetate extracts is other possible responsible bioactive metabolite of S. circinata since it has been reported that alone or combined with β- amyrin produces inhibition of pain like orofacial induced by formalin and capsaicin (Holanda-Pinto et al. 2008). Its antinociceptive effects have been associated with inhibition of COX-2 enzyme and diminution in the pro-inflammatory cytokines (Medeiros et al. 2007). The effects of the extracts were observed in a dose-dependent manner when fenolic acids and flavonoids were present together with terpenoids. These results suggest likely synergistic interactions in this plant that will be interesting to study in a future investigation.
This is the first time that antinociceptive activity is evidenced for S. circinata in agreement and reinforcing this activity already reported for other species of Salvia genus. For example, the S. wiedemannii chloroform extract from its aerial parts produced antinociceptive effects in the tail-flick and acetic acid-induced writhing tests in mice (Ustun & Sezik 2011); the S. officinalis hexane and chloroform extracts inhibited in a dose-dependent fashion the croton oil-induced ear oedema in mice (Baricevic et al. 2001), as well as in the aqueous and butanol leaf extracts in the hot plate and formalin tests in rats (Qnais et al. 2010). S. hypoleuca and S. limbata reported antinociceptive activity in the methanol and aqueous extracts of the aerial parts using the hot plate model in mice (Karami et al. 2013). Nevertheless, S. circinata demonstrated better antinociceptive potency in comparison to these species from the same genus, since we observed significant and maximal response from 1 to 10 mg/kg, i.p. in comparison to the significant response observed at 500 mg/kg, i.p. in the case of S. wiedemannii in the same nociceptive test (Ustun & Sezik 2011). Other species has demonstrated antinociceptive effect in other tests using higher dosage; for example: Salvia hypoleuca and S. limbata using a minimal dose of 100 mg/kg and a maximal of 1,500 mg/kg, i.p. (Karami et al. 2013). In contrast, there are species from this genus without antinociceptive efficacy as reported for S. halophila and S. virgata in the writhing test (Küpeli et al. 2008). This difference is probably associated with the chemical composition. According to the phytochemical background analyzed in S. circinata in this investigation, mainly the identified terpenoid content, might play an important role in the antinociceptive activity of this genus species suggesting its potential for the pain therapy and reinforcing the medical traditional use of this plant.
The chromatographic fractionation of the methanol extract allowed identify and purify also some flavonoids like pedalitin, which was the most abundant compound showing significant antinociceptive effects from a dose of 1 mg/kg. This pharmacological activity might be associated to the inhibition property on the mediators like NO, TNF-α and IL-12 production (Rao et al. 2009). Other biological activities of pedalitin are the antioxidant effects by inhibition of myeloperoxidase and as a scavenger of free radicals (Fernandes et al. 2008), as well as antihyperglycemic (Flores-Bocanegra et al. 2017).
Regarding to S. circinata aqueous extract, this was less active than the organics extracts since its significant response was observed at 10 mg/kg in comparison to 1 mg/kg, respectively. The most abundant chemical metabolites were amarisolide A, chlorogenic acid, and rutin. Chlorogenic acid has been involved in the antinociceptive effects of other species with this property (Küpeli et al. 2012, Martínez-González et al. 2016). In case of rutin, this flavonoid glycoside possesses antinociceptive properties mediated by central opioidergic neurotransmission (Selvaraj et al. 2014, Hernandez-Leon et al. 2016). This flavonoid has been even combined with a clinical analgesic to improve the efficacy against pain (Alonso-Castro et al. 2017).
The acute toxicity evaluation in vivo allowed to calculate a LD50 > 2,000 mg/kg, i.p., for all the aqueous and organic extracts of S. circinata, at least at a maximal dosage recommended in the normativity of the OECD (2001), placing these extracts in category 5 of the globally harmonized system of classification and labeling of non-toxic chemical products. These results are consistent with previous data conducted in the aerial parts of the same species using 5 g/kg, p.o. (Flores-Bocanegra et al. 2017) and in other Salvia species like in S. leriifolia seeds aqueous extract determined as LD50 = 19.5g/kg, i.p. in mice (Hosseinzadeh et al. 2003) and in S. officinalis leaves hydroalcoholic extract with a LD50 = 44.75 g/kg, p.o. (Rodrigues et al. 2012).
In conclusion, the present investigation gives pharmacological evidence of the potential use of S. circinata in the pain therapy due to the presence of diversity of bioactive compounds like terpenoids, phenolic acids, and flavonoids to validate the use of this species in the Mexican Traditional Medicine reported by the inhabitants of Santiago Huauclilla, Oaxaca, Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)