Palm trees, an iconic feature of desert oases around the world, are known to be represented by about 2,500 species in the Arecaceae family (Asmussen et al. 2006). They have been grouped into 189 genera, classified into five subfamilies, many of which are utilized in some way (food, timber, fuel, construction and ornamentals). In fact, palms have been recognized as one of the three most used plant families by human kind, following Poaceae and Fabaceae (Tregear 2011). Although most palm species are restricted to humid tropics, several groups occur in open savannas and desert oases (Klimova et al. 2017). Washingtonia is a genus classified into the Coryphoideae subfamily (tribe Trachycarpeae, subtribe Livistoninae; Guevara et al. 2014), which includes two species native to northwest Mexico, Washingtonia filifera Linden ex André, distributed in Baja California, and Washingtonia robusta H. Wendl., found in Baja California and Sonora (Nehdi 2011). Both species are cultivated as ornamentals around the world, particularly in California and in northwest Mexico, as well as in the Mediterranean basin, southern Europe, and some parts of Australia (Coşkuner & Gökbudak 2016).
Most palms in Sonora, Mexico, are found in remote oasis in rugged and isolated desert mountain ranges (Bogan et al. 2014). These unique habitats provide them with microclimatic conditions, while palms offer fundamental ecological services, such as food sources for pollinators, zones of high biodiversity, shelter for local and migratory birds, and food sources for diverse wildlife (Wehncke et al. 2009, Wehncke & López-Medellín 2014). Yet, expanding development and in some cases overexploitation of individuals (leaves from mature palms, fruits, and wood), as well as natural and human-caused wildfires threaten these species and their habitats (Felger & Joyal 1999). Two other palm species, besides W. robusta are found along the desert’s edge, Brahea brandegeei (Purpus) H.E. Moore and Sabal uresana Trel. (Felger et al. 2017). B. brandegeei is the most widespread of the three palms and presents the greatest abundance; Sabal uresana occupies less restricted habitats and is abundant. While W. robusta, the Mexican fan palm, occurs only in riparian habitats and is ecologically and geographically restricted to sites with water sources (Felger & Joyal 1999). The Mexican fan palm is endemic to Baja California Sur and Sierra El Aguaje, north of Guaymas, Sonora. Due to restricted native occurrence in isolated oases, its modern-day distribution is considered a relict of a once wider distribution (Felger & Joyal 1999, Klimova et al. 2017). It is a fast-growing palm with a thick, reddish trunk and large dark green shiny leaves. Inflorescences are long with small, white aromatic flowers, occurring by the end of summer, developing into dark-brown fruits; ellipsoid-shaped, 7.5-9 mm long, often ripening in fall-winter, which produce a thin and edible sweet pulp (date-like tasting), each fruit holding one seed (Coşkuner & Gökbudak 2016, Felger et al. 2017). It is recognized that insect pollination commonly occurs in palms (Listabarth 2001, Barfod et al. 2011), and beetles constitute the most important group of pollinators, followed by bees and flies (Henderson 1990, Barfod et al. 2011). According to personal observations, bees could be responsible for the pollination in W. robusta at the study site.
The Mexican fan palm is a keystone species in the oasis where it thrives, forming an important part of diet for many birds, bats, and some terrestrial mammals like coati, ringtail-cat, raccoon, coyote and gray fox (Wehncke et al. 2009), as well as offering habitat for many species of reptiles, insects, and migratory birds (Wehncke & López-Medellín 2014). It is economically important, since has been exploited for thatch, wood and have a widespread use as ornamental (Bullock & Heat 2006). Use and trade has been proposed as a key mechanism to provide incentives for this species conservation and the habitats they provide, as well as both timber and a wide range of non-timber forest products; In this way they constitute ecologically and economically important natural resources that can be use and profited from (Brokamp et al. 2011).
Palms provide many useful by products, in general, they are rich in oils, terpenoids and phenolic compounds. Mesocarp and endocarp oils from many palms include a range of volatile compounds and other terpenoids that are reported beneficial for health, such as phytosterols, carotenoids and pro-vitamin A, tocols and vitamin E and triterpene pentacyclics. Among the phenolic compounds, phenolic acids, resveratrol and other stilbenes, anthocyanins, flavones, flavonols, dihydroflavonoids, flavan-3-ol, procyanidins and lignans have been described in different tissues of this palm, especially in fruit pulp, seeds and leaves (Agostini-Costa 2018). According to Benahmed-Bouhafsoun et al. (2015) the phytochemical composition of methanolic extracts from W. robusta leaflets, rachis and roots showed the presence of tannins, quinons, coumarins and flavonoids; while tested negative to alkaloids. Despite being one of the most widely cultivated palms in the world, to our knowledge, so far nobody has investigated the chemical composition and nutritional potential of the Mexican fan palm fruits. In this paper we characterize the phytochemical attributes in its fruits to provide foundational data for the tropic webs that emanate from this locally and globally important palm species.
Material and methods
Collection. Ripe fruits of W. robusta were collected at two locations in Sierra El Aguaje: Barajitas Canyon (28° 03' 17.7'' -111º 09' 14.5'') and Nacapule Canyon (28° 00' 55.71'' -111º 03' 21.07''). Sampling included several fruits from one palm at each location, in the months of February and April of 2016. Samples were stored in hermetic plastic bags and kept at 4 ºC for eight months, until further analysis. Pulp and seeds from ripe fruits sampled at both localities were manually separated and kept in Petri dishes. Fruits from the two locations mentioned above were mixed to attain enough sample mass. Then, pulp and seeds were separated and analyzed for phytochemical determinations.
Extraction. Plant material was extracted according to Domínguez (1982). Pulp and seeds were dried, milled, and sieved through a Wiley mesh 20. A 5 g aliquot of sample was extracted with 100 ml (50 ml of methanol + 30 ml of HCl + 20 ml of distilled water) for 24 hours at room temperature. For alkaloid and saponin determinations, an aliquot of 1 ml was taken in triplicate for each test. Whereas for tannins, cyanogenic glycosides and flavonoids, another 5 g-aliquot was placed in an Erlenmeyer flask, and ethyl alcohol was added until the entire sample was covered to gain fluidity.
Proximal chemical analyses. Proximate analysis was done for moisture, fat, calcium and ash contents. They were carried out in triplicate, according to standard methods (AOAC 1990); proteins (N × 6.25) by Kjeldahl; ashes by incineration in a muffle stove; and sugar content was estimated by difference of mean values: 100-(sum of percentages of moisture, ash, protein and lipids) Nehdi (2011).
Phytochemical screening (secondary metabolites). Chromogenic agents were used for secondary metabolite identification, samples were prepared as described by Domínguez (1982). Alkaloid determination was done according to Martínez et al. (2003), in 1 ml of extract, two drops of Mayer reagents, with Wagner reagent or Dragendroff reagent added depending on each colorimetric test. Development of cream, orange and brown colorations in the precipitate after adding the respective reagent, indicated the presence of alkaloids. For tannins, 1 ml of the aqueous filtrate was placed into an assay tube, with 1 ml of the gelatin-salt reagent, centrifuged at 2,000 rpm for 5 minutes. Supernatant was discarded. The precipitate was dissolved in 2 ml of 10 M urea and added with 2-3 drops of 10 % ferric chloride. A precipitate formed after adding the gelatin reagent, yielded the appearance of green, blue, or black colors as a positive test for the presence of tannins. Saponins were determined according to Mena-Valdés et al. (2015) with some modifications; 3 g of ground sample were suspended in 20 ml of distilled water for 6 h and centrifuged at 3,000 rpm. Three 1 ml replicates were taken and vigorously stirred for 3 min; foam development being an indicative of saponin presence. Flavonoids were also determined following Harborne (1975), several magnesium filings were placed in an assay tube, 2 ml of filtrate were added, following the addition of several drops of HCL; the appearance of orange, pink, red, or violet colors is a positive test for flavonoids. Following the technique of Jaramillo-Jaramillo et al. (2016), cyanogenic glycosides were confirmed qualitatively through the change in color from yellow to red or yellowish red, due to the release of HCN, according to Singh (2010), on paper impregnated with a solution of carbonated picric acid (1 g of sodium carbonate + 100 mg of picric acid and water up to 10 ml), which covered the upper part of the test tube containing the fresh plant material with 1.5 ml of water and six drops of toluene, and left at room temperature for 2 h. Next, for the quantitative determination of cyanogenic glycosides, according to Nwokoro et al. (2010), 1 g of sample were placed in a container, with addition of toluene, to cover all sample. The HCN released product of cell destruction and the addition of HCl to 10 %, it was dragged by steam at 80 °C and picked up in a vial containing 5 ml of a sodium picrate solution (2.2 Mmol/l) adjusted to pH 11.8, with a solution of NaOH (0.1 mol/l). The resulting solution was diluted to 10 ML with deionized water; After 1 h, absorbance was taken at 550 nm, in a UV-Vis spectrophotometer (Spectronic Genesis 8, Rocherster, NY, USA). For the absorbance calibration curve NaCN solutions were used in water in a concentration range of 1-10 µg/ml. The same procedure was used for the plant material sample. These trials were conducted in triplicate.
Phenolic Compounds Extraction (PC). The extraction was done following the methodology of Mattila & Kumpulainen (2002), with some modifications, 0.2 g of dry sample were weighed, and added with 7 ml of methanol /BHT (2, (6) - Di-tert-Butyl-p-cresol) (2 g/L), as well as 85 % acetic acid (90:10). The sample was placed in a sonifier Bransonic 2510 (Bransonic Ultrasonic Co Danbury, USA) for 30 min. A 1 ml aliquot was taken at the end of the cycle to obtain the PC free fraction and kept in refrigeration. After this, an alkaline hydrolysis of the remaining residual was done, adding 12 ml of distilled water, 5 ml of NaOH (10 M), and nitrogen gas added at 16 psi. The sample was left at ambient temperature in constant agitation for 16 h. Once this period elapsed, sample pH was adjusted with HCl and proceeded to the PC extraction from the alkaline fraction hydrolysis, via three repeated washings with a mixture of 15 ml of ethyl ether and ethyl acetate (50:50). The liquid phase was obtained and concentrated by drying in a rotovapor (Yamato RE 200), the extract was re-suspended in 1.5 ml of methanol and kept refrigerated. For the acid hydrolysis, 2.5 ml of HCl was added to the extraction residue, following incubation at 85 °C for 30 min, it was allowed to cool down and adjusted to pH 2.0. The extraction of ethyl ether and ethyl acetate (50:50) was repeated and dried with 1.5 ml of methanol. Aliquots were used for determination of total phenol content, total flavonoids, and anti-oxidant capability.
Total Phenols (TP). Determination of TP was done according to Singleton & Rossi (1965). The assay was carried out with (NUNC 96), 30 μl of sample were placed in micro-plaques, with 150 μl of Folin reagent, and 120 μl of 7.5 % sodium carbonate, and kept in darkness for 30 min. Measurements were carried out in an Omega FLUOstar spectrophotometer (BMG Labtech, Chicago, IL, USA) at a wavelength of 765 nm, results are expressed as mg gallic acid equivalent (mg GAE/g).
Total Flavonoids. Total flavonoid contents were determined with colorimetric identification of the aluminum chloride method described by Zhishen et al. 1999, with some modifications, samples were placed in a micro plate (NUNC 96) to read the absorbance in the spectrophotometer FLUOstar Omega (BMG Labtech, Chicago, IL, USA) at 496-nm wavelength, results are expressed as mg quercetin equivalent (mg QE/g).
Antioxidant capacity. According to the method proposed by Brand-Williams et al. (1995) 2.5 mg of the stable radical DPPH (2.2-diphenyl-1-Picrilhidrazilo) were added in 100 ml of pure methanol. DPPH solution absorbance was adjusted to a value of 0.70 ± 0.02 at 515 nm with an Omega FLUOstar spectrophotometer (BMG Labtech, Chicago, IL, USA). Subsequently, 20 μl of sample were mixed with 280 μl of DPPH solution; they were kept in darkness for 30 minutes and the absorbance loss was measured again at 515 nm. The antioxidant activity was expressed as mg Trolox equivalent (water-soluble analogue of vitamin C; mg TE/g) that were calculated using a regression equation between the standard concentration and the percentage of inhibition of the radical DPPH.
Identification and quantification of phenolic compounds (PC). PC identification and quantification were done by ultra-resolution liquid chromatography, equipped with a photodiode-array detector (UPLC-DAD, Acquite H Class, Waters, Milford, Mass, USA) and read at 270 nm, using an UPLC BEH 18 column (1.7 µm, 3.0 × 100 mm), at a column temperature of 60 °C and an auto sampler at 5 °C. Two solutions were used as mobile phases: water with 0.5 % formic acid and 100 % methanol. The flow and gradient changes were followed according to the technique described by Velderrain-Rodríguez et al. 2018. All standards come from Sigma-Aldrich. Results were expressed as µg/g of dry weight.
Results
The total weight of collected fruits was 500 g; 151 g from Nacapule Canyon and 349 g from Las Barajitas Canyon.
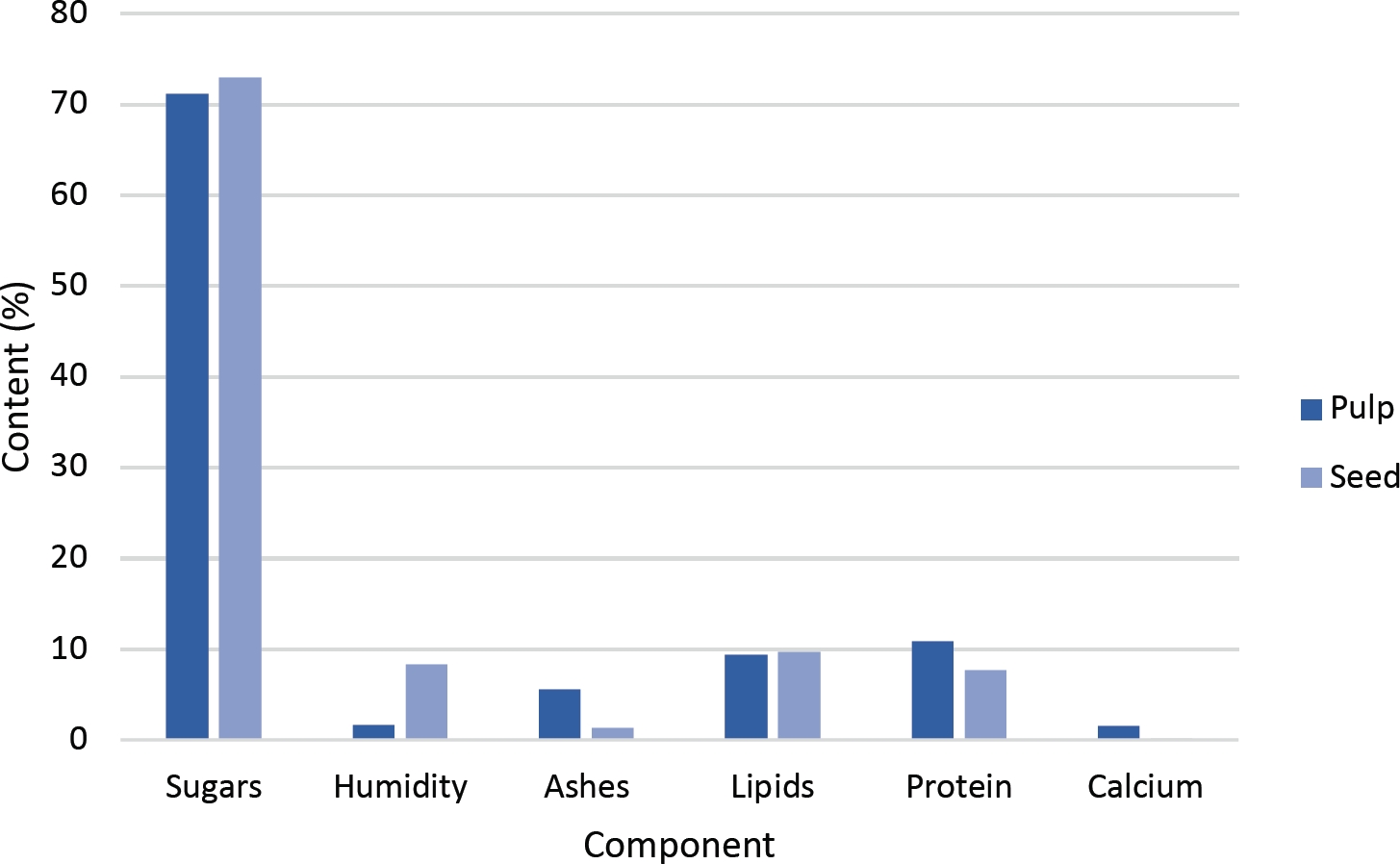
Pulp and seed proximal analysis. Main compounds found were sugars, followed by protein and fat. Protein and ash content were highest in pulp. Pulp and seeds proximal data showed, in that order, sugars 71 and 73 %, protein 10.8 and 7.4 %, humidity 1.6 and 8.4 %, ashes 5.5 and 4.3 %, fat 9.4 and 8.7 %, and calcium 1.5 and 0.2 (Figure 1).
Secondary metabolites (SM) and cyanogenic glycosides. The presence of tannins, alkaloids, saponins and flavonoids was identified, while cyanogenic glycosides reached 0.2 µg/g in pulp and 0.78 µg/g in seeds. The most abundant SM were alkaloids and flavonoids, and their presence was higher in seeds than in pulp (Table 1).
Table 1 Secondary metabolites in pulp and seeds of Washingtonia robusta.
| Tannins | Alkaloids | Saponins | Flavonoids | Cyanogenic glycosides | |
|---|---|---|---|---|---|
| Pulp | +++ | +++ | +++ | +++ | 0.20 μg/g |
| Seeds | ++ | ++++ | +++ | ++++ | 0.78 μg/g |
Scarce (+), Moderate (++), Abundant (+++), Highly abundant (++++).
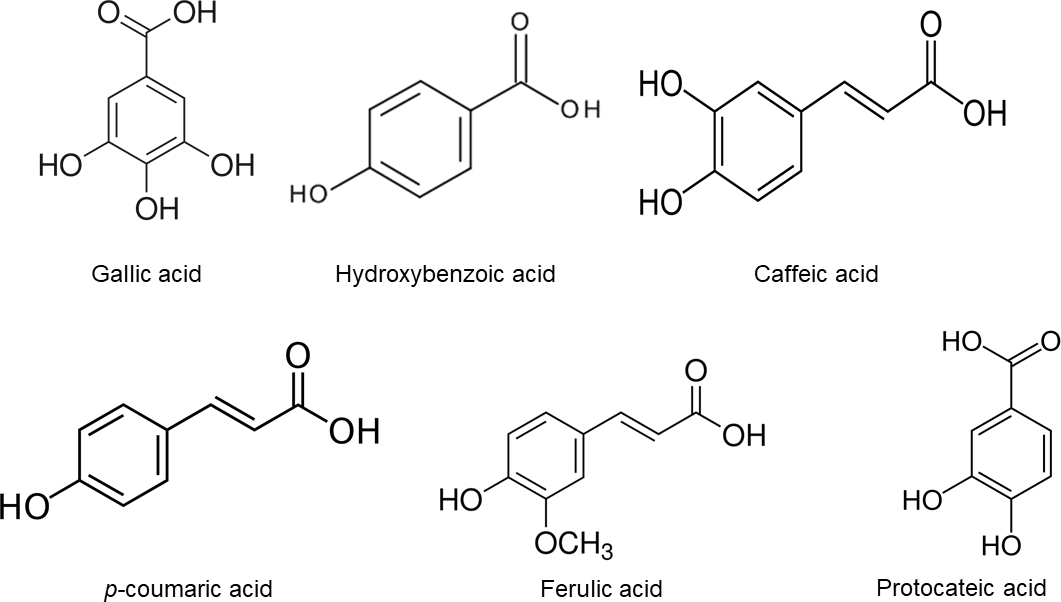
Phenolic compounds (PC). Total phenols reached 57.02 mg GAE/g in pulp and 222.10 mg GAE/g in seeds. Phenolic compounds were analyzed in the free, alkaline, and acid fractions of pulp and seed extracts. Although PC were not found in the free fraction, the alkaline and acid fractions yielded different composition and proportions. Five compounds (gallic, hydroxybenzoic, caffeic, -p-coumaric, and ferulic acids) were found in pulp, and only three (hydroxybenzoic, ferulic, and protocateic acids) were found in seeds. Furthermore, total PC content was practically the same in both tissues. In pulp however, the acid fraction was considerably higher than the alkaline fraction (Table 2, Figure 2).
Table 2 Phenols identification and quantification in pulp and seeds of Washingtonia robusta. Means are expressed in μg/g.
| Extraction fraction | |||||
|---|---|---|---|---|---|
| Sample | Compound | Free | Alkaline | Acid | Total |
| Pulp | Gallic acid | NF | 262 ± 10 | 278 ± 4 | 540 ± 14 |
| Hydroxybenzoic acid | NF | 184 ± 8 | 448 ± 5 | 632 ± 13 | |
| Caffeic | NF | 315 ± 22 | 445 ± 6 | 760 ± 28 | |
| p-Coumaric | NF | 295 ± 11 | 629 ± 9 | 924 ± 20 | |
| Ferulic acid | NF | 500 ± 3 | 1098 ± 2 | 1598 ± 5 | |
| Seed | Hydroxybenzoic acid | NF | 2709 ± 58 | 774 ± 8 | 3483 ± 66 |
| Ferulic acid | NF | 220 ± 1 | 109 ± 4 | 329 ± 5 | |
| Protocateic acid | NF | NF | 672 ± 1 | 672 ± 1 | |
NF: not found.
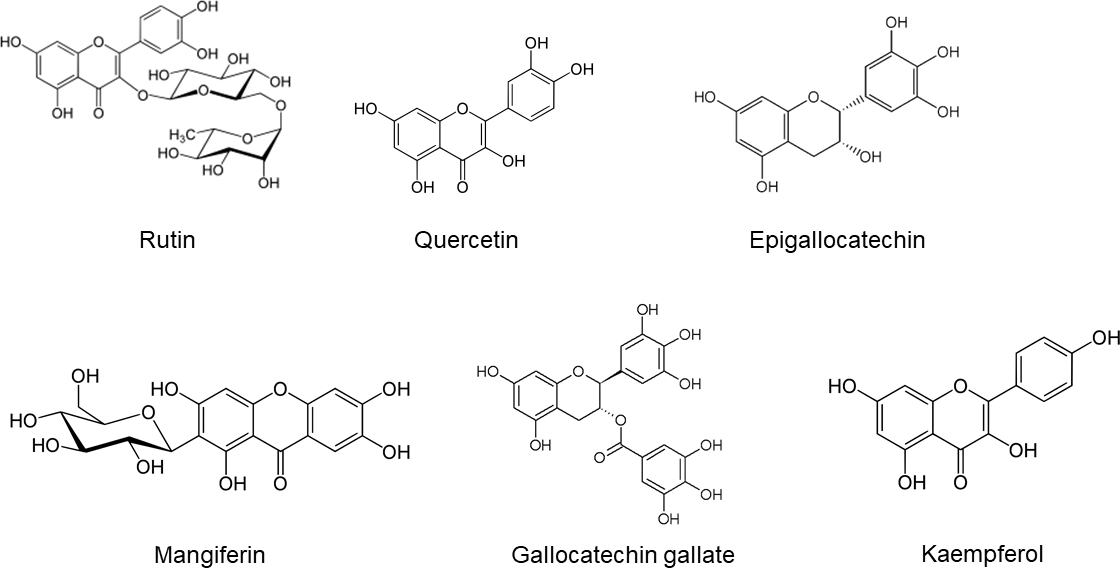
Flavonoids. Total containing of flavonoids in pulp was 248.96 mg QE/g and 2652 mg QE/ g in seeds. Flavonoid content was different between pulp and seeds. While rutin and quercetin were found in pulp, four flavonoids (epigallocatechin, mangiferin, gallocatechin gallate, and kaempferol) were identified and quantified in seeds. The highest flavonoid concentration was found in the free fraction of seeds. For the most part, seed flavonoids were minimal in the alkaline and acid fractions, while pulp flavonoids were found only in the acid fraction (Table 3, Figure 3). These phenolic acids have been identified according to their retention times and spectral characteristics of their peaks against those of standards (Figure 4).
Table 3 Flavonoids identification and quantification in pulp and seeds of Washingtonia robusta. Means are expressed in μg/g.
| Sample | Compound | Extraction fraction | Total | ||
|---|---|---|---|---|---|
| Free | Alkaline | Acid | |||
| Pulp | Rutin | NF | NF | 149 ± 3 | 149 ± 3 |
| Quercetin | NF | NF | 153 ± 9 | 153 ± 9 | |
| Seed | Epigallocatechin | 4387 ± 423 | NF | NF | 4387 ± 423 |
| Mangiferin | NF | 103 ± 32 | NF | 103 ± 32 | |
| Gallocatechin gallate | NF | NF | 148 ± 4 | 148 ± 4 | |
| Kaempferol | NF | NF | 53 ± 1 | 53 ± 1 | |
NF: not found.
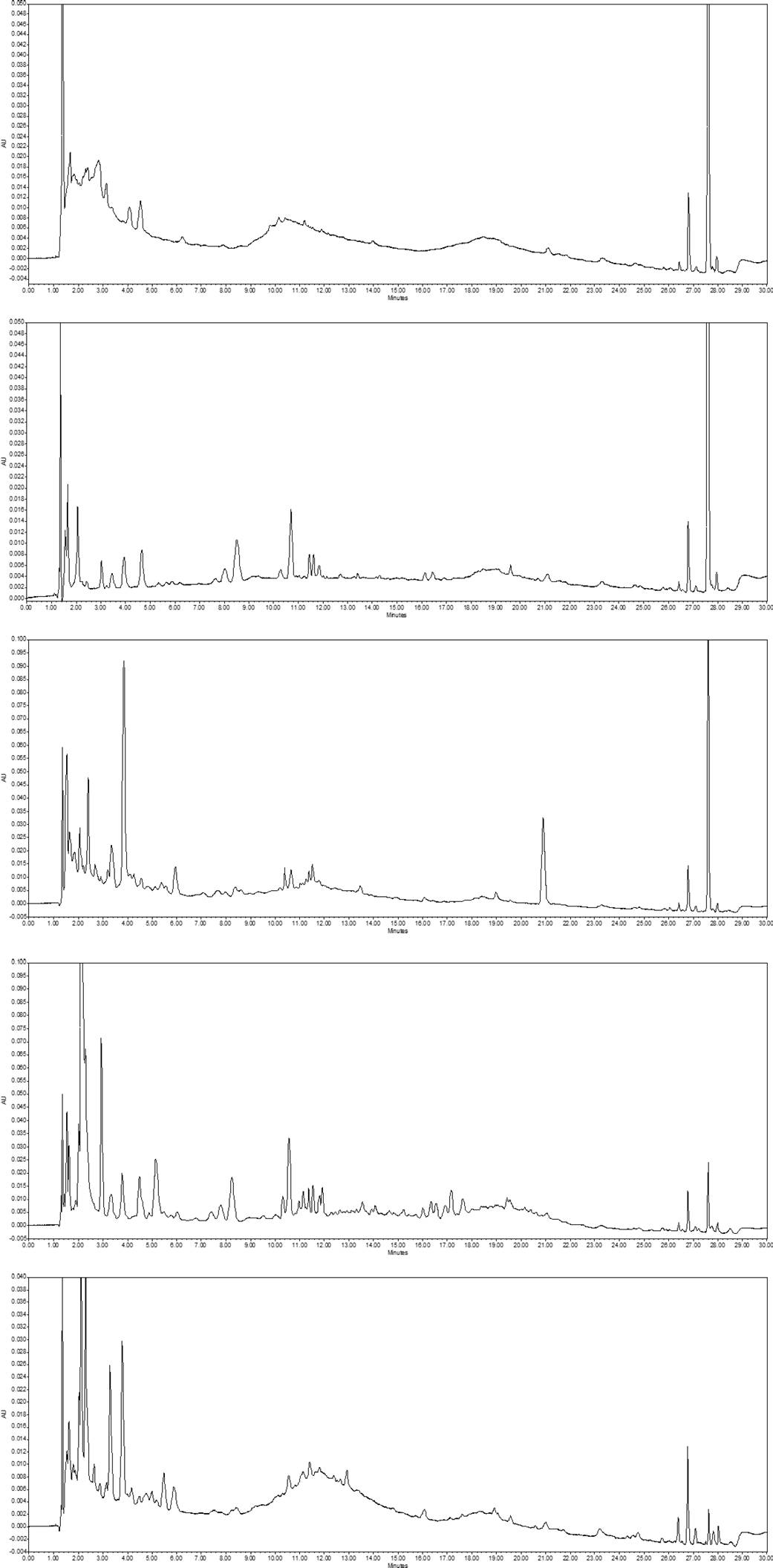
Figure 4 UPLC-DAD chromatograms at 270 nm, phen Centro de Investigación en Alimentación y Desarrollo, A.C. Hermosillo, Sonora olic compounds (PC) and retention time in free, alkaline and acid fractions in the pulp and seeds of Washingtonia robusta fruits. PC were not found in the free fraction of the pulp.
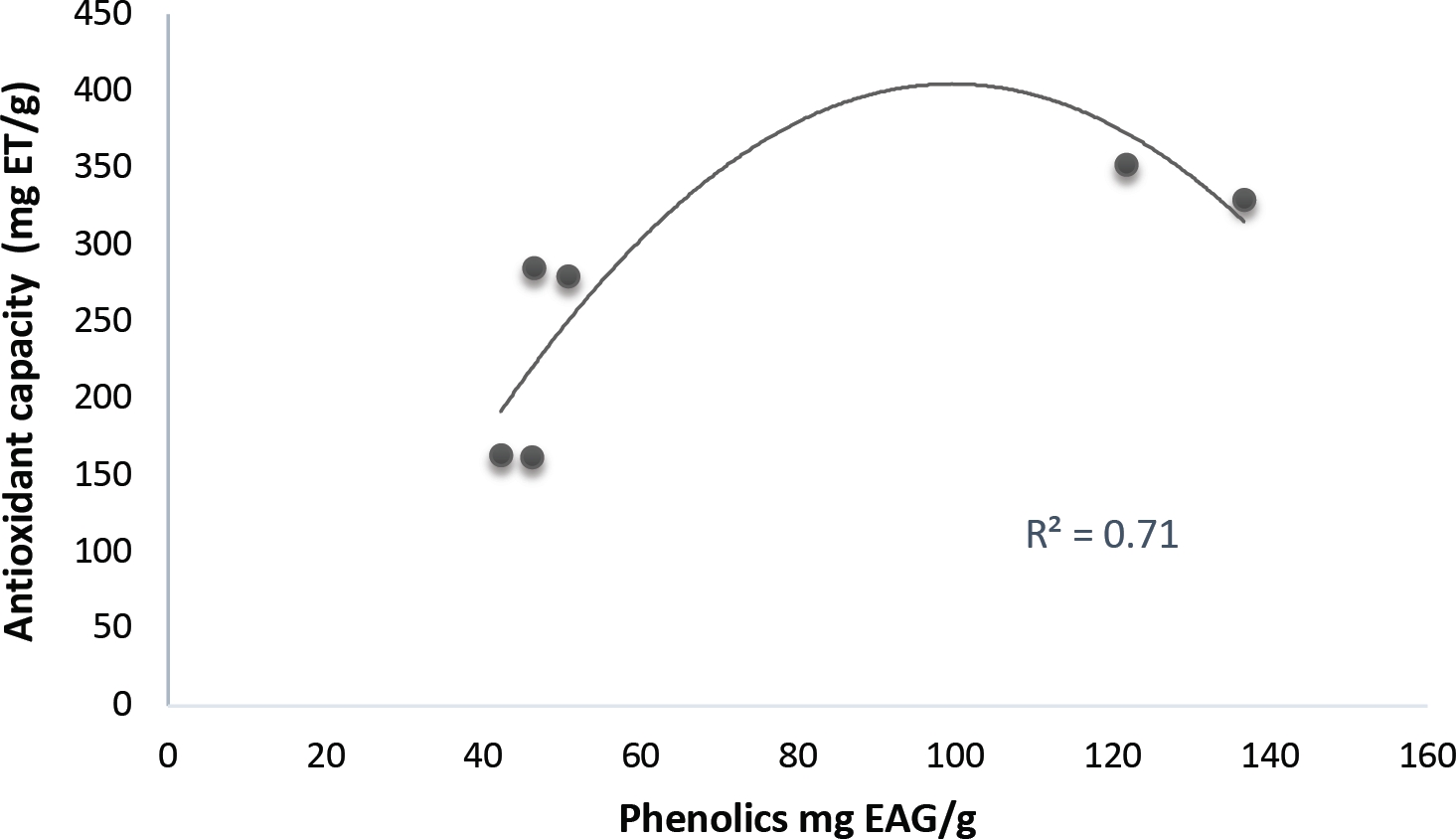
Antioxidant capacity. DPPH data show an activity mean in pulp of 491.6 ± 84.7 mg TE/g, while seeds reached up to 785.7 ± 90.8 mg TE/g, thus indicating a high antioxidant capacity, possibly correlated to the presence of the phenols of the pulp (Figures 5 and 6).
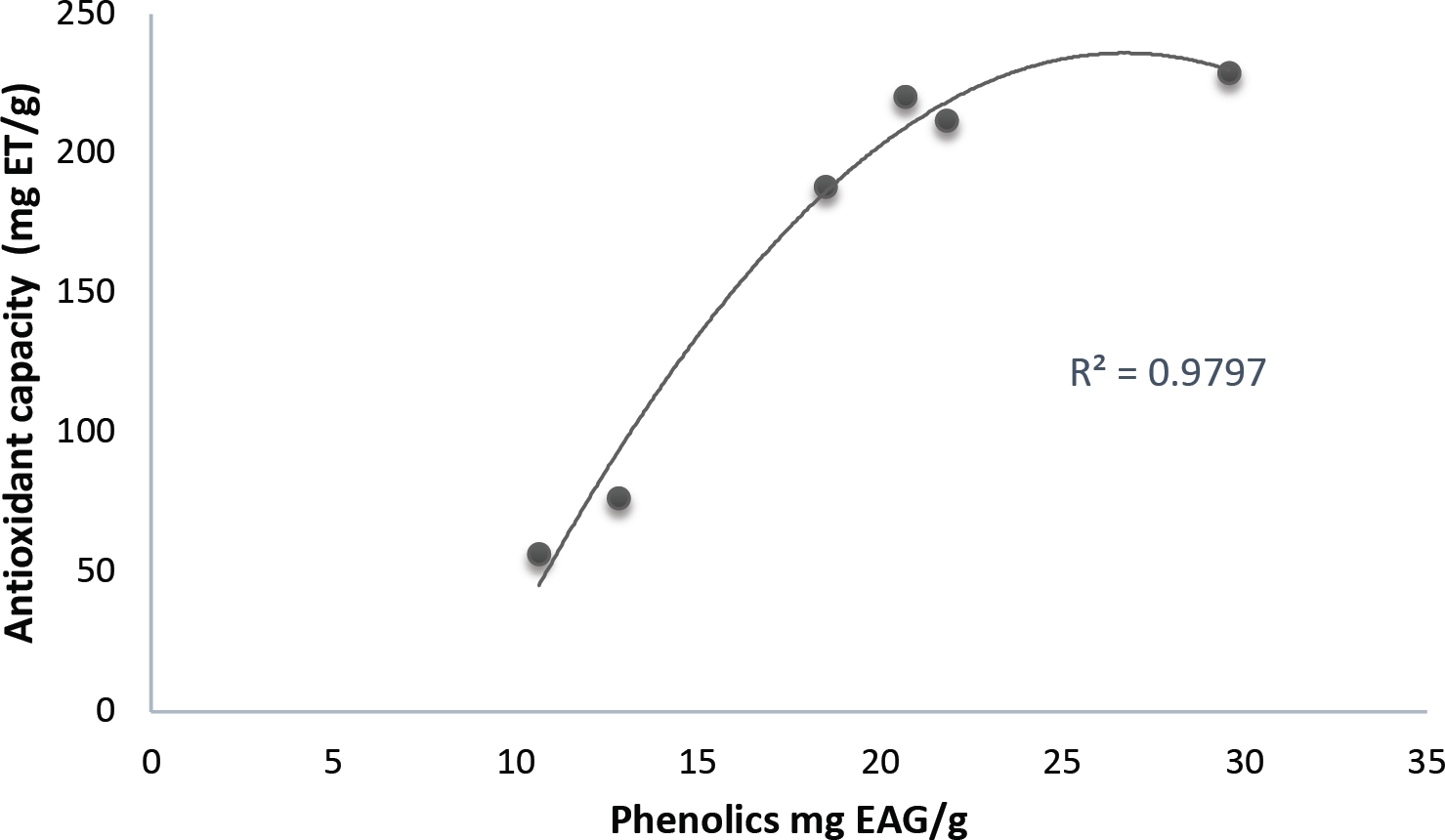
Figure 5 Non-linear correlation between the averages of phenolics (mg GAE/g) and the antioxidant capacity (mg TE/g) of the hydrolyzed fractions of fruit pulp of Washingtonia robusta.
Discussion
Macronutrients. Simple sugars are the main nutrients in most fruits (Martínez-del Río 1994), in W. robusta fruit pulp represents 71 % of sugar content; therefore, carbohydrates represent the highest nutritional contribution. Mazmanci (2011) reported that W. robusta fruits contain fermentable sugars, like glucose and fructose.
Biomass partitioning is pulp 53.7 % and seed 46.3 % (Coşkuner & Gökbudak 2016). Comparing with sugars in others edible fruits occurring in the study area like, Vitex mollis (92 %) (Montiel-Herrera et al. 2004), Washingtonia filifera (77 %) and the introduced Phoenix dactylifera (94 %) (Cornett 1987, Besbes et al. 2004) therefore W. robusta (71 %) represents a comparative sugar resource. The nutritional value content in seeds of W. filifera Nehdi (2011) and what we are reporting for W. robusta, indicate that these seeds are poor in protein, but rich in carbohydrates such as in Phoenix dactilyfera seeds (Besbes et al. 2004, Al-Farsi & Lee 2008). Fruits from both Washingtonia species are edible and have been a food source for ancient cultures; in Sonora inhabitants at San Carlos Bay, used the seeds for food, whereas the Seri ate the fruits (Rea 1981, Felger & Moser 1985, Hodgson 2001). According to oral history, starting in the mid-19th century (from 1697 to 1768), in Baja California the Jesuits implemented new agricultural systems introducing the date palm, which replaced consumption of native palm fruits (Rea 1981, Cornett 1987, Joyal 1996. Grenade et al. 2016). It has been acknowledged that they are an important natural resource, sustaining ecological interactions as well (Wehncke et al. 2009, Grenade et al. 2016). These fruits are considered as precious remnants of native desert cultures (Rea 1981). Ripe fruits generally remain in the fan palm through the winter (Felger et al. 2017), preserved by cold arid conditions (Murdoch et al. 2009), offering fruits with highly nutritious mesocarps and endosperms that constitute a critical reward for animal dispersers (Zona & Henderson 1989), consequently, they serve as an important energy source for wildlife through those rough conditions, having fundamental attributes as temporal availability, abundance and nutritional quality.
Antinutritional factors. During evolution, plants have developed the ability to produce secondary metabolites (SM). These are of vital importance because of their environmental interactions, reproductive strategies, and the defense mechanisms they trigger (Dirzo 1985, Cheynier et al. 2013). It is generally accepted that a primary function of secondary metabolites in immature fruits is defense from all consumers, the occurrence of such compounds in ripe fruits does suggest adaptive roles (Cipollini 2000). These compounds allowed species that employ them to establish an ecological relationship with the environment (Wink 2008). Nitrogen compounds, for example, such as alkaloids and cyanogenic glycosides are metabolized during germination as C and N sources for seedling development (Acamovic & Brooker 2005). In the seeds of W. robusta alkaloids are present is abundance, these compounds are often ecologically significant, likely providing a competitive benefit for seedlings. There is little information on the frequency and taxonomic distributions of cyanogenesis in palms (Lewis & Zona 2000), cyanogenic glycosides content in W. robusta seeds seem to be toxic in high intakes (Haque & Bradbury 2004), likely decreasing seed predation, which can increase the probability of germination and seedling development, by considering other organisms involved in these interactions, e.g., microbes, insect pests, vertebrate seed predators (Cipollini 2000). Tannins are high molecular weight phenols that have the capacity to complement carbohydrates and protein contributions (Cheynier et al. 2013), and the establishment of symbioses with nitrogen-fixing bacteria in the rhizosphere (Downey et al. 2006). Saponins on the other hand, are glycosides that provide a bitter taste to the plant parts they are found in (Cuéllar 1983). The abundance of tannins, flavonoids, and saponins found in W. robusta seeds represents a chemical advantage, for defense along germination and plantet development against environmental stress conditions in wild and cultivated palms, making them successful crops, since it is recognized that some of these compounds also have the function of suppressing establishment of other plants, providing protection against predation and inducing defense reactions to stressful biotic and abiotic agents (Saltveit 2017).
The Optimal Defense Theory, as outlined by McKey (1974), predicts that fruits should be among the most highly defended plant structures, because fruits have a high fitness value due to their direct link to reproductive output, and fruits may be at an increased risk of attack because their high nutritional content (Whitehead et al. 2013). Considering that the fruits are susceptible to attack during ripening, the two-common defense mechanisms in ripe and especially in unripe fruits are mechanical, in the form of a thick protective exocarps; and chemical, typically through toxic or unpalatable secondary metabolites. At the same time, fruits often increase their volatile compounds profile upon ripeness due to the need to attract seed dispersers (Nevo et al. 2017). The same was found for pulp and seed of W. robusta fruits. The presence of SM in pulp could also be important for protection against the damaging effect of UV-light and regulation of other vital functions (antioxidant activity and general and reproductive health). These attributes could be conveyed to the animals that feed upon them (Acamovic & Brooker 2005, Halliwell 2005).
Antioxidant compounds. Phenolic compounds (PC) are the most distributed and ubiquitous SM in the plant kingdom (DeGabriel et al. 2014, Cheynier et al. 2013). PC have been shown to provide health benefits including the provision of natural antioxidants, which is attributed to their ability to chelate metals and scavenge free radicals (Martínez-Valverde et al. 2000). Plant PC are also knowing to attract and recover nutrients, detect harmful radiation, and help regulate stress responses (Winkel-Shirley 2002). W. robusta pulp and seed have PC concentrations (57.02 mg GAE/g), and flavonoids (248.96 mg QE/g), which comparing with the PC in chontaduro fruit, Bactris gasipaes (30 mg GAE/100 g) (Agostini-Costa 2018), and date palm (239.5 mg/100 g) (Al-Farsi & Lee 2008) are considered to be a good source of total phenolics; and pulp and seed antioxidant capacity correlates positively with this PC content (Figures 5 and 6), according to Vasco et al. (2008) DPPH antiradical efficiency, coincides with samples with high and medium total soluble phenolic content, measured by the Folin-Ciocalteu method, which in turn neutralize free radicals and chelate metals, in addition to other biological properties as antimicrobial agents, attracting pollinators or seed dispersers (Okawa et al. 2001, Winkel-Shirley 2002, Silva & Sirasa 2018, Suganthy et al. 2016, Bjørklund et al. 2017, González-Aguilar et al. 2017). The flavonoids rutin and quercetin were found in pulp of W. robusta. The latter have been described in research focused on mammals, and has shown antioxidant, neuroprotective, antiviral, anticancer, cardiovascular, anti-microbial, anti-inflammatory, hepatoprotective, and antiobesity activities. Therefore, this phytochemical becomes a promising food component for humans for prevention of lifestyle-related disorders (Suganthy et al. 2016). It is unknown if such conditions prevail in wildlife.
These compounds found in W. robusta fruits are unique attributes and represent adaptations (Dirzo 1985). In turn, the above leads to the survival of the species, helping to minimize changes imposed by the environment. The phytochemical analyses presented here also demonstrates that W. robusta fruits are sources of carbohydrates and natural antioxidants for wildlife and humans. Palms in Sonoran Desert oases depend on intermittent water courses, their structure provide microclimatic conditions that support contrasting biotic communities and offer fundamental ecological services (Felger & Joyal 1999, Wehncke et al. 2009, Wehncke & López-Medellín 2014). On the other hand, SM play a complementary role making them a successful species in environments vulnerable to natural and anthropogenic disturbances. Being an ecological and economic important primitive palm, which can survive in many places around the world, with spectacular presence, its phytochemicals attributes indicates that they could be beneficial for biodiversity, attracting pollinators, and other fauna in the cities, as well as in natural habitat, above its properties as a natural antioxidant and sugar source this palm could be a potential source for human consumption. These data on primary and secondary metabolites in fruits of W. robusta are the first to be reported.











 nueva página del texto (beta)
nueva página del texto (beta)