The relationship between a parasitic plant and its host involves a physical attachment aboveground (shoot-to-shoot) or belowground (root-to-root), which requires a unique organ, haustorium, developed by parasitic plants. This multifunctional organ attaches to the host and establishes a phloem (holoparasites) or xylem (hemiparasites) continuum to permit exchanges of water, nutrients, hormones, proteins and nucleotides (Kim & Westwood 2015, Furuhashi et al. 2016). Characteristic for the facultative hemiparasitic plants are the lateral haustoria (Westwood et al. 2010), swollen rounded structures attached to the host surface (Estabrook & Yoder 1998) with a complex internal structure consisting of three main regions: the haustorial neck (upper haustorium or exophyte), the endophyte and the base of the haustorium (Joel 2013). Because the haustorium is the key feature of parasitic angiosperms, its study aids in understanding parasitism. Through this vascular connection, parasites obtain carbon (C) and nitrogen (N), as well as other molecules from their hosts (Marvier 1996). Root hemiparasites are able to fix some or their total C through photosynthesis, and thus they are primarily parasitic for N (Westwood 2013). Facultative parasites obtain between 10 and 60 % of their C requirement from their hosts, depending on factors such as parasite growth stage, host species and the environmental availability of resources for hosts and parasites (Yoder et al. 2009, Westwood 2013). For instance, Rhinanthus minor L. and Euphrasia rostkoviana Hayne (Orobanchaceae) substract substantial amounts (ca. 25 to 50 %) of organic carbon from hosts such as maize and wheat (Testitel et al. 2010a). Similarly, chlorophyll contents in Thesium chinense Turcz. (Santalaceae) grown with Imperata clyndrica (L.) Raeusch. (Poaceae) or Gnaphlium affine (Asteraceae) were up to 3-fold higher than that grown without host. Moreover, differences in chl a/chl b were attributed to the host species (Luo & Guo 2010). The acquisition of fixed N from another plant is perhaps the greatest benefit of parasitization for hemiparasites. By tapping into a host, the parasite gains access to an expanded root system and a direct supply of fixed inorganic N (Westwood 2013). Facultative hemiparasites can absorb mineral N from soil using their own roots. Nevertheless, haustoria provide a significant supplement: Rhinanthus minor acquires 17 % of its N from the xylem of its grass host Cynosurus cristatus L. (Poaceae) (Cameron & Seel 2007). Using transgenic tomato plants expressing green fluorescent protein (GPF) it was demonstrated bi-directional long-distance movement of proteins between the parasitic weed Philipanche and its host (Aly et al. 2011). Thesium chinense grown with I. cylindrica or Prunella vulgaris L. (Lamiaceae) had higher levels of minerals such as K, P and Cu (Luo & Guo 2010).
Castilleja tenuiflora Benth. (Orobanchaceae) is a root hemiparasitic plant of medicinal value (Sanchez et al. 2013; Herrera-Ruiz et al. 2015). Our group described the formation of haustoria under field conditions without host attachment (Montes-Hernández et al. 2015). Baccharis conferta Kunth (Asteraceae) is a shrub described as a host of C. tenuiflora (Montes-Hernández et al. 2015). Here, we describe the anatomy and histochemistry of the host-parasitic interactions between B. conferta and C. tenuiflora, and the resulting changes in C-, N- and chlorophyll levels. This is the first study that characterizes a hemiparasitic interaction in the field and specifically between C. tenuiflora and B. conferta.
Materials and methods
Plant materials. Plant materials were collected in the Iztaccíhuatl-Popocatépetl National Park (N 19° 0.5´ 9.6'', W 98° 40´ 24.2'', 3,480 masl.), State of Mexico, Mexico, in December 2013. The study site presents high annual precipitation between 800 and 1,200 mm with annual average temperature of 14.3 ºC; the area has a sandy loam soil with predominant vegetation of pine-oak forest and grasslands. Some of the plant species found are: Bidens triplinervia Kunth, Abies religiosa (Kunth) Schltdl. & Cham. (Kunth) Mirb., Trisetum spicatum (L) K. Richt and Lupinus montanus Kunth (Montes-Hernández et al. 2015). Individuals of B. conferta and C. tenuiflora growing independently or in hemiparasite interactions: 1) C. tenuiflora (-H); 2) C. tenuiflora parasitizing B. conferta (+H); 3) B. conferta (-P) and 4) B. conferta parasitized by C. tenuiflora (+P) were randomly collected within an area of 10 × 10 m; plants were in vegetative state and similar in size (Table 1). Plants were identified as Baccharis conferta Kunth and Castilleja tenuiflora Benth. by specialists of the Universidad Nacional Autónoma de México (UNAM), associated at MEXU. Vouchers have been deposited in FCME (150228, B. conferta) and MEXU (13234, C. tenuiflora). For the anatomical and histochemical studies, C. tenuiflora haustoria attached to roots of B. conferta and roots of B. conferta were collected from 10 individuals. Fully extended young leaves of 2 to 3 cm in length localized in the upper part of the branches were collected from 10 individuals of both species to analyze C, N and chlorophyll contents. To determine the C and N levels, leaves were dried at room temperature, ground and sifted through a 4-mm sieve. Powder was stored at room temperature until use. For chlorophyll determination, leaves were fixed in the field with liquid nitrogen to prevent chlorophyll degradation.
Table 1 Mean values for growth characteristics of C. tenuiflora and B. conferta growing alone and in interaction conditions
| Growth characteristic | Castilleja tenuiflora | Baccharis conferta | |||||||
|---|---|---|---|---|---|---|---|---|---|
| -H | +H | Significance (F) | -P | +P | Significance (F) | ||||
| Plant height (cm) | 62.60 ± 10.89 | 65.90 ± 13.51 | 0.361 | ns | 112.1 ± 24.46 | 95.60 ± 22.32 | 2.481 | ns | |
| Carbon (µg/g DM) | 183 ± 22.63 | 296 ± 16.46 | 163.009 | * | 142 ± 18.73 | 118 ± 14.75 | 10.125 | * | |
| Nitrogen (µg/g DM) | 14.66 ± 5.76 | 19.32 ± 6.97 | 2.652 | ns | 12.79 ± 1.05 | 14.01 ± 1.93 | 3.073 | ns | |
| C: N ratio | 12.48 | 15.32 | 10.14 | 9.22 | |||||
| Total Chlorophyll (μg/mg FM) | 9.08 ± 2.56 | 11. 01 ± 3.34 | 2.109 | ns | 8.84 ± 2.33 | 9.55 ± 3.55 | 0.269 | ns | |
| Chl a/Chl b | 2.25 ± 0.14 | 2.24 ± 0.13 | 0.06 | ns | 2.25 ± 0.19 | 2.26 ± 0.18 | 0.041 | ns | |
Values are means ± standard deviation, n = 10. df = 19. * Indicates significant difference between the groups according to the comparison test of Tukey (P < 0.05), ns indicates not significant
-H: C. tenuiflora; +H: C. tenuiflora parasitizing B. conferta; -P: B. conferta; +P: B. conferta parasitized by C. tenuiflora
Anatomical and histochemical characterization. C. tenuiflora haustoria attached to roots of B. conferta were fixed in FAA (10 ml 4 % formaldehyde, 50 ml 96 % alcohol, 35 ml distilled water and 5 ml glacial acetic acid) for 24 h and dehydrated sequentially in butyl alcohol solutions over 24 h at different (30, 50, 60, 70, 85, 95, and 100 %) concentrations. Subsequently, haustoria were dried to the critical point in an EMITEC K850 dryer and were covered with gold in a coater, QUORUM Q150R-ES. Samples were observed on a Hitachi Model SU1510 scanning electron microscope. This microscope was used to observe the surface characteristics of the haustoria.
To observe the morphology of C. tenuiflora haustoria and B. conferta roots the samples were fixed as described before (Montes-Hernández et al. 2015) and then observed and photographed under a confocal stereomicroscope (LEICA model Z16 AP0A) with camera (KL 1500 LCD).
Haustoria were processed in the same way as described by Montes-Hernández et al. (2015). To observe nuclear structures, lignified, suberized and cutinized walls, samples were stained with safranin O, which produces a red color; fast green was used to visualize cytoplasmatic structures and cellulosic walls (Sandoval et al. 2005). For starch determination, sections were immersed in Lugol’s iodine and condensed tannins were stained with acidic vanillin. After deparaffinization, samples were treated with ruthenium red and essential oils showed a red coloration. In all cases, the stained samples were observed under a Zeiss Axioskop photomicroscope in bright field, phase contrast and polarization, and digitally photographed (Sandoval et al. 2005).
C and N determinations. For the determination of C and N levels, 0.5 g of powdered leaves were used. The C content was determined by the calcination method and the N content was determined by the semi-micro Kjeldahl method. The results are expressed in µg per g biomass dry matter (DM).
Chlorophyll determination. The total chlorophyll concentration was determined by the method proposed by Lichtenthaler (1987) with some modifications. Briefly, 15 mg of fresh tissue was manually crushed in a porcelain mortar to a fine powder. The powder was mixed with 1 ml of acetone 80 % (v/v), gently shaken and allowed to stand for 30 min. Subsequently, it was centrifuged at 16,060 ×g (Heraeus®, Biofuge fresco) for 10 min at 10 °C, and the supernatant was recovered. The total chlorophyll concentration was determined by measuring the absorbance of the supernatant in a UV/VIS spectrophotometer (UV 160-A, Shimadzu, Japan) at 663.2 and 646.8 nm and calculated by the following equations:
where A663.2 represents the absorbance reading at 663.2 nm and A646.8 represents the absorbance reading at 646.8 nm, and the chlorophyll concentration was expressed in µg per mg fresh matter (FM).
Statistical analysis. C, N and chlorophyll data were analyzed using a one-way ANOVA (growing independently or in hemiparasitic interaction) to determine the significance of the data. Comparisons between means within each species were determined using Tukey’s test with a significance level of 5 %. Statistical analysis was performed using IBM SPSS Statistics v 24 (IBM Corp).
Results
Anatomical and histochemical characterization of haustoria. Haustoria of C. tenuiflora were attached to both the main and lateral roots of B. conferta; however, the proportion of lateral roots having haustoria was higher (80 %) than the proportion of main roots parasitized by these structures (40 %). Usually five to seven haustoria surrounded the lateral root of B. conferta, while the main root was surrounded by one to three haustoria.
Haustoria were globular, with an approximate diameter of 0.5 to 1 mm, and presented haustorial hairs (Figures 1A, 1B). The haustoria had two clear regions the exophyte and endophyte, and the connection between B. conferta roots and C. tenuiflora haustoria was evident (Figure 1C).

Figure 1 Morphology of haustoria of C. tenuiflora (HCt) attached to the root of B. conferta (RBc). A Confocal stereomicroscopic view of globular HCt. B Scanning electron microscopic view of HCt showing haustorial hairs (hh). C Light microscopic view of a section of HCt showing the exophyte (Exo) and endophyte (End). Scale bar = 0.5 cm (A), 1.0 cm (B), 100 µm (C).
The exophyte comprised dermal, fundamental, and vascular tissues of hemiparasitic C. tenuiflora (Figure 2A). The dermal tissue had an epidermis with four to five layers of thin-walled and flattened parenchymal cells, which stored essential oils, stained in red (Figure 2B), and starch appeared as white spots in phase constrast views (Figure 2C). The fundamental tissue or hyaline body (cortex) consisted of thin-walled, isodiametric and cellulosic parenchymal cells. The parenchymal cells stored starch (Figures 2D, 2E). The vascular tissue, which is observed dark when stained with lugol, was located in the center of the haustorium and was surrounded by cells from the hyaline body. This tissue was comprised solely of xylem (Figure 2F) characterized by vessel elements with alternate edged pits (Figure 2G) and helical thickening tracheids (Figure 2I).
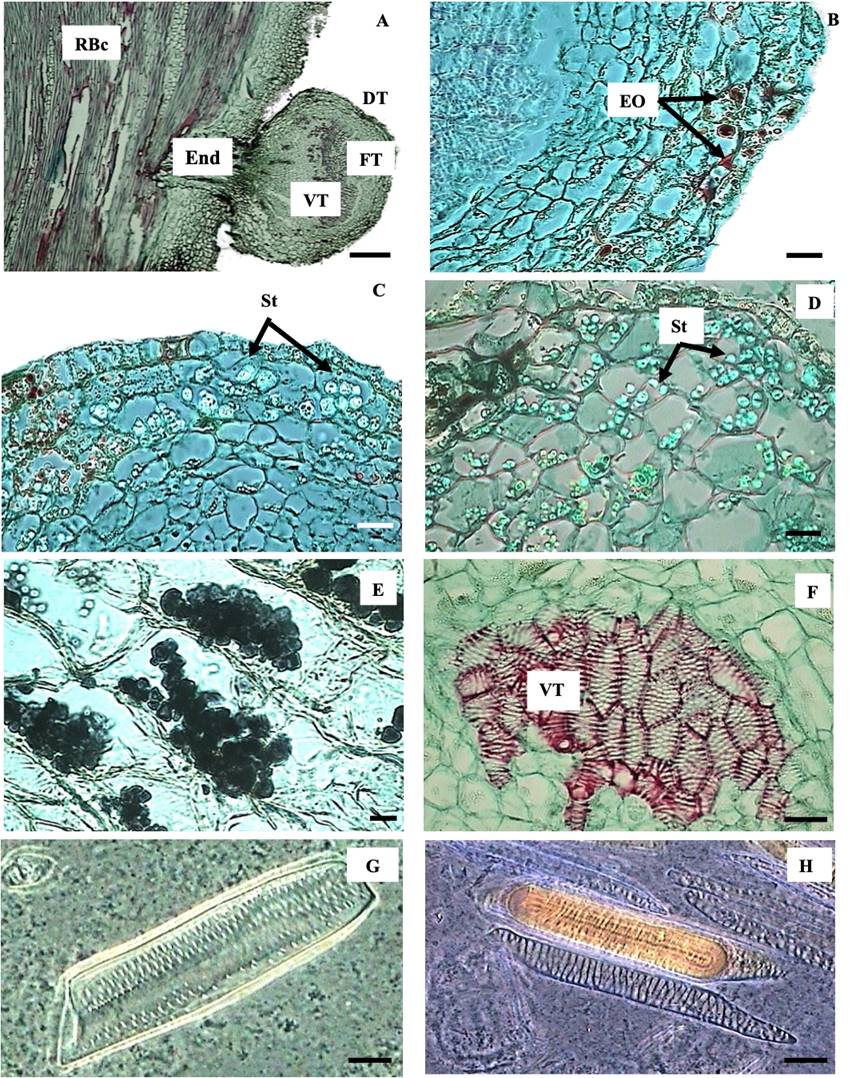
Figure 2 Anatomical arrangement of C. tenuiflora haustoria attached to the root of B. conferta (RBc). A Section showing the endophyte (End) and that the exophyte is comprised by dermal (DT), fundamental (FT) and vascular tissues (VT). B, C Phase contrast view of haustorium dermal tissue showing storage of essential oils (EO) and granules of starch (St). D Phase contrast view of fundamental tissue showing starch (St) storage. E Light microscopic view of fundamental tissue showing starch storage after lugol reaction. F Light microscopic view of haustorium vascular tissue stained in red surrounded by hyaline body stained in green. G Phase contrast view of alternate edged pits comprising xylem of the haustorium. H Phase contrast view of the tracheids found in the xylem of the haustorium. Scale bar = 300 µm (A), 5 µm (B, C, D, F), 0.5 µm (E), 7 µm (G, H).
The endophyte was located inside the root of B. conferta and consisted of parenchyma cells, vascular tissue and a meristematic zone. The parenchymal cells were thin-walled, isodiametric and cellulosic. The vascular tissue was characterized by xylem connected to the vascular tissue of the root of B. conferta root (Figure 3A). A zone of endophytic growth and development, characterized by the presence of large numbers of nuclei and a meristematic tissue, was observed (Figure 3B). All of the differentiation stages from newly formed to mature vessel elements (Figure 3C) of the alternate-pit type were found attached to the root xylem of B. conferta (Figure 3D). Parenchymal cells presented condensed tannins, observed in brown when they were stained with vanillin (Figure 3E), and starch (Figure 3F).
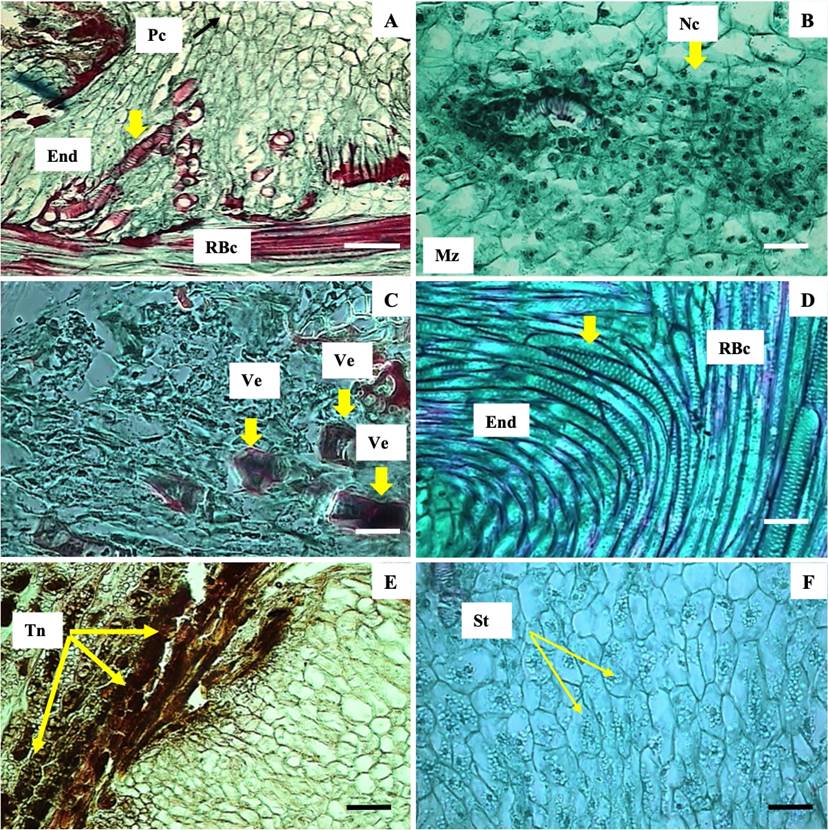
Figure 3 Haustorium endophyte of C. tenuiflora attached to the root of B. conferta. A Xilem of B. conferta (RBc) with xylem haustorium of C. tenuiflora (End) and parenchyma cells (Pc). B Meristematic zone (Mz) with presence of nuclei. C Differentiation of vessel elements (yellow arrows). D Vessel elements with alternate pits (yellow arrow). E Tannins (Tn, yellow arrow). F Starch (yellow arrow). Pc = parenchyma cells; End = Endophyte; RBc = root of B. conferta; Mz = meristematic zone; Nc = nuclei; Ve = vessel elements; Tn = condensed tannins; St = starch. Scale bar = 32 µm (A), 5 µm (B - F).
Anatomy and histochemistry of B. conferta root. B. conferta roots possessed rhizodermis, cortex, phloem and xylem (Figure 4A). The rhizodermis had at least 10 layers of isodiametric shaped and thickened wall parenchymal cells, some of which were suberized (Figure 4A). The cortex consisted of thin-walled isodiametric parenchymal cells. Phloem cells were elongated, thin-walled and had callose between phloem tubes. Xylem was characterized by vessel elements with thickened alternate-pits and opposite-pits. Both elements had thickened highly lignified walls (red coloration) and simple perforation plates. Alternate-pits were wider and shorter than opposite-pits (Figure 4B).
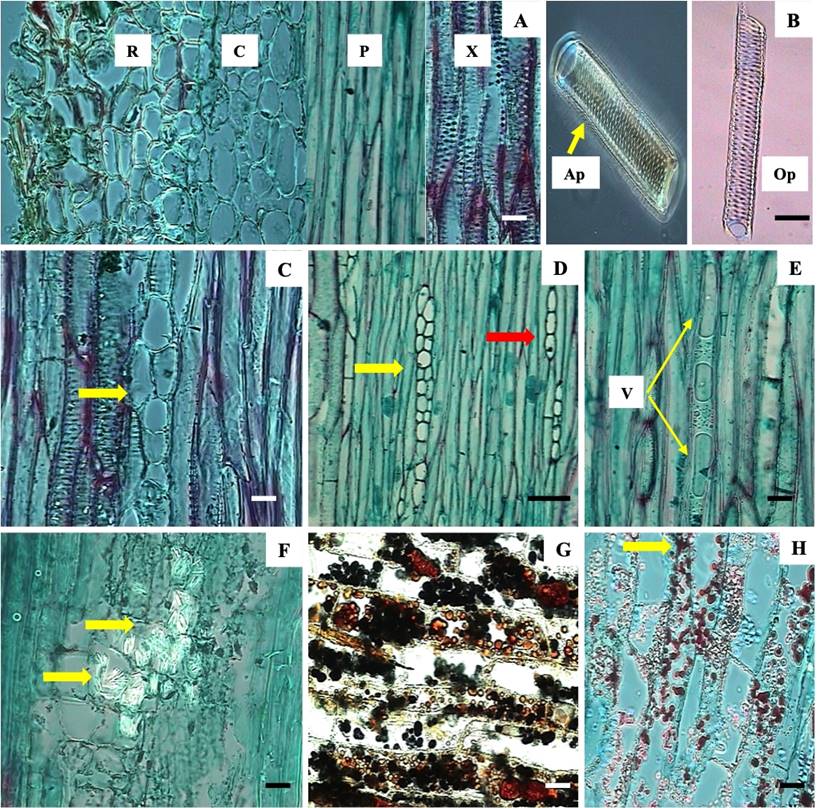
Figure 4 Root of Baccharis conferta. A Rhizodermis (R), cortex (C), phloem (P) and xylem (X). B Xylem with alternate (Ap) and opposite-pits (Op) and opposing lined pits with xylem radios (yellow arrow). C Phloem with multiseriate rays (yellow arrow). D Phloem with uniseriate (red arrow) and multiseriate rays (yellow arrow). E vacuoles (V). F oxalate crystals (yellow arrow). G Tannins (brown coloration) and starch (black coloration). H Essential oils (yellow arrow). R = rhizodermis; C = cortex; P = phloem; X = xylem; Ap = alternate-pits; Op = opposite-pits; V = vacuoles. Scale bar = 5 µm (A, C, E, F-H), 7 µm (B), 32 µm (D).
Baccharis conferta roots possessed xylem and phloem rays. These were uniseriate (a single row of cells) and multiseriate (more than one row of cells), and heterogeneous rays had erected and procumbent cells (Figure 4C and 4D). Thin-walled vacuoles near the root phloem in B. conferta were observed (Figure 4E). Other types and styloids of crystals, which were long, thin and sharp in form and composed of calcium oxalate were found (Figure 4F), while parenchymal cell rays stored tannins (brown coloration), starch (black coloration) (Figure 4G) and essentials oils (red coloration) (Figure 4H).
C, N and chlorophyll contents. The C content was significantly (P < 0.05) higher in individuals of C. tenuiflora parasitizing B. conferta (+H: 296 ± 16.46 (µg C/g DM) compared with those without the host (-H: 183 ± 22.63 µg C/g DM) (Table 1), while individuals of B. conferta parasitized by C. tenuiflora showed a lower C content (+P: 118 ± 14.75, µg C/g DM, P < 0.05) compared with those growing without it (-P: 142 ± 18.73, µg C/g DM). N content in individuals of C. tenuiflora (H-: 14.66 ± 5.76 μg N/g DM) was not affected by growing with a host (H+: 19.32 ± 6.97, µg N/g DM) (P > 0.05) (Table 1). The N concentrations in interacting and non-interacting individuals of B. conferta varied between 12.79 ± 1.05 (-P) and 14.01 ± 1.93 (+P) µg N/g DM (P > 0.05). The C:N ratio was higher in C. tenuiflora parasitizing its host than when growing alone. Similarly, the C:N ratio was higher in B. conferta without the hemiparasite (Table 1).
The total chlorophyll concentration in leaves of interacting and non-interacting individuals of C. tenuiflora varied between 11.01 ± 3.34 (+H:) and 9.08 ± 2.56 (-H) μg/mg FM (P > 0.05) (Table 1). Similarly, chlorophyll contents in parasitized and non-parasitized individuals of B. conferta (+P: 9.55 ± 3.55; -P: 8.84 ± 2.33 μg/mg FM) were not significantly different (P > 0.05) (Table 1).
Discussion
Parasitic plants invade host plants through specialized structures, called haustoria, and remove materials such as water, minerals, nutrients, hormones and nucleotides (Yoshida et al. 2016). Two characteristic regions constituted the haustoria of C. tenuiflora parasitizing B. conferta roots: the exophyte and the endophyte, as described for other hemiparasitic plants (Joel 2013). The exophyte is compossed of dermal, fundamental and vascular tissues of the parasitic plant. The main characteristic of the C. tenuiflora haustorial dermal tissue is the presence of starch and essential oils. Starch has been described in other haustoria of Castilleja (Dobbins & Kuijt 1973, Montes-Hernández et al. 2015) as a promotor of the haustorial growth (Qing-Liang et al. 2011). Because essential oils were not previously found in C. tenuiflora haustoria (Montes-Hernández et al. 2015) nor described in haustoria of other hemiparasitic plants, while there are several reports on the presence of essential oils in species of the genus Baccharis (Vannini et al. 2012, Xavier et al. 2013, Perera et al. 2016), we hypothesize that these compounds are traslocated from the host into C. tenuiflora.
The fundamental tissue, known also as the hyaline body, of C. tenuiflora presented similar characteristics to those described for the hemiparasites Pedicularis canadensis L. (Piehl 1963) and Rhinathus minor L. (Rümer et al. 2007). The role of the fundamental tissue in parasitic plants is not completely clear but it may be involved in the storage and translocation of primary and secondary metabolites (Visser et al. 1984). The vascular tissue of C. tenuiflora haustoria consists only of xylem vessel elements and tracheids, which agrees with the observations in other hemiparasites (Piehl 1963; Rümer et al. 2007).
Baccharis conferta roots contain parenchymal rays, xylem and phloem. Rays extend through the vascular root system and facilitate the radial transport of water, substances and gases (Van Bel 1990). The roots of B. conferta store oxalate crystals (mainly styloid), starch, condensed tannins and essential oils. Oxalate crystals are formed in the vacuoles of specialized cells, called crystal idioblasts, and may be involved in the regulation of calcium levels, plant protection and metal detoxification (Nakata 2003). The type, and the presence or absence of crystals are taxonomic characteristics in Asteraceae and other plant families (Kartal-Meric 2016). Oxalate crystals in the shape of styloids have been described before in leaves of Baccharis glaziovvi Baker (Jasinski et al. 2014), Baccharis brevifolia D.C. and Baccharis microdonta D.C. (Bobek et al. 2016). There are no studies reporting the presence of starch in the roots of Baccharis; however, this polysaccharide is used for growth and reproduction (Stitt & Zeeman 2012). Condensed tannins are found in the parenchymal rays of the roots of B. conferta. Tannins are abundant in the leaves of many plants and in vascular tissues, and they have a role in chemical ecology, as protectors against herbivorous insects (Salminen & Karonen 2011) and as allelopathics (Furlan et al. 2011). These phenolic compounds also confer structural rigidity to the plant, as well as providing protection against dehydration and decomposition (Espírito-Santo et al. 1999). Essential oils have been widely reported in leaves of this genus (Lago et al. 2008, Vannini et al. 2012). The chemical characterization of B. conferta is under further investigation.
C, N and chlorophyll contents increase when C. tenuiflora is associated with B. conferta. Thus, haustoria effectively acquire not only water and minerals but also C and N. Hemiparasitic plants have a double strategy for resource acquisition. They combine their own photosynthetic activities with the parasitism of other species with a host-to-hemiparasite flux of organic C (Tesitel et al. 2010b). In our study, C increases by 40 % in C. tenuifora individuals parasitizing B. conferta and decreases by 32 % in B. conferta individuals parasitized by C. tenuiflora. Hemiparasitic plants are green and perform photosynthesis; however, at the same time, they attack the vascular system of other species and exploit the host’s xylem sap, which contains water, mineral nutrients and organic C (Irving & Cameron 2009). Despite the low organic C concentration in xylem, the extensive amount of solutes acquired from the host can also result in significant heterotrophic C gains (Tesitel et al. 2010a), which has been observed not only in Rhinanthus but also in numerous representatives of other root-hemiparasitic species, including Euphrasia (Tesitel et al. 2010a), Striga (Press et al. 1987) and Olax (Tennakoon & Pate 1996). The acquired C from host xylem sap may come from C attached to N-based solutes, amino acids, and organic acids (Marshall & Ehleringer 1990). The acquisition of C and N (and other nutrients) may also be linked and regulated by the parasites’ rate of photosynthesis (Tesitel et al. 2015). The actual product acquired from the host (xylem sap) does not contain large amounts of C to begin with, therefore C assimilation allows for the further growth of the parasitic plant, as evidenced by an increase in leaf area, delay in leaf senescence and increase in RUBISCO activity (Watling & Press 1997). However, C acquisition depends on many factors, such as the development stage of the parasitic plant, the host species and environmental conditions (Tesitel et al. 2011). C acquisition is difficult to follow, owing to its rapid metabolization in the parasitic plant into other compounds, such as hexoses, mannitol and starch (Westwood 2013).
The acquisition of N in associated parasitic plants has been reported, and research has focused specifically on understanding the effects of N on parasitic plants. Cechin & Press (1993) reported an increase in the N concentrations in leaves of Striga hermonthica (Delile) Benth., associated with Sorghum sp., compared with leaves of non-associated S. hermonthica. Furthermore, it was observed that the rate of photosynthesis of S. hermonthica increases with N concentration augmentation, owing to increase in chlorophyll and RUBISCO activity (Cechin & Press 1993). These data are consistent with the results obtained in this work, in which the concentrations of N (17 %) and chlorophyll increased slightly in leaves of C. tenuiflora growing in hemiparasitic relationships with B. conferta (no significant difference). Thus, it was hypothesized that the increase in the N and chlorophyll contents may have significant effects on the rate of photosynthesis in C. tenuiflora. Studies on different species of parasitic plants (Bartsia trixago L., R. minor, S. hermonthica and Thesium chinense Turez) indicate that the N content is greater when parasites interact with their hosts, and that a high content N content is reflected in the increase in the aboveground biomass, photosynthesis rate and chlorophyll content. Additionaly, it is also reflected in higher growth and reproduction rates for the hemiparasites (Luo & Guo 2010).











 nueva página del texto (beta)
nueva página del texto (beta)


