Coastal vegetation, specially these of the coastal dunes, plays a relevant ecological role by providing important environmental support services. This plant community, located in the transition zone between the marine and terrestrial environments; it is an important source of food, refuges and habitats for diverse species of animals and plants, and significantly favors the presence and maintenance of biodiversity (Martínez & Moreno-Casasola 1993, Flores & Espejel 1994, Moreno-Casasola 2004, Acevedo-Rodríguez & Strong 2008, Carboni et al. 2009). Likewise, it offers significant regulatory services, by contributing to soil formation (Wolfe & Nickling 1993) and the control of erosion and floods, since the plant dune communities act as natural barriers against the pounding of winds and tides caused by cyclonic events characteristic of the tropical zone (Miller et al. 2010).
Despite the importance of this coastal plant community of the tropical and subtropical regions, it is one of the most threatened and vulnerable natural system in Mexico and the world (Martínez & Moreno-Casasola 1993, Moreno-Casasola et al. 1998, Herrera-Silveira et al. 2005, Isla 2013). Seingier et al. (2009) report for Mexico the loss of approximately 14 % of the coastal dunes of the country, with the Mexican Caribbean region having the greatest loss of coastal dunes vegetation. Due to the threat to which these natural systems are exposed, it is necessary to take actions to protect them. Improving scientific knowledge of these natural coastal communities can help to fill in conservation gaps and promote the development of protective strategies (Jiménez-Orocio et al. 2015).
In general, the coastal vegetation of Mexico is classified by Rzedowski (2006) within other types of vegetation. In this group, it refers to halophilous vegetation. In this type of vegetation, plant associations typical of sandy beaches and coastal dunes vegetation are described, both in the Atlantic and Pacific coasts, as well as in the coasts of Baja California, and other associations typical of the beaches of brackish lagoons. Some of the representative species mentioned by Rzedowski (2006) are Cakile lanceolata, Canavalia rosea, Ipomoea pes-caprae in the herbaceous stratum; and Coccoloba uvifera, Suriana maritima, Thournefortia gnaphalodes and Bonellia macrocarpa in the shrubby stratum.
On the other hand, Miranda & Hernández (1963) refer to halophilic groupings, such as vegetation that grows near the coast, but which predominates in saline bottoms of closed basins of arid and sub-arid zones, with different degrees of flooding. In addition to the grouping of halophiles, Miranda & Hernández (1963) cite coastal dunes vegetation, characteristic of coastal areas with sandy soils of different degrees of mobility. As representative species of this type of vegetation they mention I. pes-caprae and C. uvifera.
The coastal vegetation of the Yucatan Peninsula is classified by Flores & Espejel (1994) as coastal dunes vegetation. According to these authors, this type of vegetation extends along the entire coast of the Peninsula and is only interrupted by strip mangroves and lime cliffs located at specific points on the coastline. In general, it grows on sandy soils and, on the coast of Quintana Roo; it may be adjacent to rocky areas where pioneer herbaceous and creeping species, as well as other shrubs are developed (Flores & Espejel 1994). The coastal dunes vegetation is subdivided into two main types (Espejel 1984, Chan et al. 2002, Torres et al. 2010), according to the zone where it is located along a stabilization gradient (Jiménez-Orocio et al. 2015). The first type consists of species of the pioneer zone and is located on the coastline adjacent to the sea on the embryonic dunes and to the windward side of the first dune cord. This group is composed of annual herbs, shrubs and halophytes from one to two meters high. The second type consists of the coastal scrub zone species, located on the windward face of the first dune, and from that point to the boundary with the mangrove or tropical forest (Durán et al. 2010). This group is composed of shrub species and sometimes small trees, with or without spines, and variable heights that reach up to three meters (Chan et al. 2002).
In the Mexican Caribbean, the pioneer species that colonize the beach, the embryonic dunes (which represent the initial stages of sand dunes formation), and the first frontal cord of sand dunes are Sesuvium portulacastrum, T. gnaphalodes, C. uvifera, S. maritima y Euphorbia buxifolia; and the characteristic species of shrub strata in this region are Thrinax radiata, Coccothrinax readii, Bravaisia berlandieriana, Pithecellobium keyense, Cascabela gaumeri, Cordia sebestena, Sideroxylon americanum, B. macrocarpa, Erithalis fruticosa, Agave angustifolia, Leucaena leucocephala, C. uvifera, Metopium brownei, Bursera simaruba, Coccoloba barbadensis, Piscidia piscipula y Diospyros salicifolia (Moreno-Casasola et al. 2014).
For the Island of Cozumel, the third largest island of Mexico which is located in the Mexican Caribbean, Téllez et al. (1989) recognize the presence of coastal vegetation, which is classified for them as halophilous vegetation or coastal dunes vegetation. They describe their development in sandy and gravelly soils, and report the eight plant associations: (1) Ambrosia hispida - Opuntia stricta - I. pes-caprae; (2) Canavalia rosea - Tephrosia cinerea - Sophora tomentosa; (3) Thournefortia gnaphalodes - S. maritima - C. uvifera; (4) Thrinax radiata - Hymenocallis littoralis- I. pes-caprae; (5) Thrinax radiata - Caesalpinia bonduc; (6) Rachicallis americana - E. fruticosa - Ernodea littoralis; (7) Salicornia bigelovii - Batis maritima; (8) Vallesia antillana -Quadrella incana - Enriquebeltrania crenatifolia. Although, at the island level some research has been carried out to evaluate the general characteristics of the vegetation in coastal dunes (Téllez et al. 1989, Flores 1992), there is no plant community characterization of the North East of the island, particularly from the terrestrial vegetation of the coastal system. In order to address the following question: How are the structure and composition of well-preserved coastal vegetation communities in the island of Cozumel? This work describes the composition, structure and diversity (alpha and beta) of the vegetation of the rocky and sandy vegetal communities of the North East of Cozumel, Mexico.
Materials and methods
Study site. The island of Cozumel is located in the Caribbean Sea, off the coast of the Yucatan Peninsula, 17.5 km from the city of Playa del Carmen (20° 20′ 00′′ - 20° 30′ 00′′ N and 87° 00′ 00′′ - 86° 50′ 00′′ W) (Figure 1). It is an oceanic island of approximately 490 km2 and has a humid warm Am (f) (i) climate, with summer rains, an average annual rainfall of 1570 mm and an average annual temperature of 25.5 °C (García 1981). The predominant soil in the island are rendzina; they are shallow, and they have good structure and drainage. Geologically, the most accepted hypothesis about its geological origin is that the island was formed from a detachment of the eastern margin of the peninsula, when the basin of Yucatan was formed, between the Late Mesozoic and the Early Cenozoic (Uchupi 1973). Like the Yucatan Peninsula Plate, the island is constituted by sediments and calcareous rocks of marine origin from the Tertiary and recent Quaternary. The island of Cozumel is practically flat with shallow slopes, mainly flat in its western portion and steep in its northern and eastern coasts (INEGI 2002). According to the physiognomic-floristic criteria proposed by Miranda (1959), the most important vegetation types of the island of Cozumel are medium stature subdeciduous forest, low stature deciduous forest, mangroves, coastal dunes vegetation and palm grove (Téllez et al. 1989, Flores 1992).
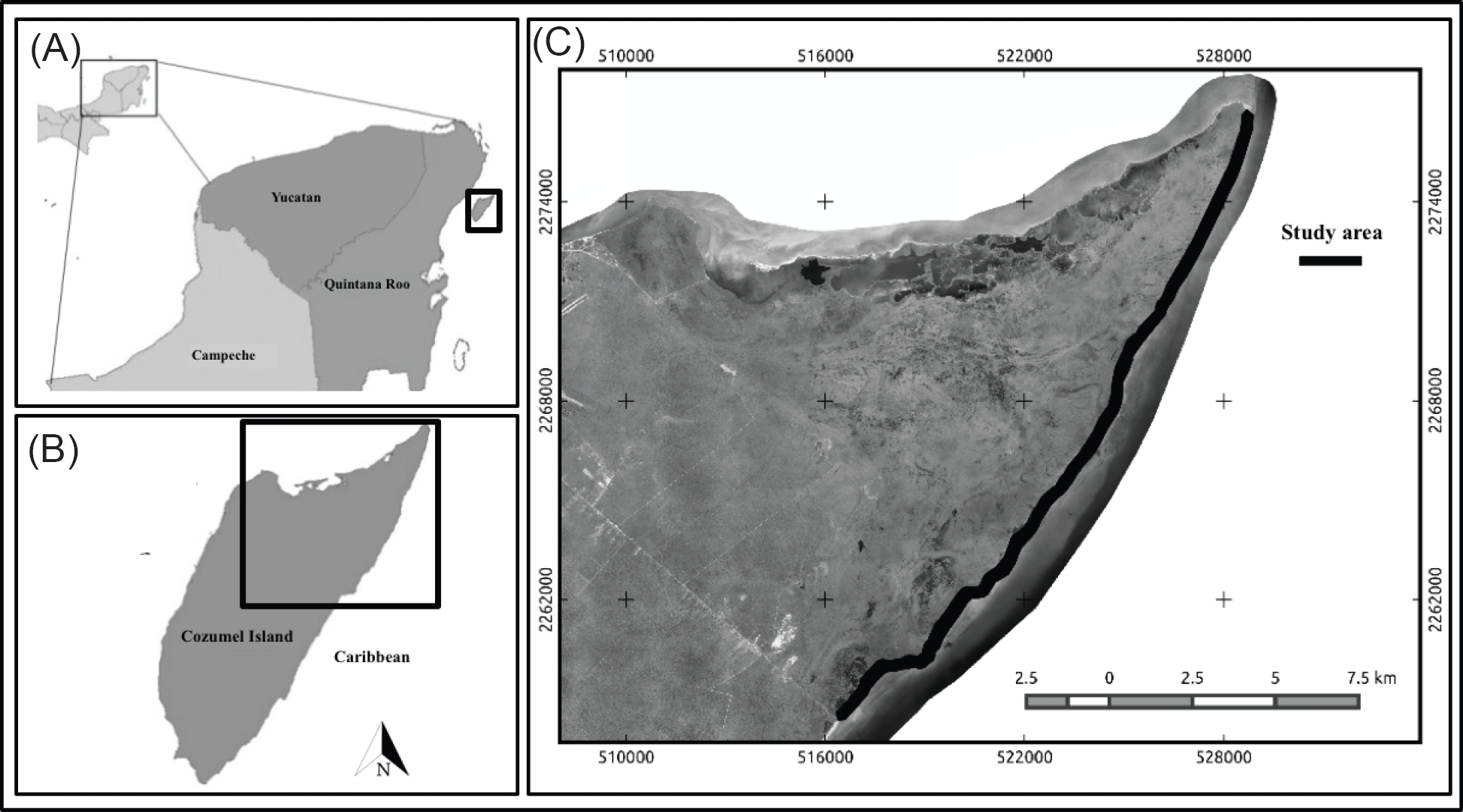
Figure 1 A. Location of the Yucatan Peninsula, Mexico. B. Island of Cozumel. C. The study area highlighted with dark line, located along the northeast area of the Flora and Fauna Protection Area of Cozumel Island (APFFIC).
The main economic activity on the Island is tourism, which has developed in the western coastal area of the island of Cozumel (Palafox-Muñoz & Collantes-Chávez Costa 2008). Approximately 80 % of the island of Cozumel is sheltered by different protected natural areas, one municipal, 2 state and 2 federal ones.
The Flora and Fauna Protection Area of Cozumel is located at the north east part of the island of Cozumel which was decreed on the 25th of September 2012 and covers a total surface of 37,829 ha (DOF 2012). It is a federal protected area that has a coastal line of approximately 50 Km long, from which a stripe of 25 km long by 150 m width corresponds to a coastal vegetation ecosystem. Vegetation of this strip is classified as coastal dunes vegetation, and different plant associations can be distinguished, all in good conserved condition (DOF 2012).
Floristic composition. For the evaluation of the coastal vegetation types of the Island of Cozumel Flora and Fauna Protection Area, fifteen canfield lines were carried out in the summer of 2014 (Canfield 1941, Matteucci & Colma 1982, Brower et al. 1998). Five canfield lines of 80 m located perpendicular to the coastline were randomly established every 100 m from each other in three environments: rocky beaches, sandy beaches and coastal dunes. The species registered were identified on site at the species level by qualified personnel of the herbarium of the Centro de Investigación Científica de Yucatán (CICY) and Universidad de Quintana Roo (UQRoo), and the vouchers of plants collected were deposited at the herbarium of Plant Ecology Laboratory-UQRoo. For the taxonomic nomenclature of families, genera and species we followed the APG III (2009), and the scientific names and families were corroborated in The International Plant Names Index (IPNI 2012).
Community structure. In each of the canfield line, the coverage and frequency by species were determined, the coverage being the measure obtained from the perpendicular projection of the aerial structures of each plant to the line; and the frequency, the number of plant clumps or individual plants per specie presents along the line. In order to calculate the Importance Value Index (Brower et al. 1998), the plant community was characterized horizontally in terms of its relative density of the species, and its coverage and relative frequency. The first one (relative density of the species i, RD i ) was obtained by the formula:
where ni is the total number of individuals of the species i collected and Σn is the total number of individuals of all species. The relative coverage of the species i (RCi ) was determined by:
where l i is the sum of the interceptions in the length for the species i (total length of transects intercepted by the species i) and Σl is the total of the interceptions in the length.
The relative frequency of the species i (RFi ) was calculated by the following equation:
where fi is the frequency of the species i, j i is the number of interception lines in which the species i is present, k is the total number of interception lines. The Importance Value Index of the species i (IVI i ) was calculated by:
The Shannon index (H′) was used to determine alpha diversity, which is based in the community structure, that is, the proportional distribution of the value of each species (Moreno 2001). For its calculation the following equation was used:
where pi = n i /N and ln is the natural logarithm, N is the total number of individuals and ni is the number of individuals of the species i.
After verifying the statistical assumptions of normality, homoscedasticity and independence among the observations, one factor (plant community) Analysis of Variance (ANOVA) was carried out at three levels determined by the type of environment (rocky beaches, sandy beaches and coastal dunes) between the ecological parameters of density and coverage and species richness and Shannon index (P ≤ 0,05). As a Post Hoc test the Tukey HSD test was used. The statistical program used was the SPSS version 22.0 (SPSS 1976).
Floristic similarity. To determine the similarity in the composition of the species among the sampling units, beta diversity was used. For this, the Bray-Curtis sorting analysis was developed (Bray & Curtis 1957). The results are represented in a dendrogram, thereby determining the similarity-dissimilarity between the sampling areas. The analysis was done using the BioDiversity Professional Version 2 computer package (McAleece et al. 1997).
Results
Floristic composition. Twenty-three families, 35 genera and 37 species were registered. (Table 1). The most representative families are Poaceae with four species, and Rubiaceae and Asteraceae with three species each; Sideroxylon and Euphorbia were the richest genera, with two species each. The rest of the families are represented by a number of 1 to 2 species. According to the life habit, 18 herbaceous, 12 arboreal (treelets), 6 shrubby, and 1 palm species were recorded.
Table 1 Scientific and common name, family and life habit of the species registered in the study area.
| Scientific Name | Common Name | Family | Life habit |
|---|---|---|---|
| Acanthocereus tetragonus (L.) Hummelinck | tsakam, nuum tsutsuy (maya). | Cactaceae | Herbaceous |
| Ageratum maritimum Kunth | unknown | Asteraceae | Herbaceous |
| Ambrosia hispida Pursh | sea altanisa, sea daisy ; muuch' kook, k'an lool xiiw (maya). | Asteraceae | Herbaceous |
| Batis maritima L. | alambrillo, mañanita de la mar, wild parsley; ts’aay kaan (maya). | Bataceae | Herbaceous |
| Borrichia arborescens (L.) DC. | sea daisy; k'an lool xiiw (maya). | Asteraceae | Shrub |
| Bursera simaruba (L.) Sarg. | mulatto stick; chakaj (maya). | Burseraceae | Tree |
| Cakile lanceolata O.E. Schulz | unknown | Brassicaceae | Herbaceous |
| Canavalia rosea (Sw.) DC. | frijolillo, sea bean | Fabaceae | Herbaceous |
| Chrysobalanus icaco L. | icaco, nut | Chrysobalanaceae | Shrub or Tree |
| Coccoloba uvifera L. | sea grape, beach grape; ni' che' (maya). | Polygonaceae | Tree |
| Conocarpus erectus L. | botoncillo | Combretaceae | Tree |
| Cordia sebestena L. | anacahuite, siricote blanco, beach siricote; k'oopte', sak k'oopte' (maya). | Boraginaceae | Tree |
| Crotalaria pumila Ortega | garbancillo | Fabaceae | Herbaceous |
| Dactyloctenium aegyptium (L.) Willd. | crow's foot chimes su'uk, k' an toop su'uk (maya). | Poaceae | Herbaceous |
| Digitaria insularis (L.) Fedde | nej boob (maya). | Poaceae | Herbaceous |
| Distichlis spicata (L.) Greene | baakel aak' (maya). | Poaceae | Herbaceous |
| Ernodea littoralis Sw. | unknown | Rubiaceae | Tree |
| Euphorbia dioeca Kunth | xana mukuy (maya). | Euphorbiaceae | Herbaceous |
| Euphorbia mesembryanthemifolia Jacq. | siis ja', sak iits (maya). | Euphorbiaceae | Herbaceous |
| Gomphera sp. | Amaranthaceae | Herbaceous | |
| Hymenocallis littoralis (Jacq.) Salisb. | unknown | Amaryllidaceae | Herbaceous |
| Ipomoea pes-caprae (L.) R. Br. | beach rattan or riñonina | Convolvulaceae | Herbaceous |
| Lantana involucrata L. | orégano xiiw (español -maya); sikil ja' xiiw (maya). | Verbenaceae | Shrub |
| Metopium brownei Urb. | cheechem, boox cheechem (maya). | Anacardiaceae | Tree |
| Neea psychotrioides Donn.Sm. | ta'tsi' (maya). | Nyctaginaceae | Shrub or Tree |
| Pithecellobium keyense Britton | ya'ax k'aax (maya). | Fabaceae | Shrub or Tree |
| Rachicallis americana Hitchc. | unknown | Rubiaceae | Shrub |
| Randia sp. | Rubiaceae | Tree | |
| Scaevola plumieri Vahl | chunup (maya). | Goodeniaceae | Tree |
| Sesuvium portulacastrum L. | beach purslane; ts'a'aykann, xukul (maya). | Aizoaceae | Herbaceous |
| Sideroxylon americanum (Mill.) T.D. Penn. | caimitillo, real peak (Spanish); mulche', puuts' mukuy, péech kitam, sak ts'iits'il che' (maya). | Sapotaceae | Tree |
| Sideroxylon obtusifolium (Roem. & Schult.) T.D. Penn. | zapotillo (Spanish); baalche'kéej, ja'as tóoch, pak' aal che', puuts' mukuy, káapoch (maya). | Sapotaceae | Tree |
| Sporobolus virginicus (L.) Kunth | ch'ilibil su'uk (maya). | Poaceae | Herbaceous |
| Suriana maritima L. | pats´il (maya) | Surianaceae | Tree |
| Thrinax radiata Lodd. ex Schult. & Schult. f. | chit Palm, coast guano | Arecaceae | Palm |
| Tournefortia gnaphalodes (L.) Roem. & Schult. | tabaquillo; sik'imay (maya). | Boraginaceae | Tree |
| Tribulus cistoides L. | thistle (Spanish); chan koj xnuk, chan xnuuk (maya). | Zygophyllaceae | Herbaceous |
Community structure. The species density did not vary among environments. However, a marginal difference among them is reflected (d.f. = 14; F = 3.717; P = 0.055). The density on the sandy beaches was greater than that observed on the rocky beaches, and that of the coastal dunes greater than the first; the vegetation established in sandy beaches was 42 ± 10.70 (median ± SD) individuals per line, while rocky beaches presents 31 ± 11.46, and coastal dune 56.80 ± 17.28. The most representative species for the latter were T. radiata, Ambrosia hispida, Euphorbia mesembryanthemifolia and I. pes-caprae (Relative Density, RD = 65.49 %). On the rocky beaches, T. gnaphalodes, C. uvifera, Conocarpus erectus and R. americana (RD = 59.99 %) mark a dominance over the other species. For the sandy beaches, the species C. rosea, E. mesembryanthemifolia and I. pes-caprae are the most dominant (RD = 56.88 %) (Table 2).
Table 2 Relative density (RD), relative coverage (RC), relative frequency (RF) and index value importance (IVI) of the three plant communities studied.
| Species | Coastal dune | Rocky beach | Sandy beach | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RD (%) |
RC (%) |
RF (%) |
IVI (%) |
RD (%) |
RC (%) |
RF (%) |
IVI (%) |
RD (%) |
RC (%) |
RF (%) |
IVI (%) |
|||
| Acanthocereus tetragonus | 1.06 | 0.63 | 1.69 | 1.13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ageratum maritimum | 0 | 0 | 0 | 0 | 6.45 | 1.29 | 10.87 | 6.2 | 0 | 0 | 0 | 0 | ||
| Ambrosia hispida | 14.44 | 8.23 | 6.78 | 9.82 | 0 | 0 | 0 | 0 | 11.37 | 25.5 | 30.77 | 22.55 | ||
| Batis maritima | 2.82 | 2.31 | 6.78 | 3.97 | 0.65 | 0.09 | 2.17 | 0.97 | 1.9 | 0.55 | 0 | 0.82 | ||
| Borrichia arborescens | 0 | 0 | 0 | 0 | 1.94 | 0.74 | 4.35 | 2.34 | 0 | 0 | 0 | 0 | ||
| Bursera simaruba | 0 | 0 | 0 | 0 | 1.29 | 2.15 | 2.17 | 1.87 | 0 | 0 | 0 | 0 | ||
| Cakile lanceolata | 0.35 | 0.04 | 1.69 | 0.7 | 1.29 | 0.23 | 2.17 | 1.23 | 0 | 0 | 0 | 0 | ||
| Canavalia rosea | 2.82 | 0.86 | 1.69 | 1.79 | 0 | 0 | 0 | 0 | 15.17 | 22.09 | 0 | 12.42 | ||
| Chrysobalanus icaco | 4.23 | 6.08 | 6.78 | 5.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Coccoloba uvifera | 1.41 | 1.1 | 5.08 | 2.53 | 14.84 | 30.03 | 10.87 | 18.58 | 0.47 | 0.07 | 7.69 | 2.75 | ||
| Conocarpus erectus | 0 | 0 | 0 | 0 | 14.19 | 20.29 | 10.87 | 15.12 | 0 | 0 | 0 | 0 | ||
| Cordia sebestena | 1.41 | 1.97 | 3.39 | 2.26 | 1.94 | 1.28 | 2.17 | 1.8 | 0 | 0 | 0 | 0 | ||
| Crotalaria pumila | 0.35 | 0.28 | 1.69 | 0.77 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Dactyloctenium aegyptium | 0.35 | 0.11 | 1.69 | 0.72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Digitaria insularis | 1.06 | 1.22 | 3.39 | 1.89 | 0 | 0 | 0 | 0 | 10.9 | 9.52 | 0 | 6.81 | ||
| Distichlis spicata | 5.28 | 0.92 | 6.78 | 4.33 | 3.23 | 0.4 | 4.35 | 2.66 | 7.11 | 2.24 | 0 | 3.12 | ||
| Ernodea littoralis | 0.7 | 0.8 | 3.39 | 1.63 | 0 | 0 | 0 | 0 | 0.47 | 0.18 | 7.69 | 2.78 | ||
| Euphorbia chamaesyce | 0 | 0 | 0 | 0 | 2.58 | 2.55 | 2.17 | 2.43 | 0 | 0 | 0 | 0 | ||
| Euphorbia mesembryanthemifolia | 9.15 | 5.03 | 6.78 | 6.99 | 8.39 | 1.39 | 10.87 | 6.88 | 15.17 | 4.48 | 0 | 6.55 | ||
| Gomphrena sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.79 | 0.52 | 0 | 1.44 | ||
| Hymenocallis littoralis | 1.41 | 1.27 | 5.08 | 2.59 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ipomoea pes-caprae | 9.15 | 2.2 | 5.08 | 5.48 | 0 | 0 | 0 | 0 | 26.54 | 29.2 | 0 | 18.58 | ||
| Lantana involucrata | 0.35 | 0.08 | 1.69 | 0.71 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Metopium brownei | 0 | 0 | 0 | 0 | 0.65 | 4.9 | 2.17 | 2.57 | 0 | 0 | 0 | 0 | ||
| Neea psychotrioides | 0.7 | 0.5 | 1.69 | 0.96 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Pithecellobium keyense | 0.35 | 0.08 | 1.69 | 0.71 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Rachicallis americana | 0 | 0 | 0 | 0 | 14.19 | 7.76 | 8.7 | 10.22 | 0 | 0 | 0 | 0 | ||
| Randia sp. | 0.7 | 0.19 | 1.69 | 0.86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Scaevola plumieri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.47 | 2.4 | 7.69 | 3.52 | ||
| Sesuvium portulacastrum | 0.35 | 0.11 | 1.69 | 0.72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Sideroxylon americanum | 4.93 | 9.67 | 1.69 | 5.43 | 3.87 | 8.52 | 2.17 | 4.86 | 0 | 0 | 0 | 0 | ||
| Sideroxylon obtusifolium | 0 | 0 | 0 | 0 | 1.29 | 3.62 | 2.17 | 2.36 | 0 | 0 | 0 | 0 | ||
| Sporobolus virginicus | 0.35 | 0.12 | 1.69 | 0.72 | 1.29 | 0.21 | 2.17 | 1.22 | 0 | 0 | 0 | 0 | ||
| Suriana maritima | 0.35 | 0.43 | 1.69 | 0.83 | 3.23 | 3.57 | 4.35 | 3.71 | 0 | 0 | 0 | 0 | ||
| Thrinax radiata | 32.75 | 52.05 | 8.47 | 31.09 | 1.94 | 2.13 | 4.35 | 2.8 | 0 | 0 | 0 | 0 | ||
| Tournefortia gnaphalodes | 2.11 | 3.25 | 6.78 | 4.05 | 16.77 | 8.83 | 10.87 | 12.16 | 1.9 | 2.33 | 30.77 | 11.67 | ||
| Tribulus cistoides | 1.06 | 0.45 | 3.39 | 1.63 | 0 | 0 | 0 | 0 | 4.74 | 0.92 | 15.38 | 7.01 | ||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
The relative coverage also did not vary among environments, but showed a marginal difference (d.f. = 14; F = 3.832; P = 0.052); the plant community established on the rocky beaches presented a coverage of 43.65 ± 7.43 m, whereas in the coastal dunes vegetation was 72.67 ± 16.88 m. The most representative species for Relative Coverage on Coastal dunes were T. radiata and S. americanum (RC = 61.72 %); for rocky beaches C. uvifera, C. erectus, R. americana, S. americanum, and T. gnaphalodes (RC = 75.43 %); and for sandy beaches A. hispida, C. rosea, I. pes-caprae (RC = 76.79 %).
The highest values for relative frequency in the coastal dunes correspond to T. radiata, A. hispida, Batis maritima, Chrysobalanus icaco, Distichlis spicata, E. mesembryanthemifolia and T. gnaphalodes that represent a 49.15 % of the total, all off them with the same value except for T. radiata. For the rocky beaches, Ageratum maritimum, C. uvifera, C. erectus, E. mesembryanthemifolia and T. gnaphalodes show the same higher relative frequency values, adding the 54.35 % of the total. As for the sandy beaches A. hispida and T. gnaphalodes represent the 61.54 % of the total relative frequency (Table 2).
Besides, the three plant communities showed statistical similarity in the Shannon diversity index (d.f. = 14; F = 1.929; P = 0.188) (Figure 2). The plant community established on the rocky beaches presented a value of 1.97 ± 0.21, while the one established on the sandy beaches was 1.54 ± 0.64, and the established in coastal dunes 1.98 ± 0.19 (Figure 2).

Figure 2 Means ± typical errors of the variables of relative density, coverage, Shannon index (H') and species richness (S).
The plant community established in the coastal dunes presented higher species richness per intercept line than that developed sandy beaches community (d.f. = 14; F = 6.288; P = 0.014), with 12.40 ± 1.67 and 7.40 ± 3.04 respectively (Figure 2). The rocky beaches community presented statistical equality with the other two communities, with 9.20 ± 1.78 species per interception line (Figure 2).
Floristic similarity. Similarity dendrogram using Bray-Curtis similarities, highlighting the difference in plant composition between the rocky beach environment, and the coastal dune and sandy beach environments. According to the Bray-Curtis similarity dendrogram, these two large groups show a similarity of 29.07 % (Figure 3). The first group consists of the five transects established on the rocky beaches, which had a similarity of 45.5 %; the representative species of this group are C. erectus, R. americana, B. arborescens and S. obtusifolium. The second cluster groups transects established in the coastal dunes and sandy beaches, and shows a similarity of 37.03 % (Figure 3), being A. hispida, C. rosea, D. insularis, E. littoralis, I. pes-caprae, and T. cistoides the main shared species.
Discussion
In our study, the species richness of the rocky beaches community (9.20 ± 1.78) is similar than that observed in other works carried out in similar communities. González et al. (2015) identify a total of 7 species in the a similar phytosenosis called rocky coast complex, characterized by the presence of R. americana and C. erectus (on the coast of Cuba). On the other hand, for the same study, González et al. (2015) recognize a total of 126 species for sandy coast vegetation, while for our work we find 28 species. This difference could be due to the non-sampling method used by these authors.
The difference between the observed richness from the coastal dunes vegetation contrasted to the sandy beaches vegetation is mainly due to the fact that the former presents a gradient of substrate stability, organic matter content, and salinity conditions, which confers a greater variability of environments that can harbor a greater variety of species. Species representative of the environment were Acanthocereus tetragonus, H. littoralis, Lantana involucrata, Neea psychotrioides, Pithecellobium keyense, Sesuvium portulacastrum, and T. radiata. In addition, it had several species of sandy beaches vegetation, in embryonic coastal dunes, which are the area located between the littoral zone and windward side of the coastal dunes (e.g., A. hispida, C. rosea and I. pes-caprae).
The vegetation coverage in the three environments is similar to that observed by Torres et al. (2010) for the coastal shrub of the Yucatan Peninsula, which ranges between 41 and 85. These results could be related to the general environmental conditions presented in the coastal line that limit the coverage of the characteristic vegetation (Etherington 1975). Likewise, for Caribbean coastal communities, Reys & Acosta-Cantillo (2003) describe in Cuba similar coverages to those observed in the study herein; particularly for rocky beaches they observe coverages between 5 and 25 % of C. erectus, less than 5 % of R. americana, coverage related to the scattered availability of substrate. C. erectus, R. americana and B. arborescens have different morphological and physiological adaptations that allow them to establish themselves, and grow in conditions of moderate to high salinity, caused by the splashing of seawater and occasional flooding of the scattered karst cavities of the rocky environments. There, strong winds, high insolation and temperature, and sudden changes in humidity and drought occur. All these are characteristic of the supra-tidal movement and the climatic conditions of these environments (Brunt 2012). In general, these species have a life habit of decumbent, prostrate and occasionally erect, but always of low height. The R. americana and B. arborescens species are Nanofanerophytes species, with leptophilic and microphilic leaves respectively (Martínez-Quezada 2014), to deal with strong winds and high insolation. C. erectus has two salt glands at the base of the leaves and tolerates salinity conditions of up to 120 UPS (Agraz-Hernández et al. 2006).
The existing floristic similarity between the plant communities of sandy beaches and coastal dunes can be explained by the presence of A. hispida, E. mesembryanthemifolia, C. rosea and I. pes-caprae, species shared between sandy beaches and embryonic coastal dunes. These plants are pioneer species that are established in areas where sand has not been stabilized (Espejel 1984, Chan et al. 2002, Torres et al. 2010, Jiménez-Orocio et al. 2015). They are stress tolerant, low height, and produce abundant seeds. It has herbaceous habit, lateral growth (mostly postrate or decumbent), and roots adapted to the mobility of the substrate. A. hispida, also produces abundant low sized seeds, that are dispersed by the wind, attributes that could explain its relative high IVI in these environments. C. rosea and I. pes-caprae further have a well-developed vegetative reproduction, and produce seed that can float in salt water, allowing them a wide distribution and colonization capacity of beaches and coastal dunes (Devall 1992 and Mendoza-González et al. 2014).
Sandy beaches also presented scattered individuals of T. gnaphalodes and C. uvifera (Figures 4 a and b), which are species characteristic of coastal dunes vegetation (Rzedowski 2006) (Figures 5 a and b). These two species also occur in the area opposite to the shore of the rocky beaches, in the inner limit of the rocky beaches, where the subtrate is abundant and stable (Figures 6 a and b). T. gnaphalodes and C. uvifera, in adition to Distichlis spicata, and E. mesembryanthemifolia, explain the 29 % of the similarity between the vegetation of rocky beaches, and the group formed by the sandy beaches and coastal dunes vegetation. In this area, the substrate is sandy-grainy, and it is where T. gnaphalodes and C. uvifera can be established (Parrotta 1994).
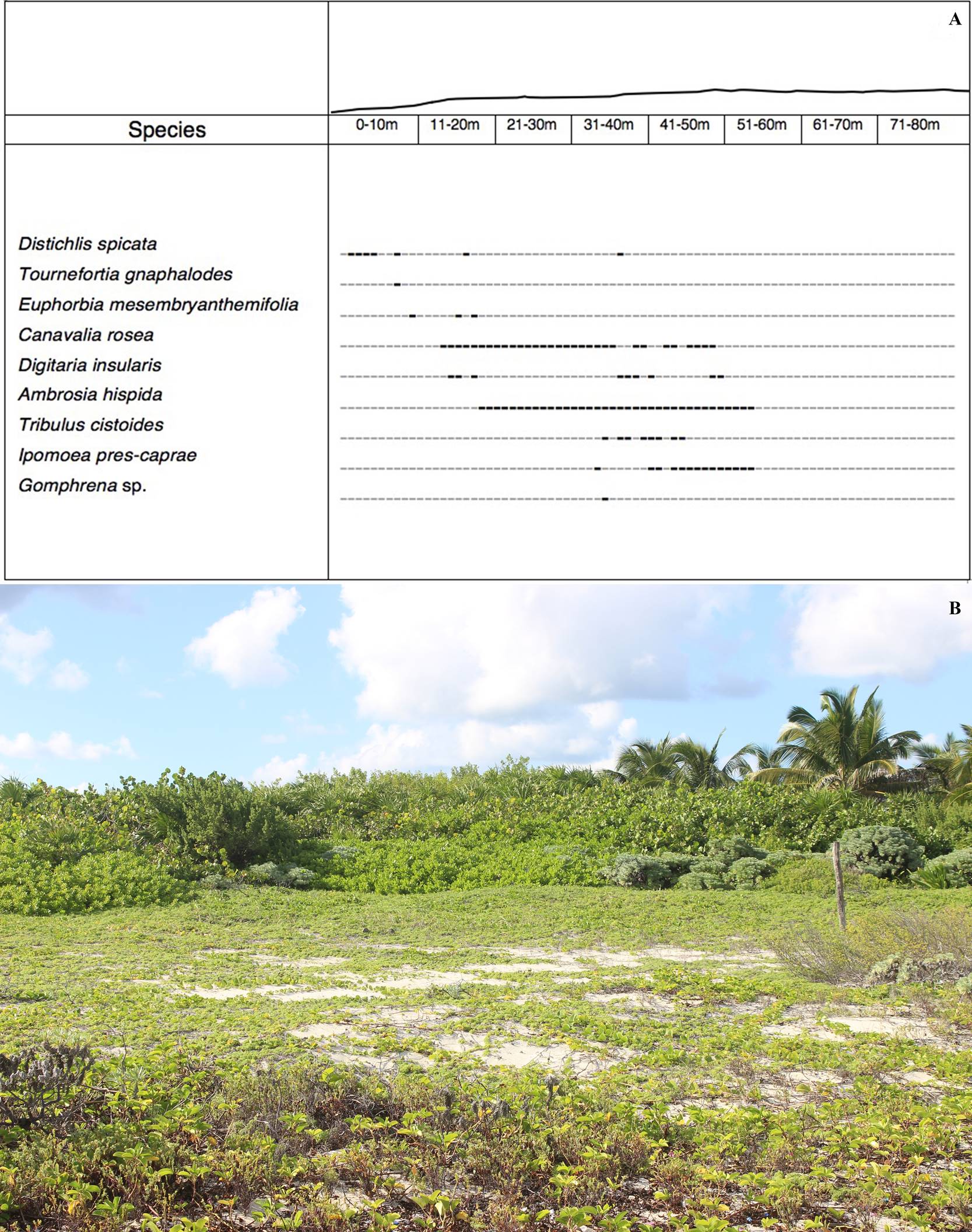
Figure 4 A. Vegetation profile of a typical sandy beaches association of the northeast coastal area of the Island of Cozumel, Quintana Roo. The intervals are in metric units; the cero meter corresponds to the tide line. The points in bold font represent the location of the species and their coverage. B. Typical sandy beaches association of the northeast coastal area of the Island of Cozumel, Quintana Roo.
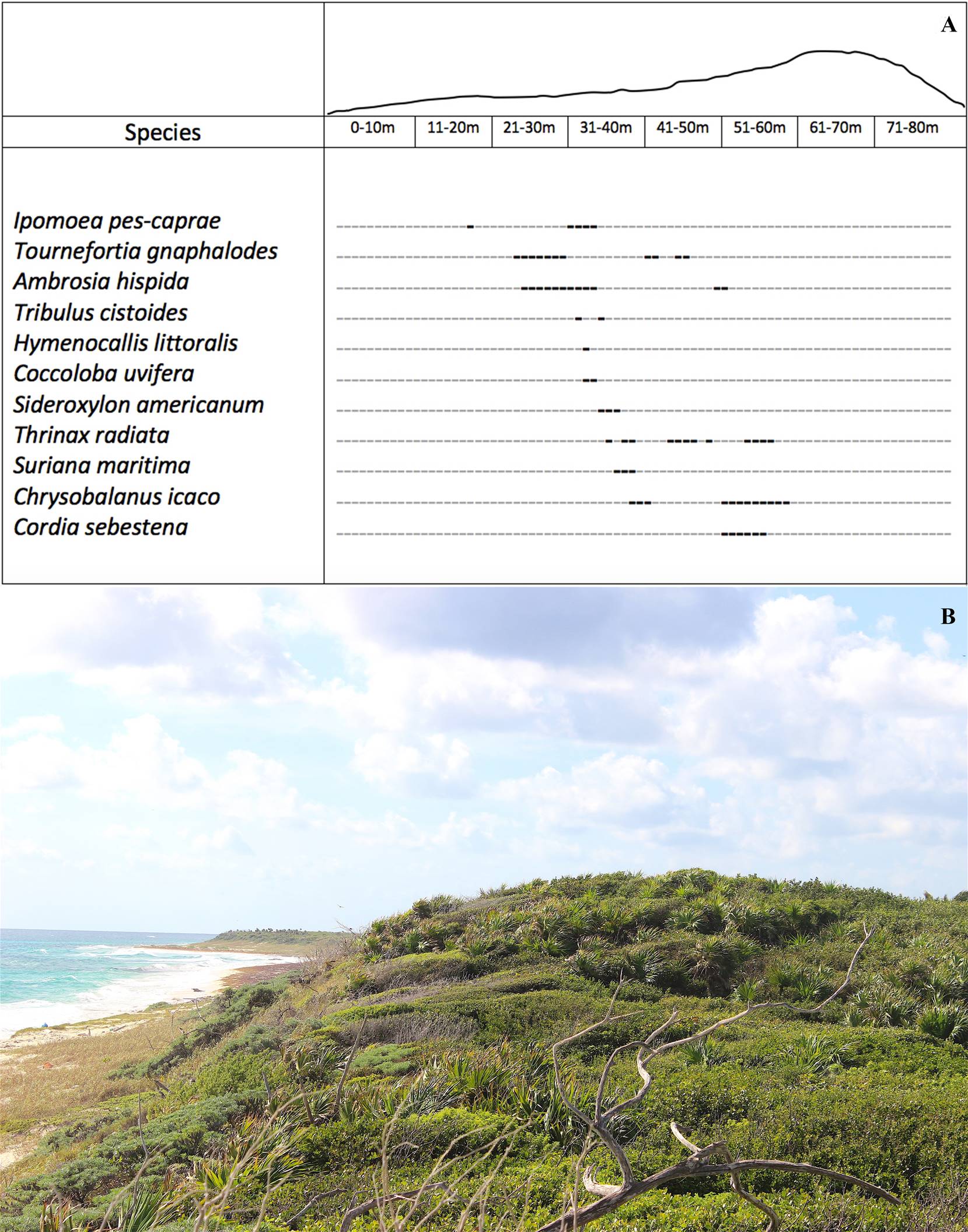
Figure 5 A. Vegetation profile of a typical coastal dunes association of the northeast coastal area of the Island of Cozumel, Quintana Roo. The intervals are in metric units; the cero meter corresponds to the tide line. The points in bold font represent the location of the species and their coverage. The height of the dune is on a scale proportional to the coverage intervals. B. Typical coastal dunes association of the northeast coastal area of the Island of Cozumel, Quintana Roo.

Figure 6 A. Vegetation profile of a typical rocky beaches association in the northeast coastal area of the Island of Cozumel, Quintana Roo. The intervals are in metric units; the cero meter corresponds to the tide line. Points in bold font represent the location of the species and their coverage. B. Typical rocky beaches association of the northeast coastal area of the Island of Cozumel, Quintana Roo.
The difference between the floristic composition of the rocky beaches in relation to the group formed by the other two communities, is mainly determined by C. erectus and R. americana, that can be established in harsh environments, with little substrate present in small hollows of the rock, and subject to the conditions of high salinity caused by exposure to the sea breeze and splashing waves (Brunt 1994). This result suggests the presence of two different plants association into the halophilous vegetation, that present differences in terms of the substrate (sandy and rocky).
The rocky beaches vegetal community described in our research has some floristic and phytosociological similarities with Cayman Islands, Jamaica, and Swan Islands (Proctor 1994, Brunt 1994). For the rocky beaches, the characteristic association found corresponds to that formed by Borrichia arborescens, R. americana and C. erectus, dominant species of phytocenosis, and consistently reported in the Caribbean-Mesoamerican biogeographic region (Rivas-Martínez 1997), both in Cayman Islands (Sauer 1983, Brunt 1994), and Cuba (Reys & Acosta-Cantillo 2003, Martínez-Quesada 2014). It is particularly recognized in the biogeographic Cuban and Yucatanian / Lesser Antillean Provinces, and it is a phytocenosis that is classified as follows:
Class: Sesuvio-Rachicallietea Borhidi (Borhidi et al. 1979, Borhidi et al. 1983)
Order: Borrichio-Rachicallietalia Borhidi (Borhidi et al. 1979, Borhidi et al. 1983)
Alliance: Rachicalli-Borrichion (Samek 1973)
Association: Borrichio-Rachicalletum americanae (Reys & Acosta-Cantillo 2003, Samek 1973).
Finally, while it is strongly suggested that the relationships between the biota of the Yucatan Peninsula were closer to the Centroamenican ones than to the ones of the center of Mexico or from the Antilles (Estrada-Loera 1991, Ibarra-Manríquez et al. 2002, Carnevali et al. 2003, and Ramírez-Barahona et al. 2009), the presence of the species R. americana (whose records are restricted to some sites the coast of Quintana Roo and Cozumel), and the Borrichio-Rachicalletum americanae phytocenosis (present on the rocky beaches), not only show the biogeographical relation of the island of Cozumel with the Caribbean zone from the shared taxa, but also in their plant associations and Caribbean landscape, so that the protection of this coastal systems of the Cozumel Island contributes to the conservation of Mexico’s biodiversity.











 nueva página del texto (beta)
nueva página del texto (beta)



