The beans (Phaseolus spp.) belong to the botanical family Fabaceae. They are native to the Americas, and are the most cultivated legumes in Latin America and Africa. They are important as food, forage and nitrogen fixers (Cardador-Martínez et al. 2002, Peña et al. 2012). During the history of bean domestication, seeds have been selected based on qualities concerning farming and for cooking ease and taste. For example, cultivated varieties are selected according to seed size and local preference. Because selection focuses on these characteristics, helpful metabolites that protect plants against diseases and plagues are overlooked (Mejía et al. 2003, Lépiz et al. 2010, Bautista-Lozada et al. 2012, Peña et al. 2012). In medicine, agricultural, and nutritional research areas, Phaseolus varieties are generally improved to increase seed production, to achieve resistance to environmental conditions and pests and to fight degenerative illnesses (Cardador-Martínez et al. 2002, Mejía et al. 2003, Peña et al. 2012, Ramírez-Jiménez et al. 2014).
In the genus Phaseolus, metabolites are located in the reproductive organs, which contribute to securing reproductive processes and seed protection. These metabolites include phenolic pigments (flavonoids, anthocyanins, and tannins), lectins, trypsin and amylase inhibitors and phenolic acids (Soto-Figueroa 1994, Franco et al. 2002, Cardador-Martínez et al. 2002, Espinosa-Alonso et al. 2006, Reyes et al. 2008, Peña et al. 2012). In general, secondary metabolites, minerals, proteins, fibers and tannins are present in greater concentrations in wild beans than in cultivated beans (Benrey et al. 1998, Peña et al.2012). A study developed by Nagl et al. (1997) suggests that species selection and genetic improvement in Phaseolus, have led to an increase in the quantity and quality of nutrients (vitamins and some proteins) and to the reduction of anti-nutrient and toxic components (lectins, phytohaemagglutinins, enzymatic inhibitors), which in nature are frequently used as a defense mechanism against herbivores.
Other characteristics like seed coat color are largely determined by their secondary metabolites content. Herrera-Flores et al. (2005) found that phenolic content varies among bean varieties and species and through different pods and seed maturity stages. They also registered the presence of polyphenols in Phaseolus vulgaris, P. coccineus and P. polyanthus Greenm. in parenchyma, whereas in P. coccineus, they found that, at day 35 of pod development, there is a wide space between parenchyma cells occupied by polyphenols. In addition, Soto-Figueroa (1994) found a close relationship between seed morphology and its phenolic content, which gives bean varieties different flavor. Furthermore, Beninger & Hosfield (2003) reported that antioxidant activity produces different colors in the coat of P. vulgaris.
Differences in nutrient and resource allocation generate variation in chemical and physical attributes between wild and cultivated plants (Gepts 2014). Broad research is concerned about how the different metabolites concentration between domesticated and wild plants varies affecting plant resistance, attractiveness and susceptibility to phytophages (Benrey et al. 1998). In Mexico, as in other countries, most of the bean studies are focused on the majority commonly cultivated species: (P. vulgaris L, P. coccineus L, P. lunatus L., P. acutifolius A. Gray and P. dumosus Macfad). Excluding wild and weedy varieties that could potentially contribute to the improvement of cultivated beans, and could also sustain the diversity of native beans (Lépiz et al. 2010).
Mexico has the greatest diversity of Phaseolus in the world, and the majority of the diversity is located in the western part of the country. This area is considered a center of domestication center of Phaseolus vulgaris (Chacón et al. 2005, Lépiz et al. 2010). The most commonly cultivated species is P. vulgaris. However, P. coccineus has excellent nutritive qualities and is found mainly in the marginal highlands and high valleys of Mexico. Nonetheless, P. coccineus is not cultivated at the same intensity as P. vulgaris (Vargas-Vázquez et al. 2011). Traditional and native landraces are better adapted to different environmental conditions, and it is possible that there are more species and major distributions than reported in herbaria and germplasm banks (Cárdenas-Ramos 1997, Peña et al. 2012).
Although Phaseolus is a very important genus, information is lacking about wild and weed species, bean varieties and their usage in genetic improvement programs (Peña et al. 2012). A project exploring wild bean species was developed between 2003 and 2009 in several Mexican states (Jalisco, Michoacán, Colima, and Nayarit), where less species were currently found than previously reported (Lépiz et al. 2010). The authors strongly recommend field collections to conserve and study Phaseolus wild germplasm, emphasizing the importance of preserving genetic resources to generate better landraces and maintain the cultural richness of these crops in Mexico. Chacón et al. (2005) studied several cultivated P. vulgaris varieties and found differences in haplotypes between wild and domesticated seeds. They obtained bean samples from Chihuahua, Durango, Nayarit, Jalisco, Guanajuato, Michoacán, Puebla, Mexico City, Oaxaca and Chiapas, reporting six similar haplotypes from the samples from Jalisco, Michoacán, Puebla and Guanajuato. Additionally, Peña et al. (2012) point out the work of several authors evaluating partial chemical composition in Durango, Tlaxcala, Chihuahua and Oaxaca. They conclude that there is more genetic richness in wild bean populations and the genetic richness could be used to improve and complement cultivated bean consumption. Also, Vargas-Vázquez et al. (2011) studied the phenology and morphology of 98 P. coccineus samples from the Huasteco Karst region in the Sierra Norte of Puebla, and found that phenology was similar among the samples, whereas seed color was highly variable.
In the state of Querétaro, Mexico, there are 12 species of Phaseolus, where P. vulgaris (common bean) and P. coccineus (chimate bean) are the most commonly cultivated beans (CONABIO 2016, QMEX 2016). There are varying domestication stages of P. coccineus within the state, ranging from completely wild in the north, to domesticated at the south. Its distribution ranges from the humid forest areas in the northern part in the Sierra Madre Oriental (locally known as Sierra Gorda), to the dry environments in the southern areas of the Mexican Plateau.
The local populations prefer negro (black) beans, P. vulgaris, to pinto (mixed colors: brown, taupe pink, red, white, black) and bayo (pale pink and yellow) beans. It is possible to find other varieties such as flor de mayo (taupe pink), mantequilla (pale yellow), red and brown beans (Freytag & Debouk 2002, CONABIO 2016, QMEX 2016).
In Querétaro, little research regarding native beans has been conducted. One of the few studies is that of Díaz-Batalla et al. (2006), who quantified flavonoid and phenolic acids in seeds of fourteen varieties of P. vulgaris, with one sample native to Querétaro. They found that ferulic and p-coumaric acids and quercetin were the most abundant metabolites in of all the samples evaluated. The goal of this study was to describe the secondary metabolite profiles of some native seeds from P. vulgaris and P. coccineus with different domestication status in Querétaro. The proposed hypothesis was that secondary metabolites concentration will be higher in the seeds of P. coccineus from wild and disturbed areas.
Materials and methods
Distribution of species in Querétaro. Data about the genus Phaseolus was searched in records in the QMEX Herbarium, the Germplasm bank of the Universidad Autónoma de Querétaro, and the CONABIO databases (REMIB 2017). The information was compiled in a database, which briefly describes the bean species and varieties collected, their distribution, use, common names, and number of specimens collected.
Collection sampled for Phaseolus vulgaris and P. coccineus. The samples for the chemical analysis were collected from seven regions in Querétaro, two located at the Mexican Plateau and five from the Sierra Madre Oriental (Table 1). Nine samples were studied (Figure 1), six belonging to P. vulgaris (PV) and three belonging to P. coccineus (PC). The samples sizes of P. vulgaris ranged from 200 to 1,000 g of seeds, depending on the local availability. All of the samples were collected from rainfed agriculture crops and provided by the landowners. The samples of P. coccineus were from three different wild and weedy populations. In these populations, five to fifteen plants with mature pods were selected. The pods were taken to the Laboratory of Botany at the Facultad de Ciencias Naturales (FCN), Universidad Autónoma de Querétaro (UAQ) in order to dry them at air temperature and obtain the seeds. The amount of P. coccineus seeds varied between 200 and 300 g. A 60 % backup of each sample was stored in the germplasm bank at the FCN-UAQ.
Table 1 Data site of collection of the samples used in the experiment.
| Species | Phaseolus vulgaris (PV) | Phaseolus coccineus (PC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | PV1 | PV2 | PV3 | PV4 | PV5 | PV6 | PC1 | PC2 | PC3 | |
| Origin | Area | Mexican Plateau | Sierra Gorda | Mexican Plateau | Sierra Gorda | |||||
| Site of collection | La Barreta | La Barreta | La Barreta | La Margarita | La Yesca | La Yesca | La Joya | Maravillas | La Yesca | |
| Geograpical coordinates | 20° 49´ 46.93´´ N 100° 30´ 11.08 W |
20° 49´ 46.93´´ N 100° 30´ 11.08 W |
20° 49´ 46.93´´ N 100° 30´ 11.08 W |
21° 21´ 24´´ N -99° 13´ 4´´ W |
21° 13´ 14´´ N 99° 7´ 32´´ W |
21° 13´ 14´´ N 99° 7´ 32´´ W |
20° 48´33´´ N 100° 31´ 43´´ W |
20° 56´ 10´´ N 99° 31´ 15´´ W |
21° 13´ 14´´ N 99° 7´ 32´´ W |
|
| Altittude (m asl) | 2,127 | 2,127 | 2,127 | 1,627 | 1,596 | 1,596 | 2,450 | 2,134 | 1,596 | |
| Variety | “Negro” | “Pinto” | Bayo | “Mantequilla” | “Negro” | “Pinto” | NA | NA | NA | |

Figure 1 Seed samples of Phaseolus spp. and their collection areas. PV1, PV2, PV3, PV4, PV5 and PV6 (P.vulgaris). PC1, PC2 and PC3 (P. coccineus).
Secondary metabolites characterization of Phaseolus seeds. The characterization of the secondary metabolites consisted of the total content of phenolic compounds, tannins, anthocyanins, in addition to the amylase inhibitors and protein determinations for the methanolic and aqueous extracts. In accordance with the methodology described by Cardador-Martínez et al. (2002), a ratio of 1:10 was used to prepare the methanolic extracts. For the determination of the total amount of phenols, Folin Ciocalteu’s technique was performed, using gallic acid as a standard in both, seed coats and cotyledons. The results were expressed as µg GAE/ml (Dewanto et al. 2002). Tannin content in seed coat was measured using the vanillin and (+)-catechin technique modified for microplate, and the results were reported as µg (+)-Cat/ml (Mejía et al. 2003). The seed coat anthocyanins were quantified employing the differential pH method with buffers of 0.025 M potassium chloride pH 1 and 0.4 M sodium acetate pH 4.5 (Giusti & Wrolstad 2001) and reported as µg of cyanidin-3-glucoside/ml. To obtain the HPLC profiles, the extracts were filtered through a 0.2 mm filter membrane and 50 µL were injected in triplicate into a reversed phase column (C18 Prep-Nova Pack HR, 60 Å, 6 um, 3.9-300 mm), using a Waters HPLC system (Waters Corporation, Milford, MA, USA) which consisted of a quaternary pump (model 600), a photodiode array detector (model 996), an in-line vacuum degasser (MetaChem Technologies Inc.), and a Rheodyne injector (4793). Control of the equipment, data acquisition, processing, and management of chromatographic information were performed by the Millennium32 software program (Waters). Chromatographic conditions were λmax 280 nm; the gradient mobile system consisted of (A) water/1 % acetic acid and (B) acetonitrile at a flow rate of 0.8 ml/min. A linear gradient was used as follows: 85 % of solvent A for 2.5 minutes, 60 % of solvent A for 2.5 minutes, 70 % of solvent A for 5 minutes, 80 % of A for 2 minutes, 40 % of solvent A for 1 minute, 50 % of solvent A for 2 minutes and 85 % of solvent A for 3 minutes. Quantification was carried out by external standardization and full standard curves were constructed. A total of 13 commercial standards were measured: caffeic, chlorogenic, ellagic, ferulic, gallic, p-coumaric and sinapic acids, apigenin, catechin, kaempferol, quercetin, rutin, and vanillin. The results were reported as μg/ml (Díaz-Batalla et al. 2006, Ramírez-Jiménez et al. 2014).
Protein and amylase inhibitors were measured employing an aqueous extract of defatted bean flour in proportion 1:5, including seed coat and cotyledon (Campos et al. 1997). The protein quantification was performed by the Bradford’s method modified for microplate with bovine albumin as standard (Bradford 1976). The amylase inhibitor content was determined by the spectrophotometric technique of disappearing substrate (Somogyi 1938) using a starch buffer substrate solution (0.05 % starch, 0.15 M NaCl, 0.1 M sodium acetate pH 5) and a developer solution (0.006 % I2 + 0.06 % KI in HCl 0.02 M). The results were reported as Amylolytic Units (AU/ml).
Statistical analysis. The values of each chemical variable were compared between species (P. vulgaris and P. coccineus) and origin by using a bootstrap method implemented in the software Data Pilot 1.03 (Two Pilots Inc. 2003). This method generates multiple averages of subsamples to determine the probability that a group average is higher than the average of another group (Henson 2015). For one of the groups multiple averages were generated from the six samples of P. vulgaris and for the other group from the three samples of P. coccineus. On the other hand, two groups were generated according to their origin, one group from the four samples from the Mexican Plateau and the other one from samples from Sierra Gorda. With the set of variables differing between species and origin, a discriminant analysis was carried out in order to determine whether the nine samples were grouped by species or origin. For the selection of the variables used in the discriminant analysis, a Spearman Rho correlation coefficient for no normal variables was used. The explanatory power of each of the correlated variables was evaluated stepwise into the discriminant model (Kachigan 1986 Quinn & Keough 2003) and the significant model (Wilks´ Lambda > 0.05 = X < 0.05) that maximized differences between species and origin was selected. The analysis was carried out in the software JMP 8.0 (SAS Institute Inc.).
Results
Distribution of species in Querétaro. Data about the genus Phaseolus was mostly obtained from the herbarium specimens with a total of 202 records and 13 species. Phaseolus vulgaris has 82 records and P. coccineus has 68, whereas the remaining species have 18 or less (P. acutifolius, P. formosus Kunth, P. glabellus Piper, P. grayanus Wooton & Standl., P. heterophyllus Humb. & Bonpl. ex Wild, P. leptostachyus Benth., P. maculatus Scheele, P. microcarpus Mart., P. pauciflorus Sessé & Moc. Ex G.Don, P. pedicellatus Benth., P. pluriflorus Maréchal, Mascherpa & Stainier). From the total records, 117 were placed in some variety or subspecies. At the infraspecific level, two subspecies (P. coccineus subsp. coccineus, P. acutifolius subsp. latifolius G. F. Freeman), and four varieties (P. leptostachyus var. leptostachyus , P. vulgaris var. vulgaris, P. vulgaris var. mexicanus and P. pedicellatus var. pedicellatus) were found. The records were obtained in 18 municipalities, and the majority were from Landa de Matamoros (35), Querétaro (27), San Joaquín (19), Pinal de Amoles (17) and San Juan del Río (15). There were 56 collections from 1990 to 1999 and 60 from 2010 to the present. For the rest of the decades there were 31 collections or less. Samples were collected largely from pine and oak forests or from agriculture fields in the municipalities of San Juan del Río and Querétaro (Figure 2).

Figure 2 Number of collections of Phaseolus in Querétaro ordered by species (A) and year (B) registered at the QMEX Herbarium, the Germplasm bank of the Universidad Autónoma de Querétaro, and the CONABIO databases.
Twenty-six common names, mainly for P. vulgaris and P. coccineus were found from the species used for consumption (Table 2). Frequently their common names refer to the beans color (black, green, red, purple, pink, yellow), shape (bolondito: round), usage (ejotero: edible pods) or some other characteristics such as flavor or texture. The consumed part of the plant is the boiled pod (P. coccineus) and the cooked seeds, either whole or refried (P. vulgaris and P. coccineus).
Table 2 Common names used for Phaseolus spp. at the state of Querétaro.
| SPECIES | COMMON NAME | SPECIES | COMMON NAME |
|---|---|---|---|
| P. vulgaris | Frijol americano | P. vulgaris | Frijol negro |
| P. vulgaris | Frijol faja de indio o San Franciscano | P. vulgaris | Frijol negro de mata |
| P. vulgaris | Frijol | P. vulgaris | Frijol negro de monte |
| P. vulgaris | Frijol bayacote | P. vulgaris | Frijol pintito |
| P. vulgaris | Frijol bayo | P. vulgaris | Frijol pinto |
| P. vulgaris | Frijol bolondito | P. vulgaris | Frijol pipiano |
| P. vulgaris | Frijol burro | P. vulgaris | Frijol rojo |
| P. vulgaris | Frijol criollo | P. vulgaris | Frijol sabino |
| P. vulgaris | Frijol ejotero | P. vulgaris | Frijol vaquita |
| P. vulgaris | Frijol Flor de mayo | P. vulgaris | Frijol verde |
| P. vulgaris | Frijol mantequilla | P. vulgaris | Frijoles morados varios |
| P. vulgaris | Frijol morado de mata | P. coccineus | Frijol chimate |
| P. vulgaris | Frijol moro | P. coccineus | Frijol chimate |
Secondary metabolite characterization of Phaseolus seeds. The sampled seeds were quite different. Seeds from cultivated plants of P. vulgaris were the following colors: black, brown, light pink and yellow. The brightness of the color varied from intense to dull, and most of them had a smooth seed coat and a uniform color (Figure 1). The seeds of wild and weedy plants of P. coccineus were mainly black spotted with brown. They were shiny and had different shapes, from ovoid to quadrangular (Figure 1).
In general, the total phenolic content was higher in the seed coat than in the cotyledon; in P. vulgaris the mean was 20 % higher while in P. coccineus was 200 % higher. Samples PV2 and PV5 did not follow this pattern, and because of that the mean value declined. For the seed coat extract of P. vulgaris samples, PV5 showed the highest value (5.92 µg eq GAE/ml extract); and sample PV4 had the lowest value (1.47 µg eq GAE/ml extract). Both samples were from the Sierra Gorda. The tannin content ranged from 0.07 to 0.69 µg eq. (+) Cat/ml, with the highest value found in the Mexican Plateau´s pinto bean (P. vulgaris; 0.69 µg eq. + Cat/ml extract). For the sample PV4, mantequilla bean (P. vulgaris), tannins were not detected. The anthocyanins concentration of the bean seed coat ranged from 0.04 to 2.48 µg eq. Cyanidin-3-glucoside/ml; the lowest value was from PC2, P. coccineus and the highest for the sample PV5, P. vulgaris, respectively (Table 3).
Table 3 Mean values from chemical data from P. vulgaris and P. coccineus seeds evaluation. TPc (cotyledon total phenols), TPsc (seed coat total phenols), TAN (tannins), ANT (anthocyanins), GAL (galic) acid, CLO (chlorogenic acid), CAF (caffeic acid), ELA (ellagic acid), PCO (p-coumaric acid), FER (ferulic acid), CAT (cathechin), VAI (vainillin), API (apigenin), KAE (kaempferol), AMI (amylase inhibitors), PRO (protein).
| Species | Phaseolus vulgaris(PV) | Phaseolus coccineus(PC) | Significant value for media comparison by species (> 0.95) | Significant value for media comparison by origin (> 0.95) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | PV1 | PV2 | PV3 | PV4 | PV5 | PV6 | PC1 | PC2 | PC3 | Media PV (DE) | Media PC (DE) | ||
| Origin | Mexican Plateau | Sierra Gorda | Mexican Plateau | Sierra Gorda | |||||||||
| Variety | “Negro” | “Pinto” | Bayo | “Mantequilla” | “Negro” | “Pinto” | NA | NA | NA | ||||
| TPc (µg eq GAE/ml extract) | 0.73 | 17.02 | 1.23 | 0.96 | 7.19 | 1.24 | 1.06 | 2.79 | 1.16 | 4.72 (6.50) |
1.67 (0.97) |
||
| TPsc (µg eq GAE/ml extract) | 2.90 | 3.90 | 5.50 | 1.47 | 5.92 | 2.50 | 2.53 | 3.40 | 4.26 | 3.69 (1.74) |
3.40 (0.86) |
||
| TAN (µg eq (+)- Cat/ml extract) | 0.36 | 0.69 | 0.46 | 0.00 | 0.28 | 0.23 | 0.07 | 0.37 | 0.42 | 0.33 (0.23) |
0.29 (0.18) |
||
| ANT (µg Cyanidin-3-glucoside /ml extract) | 0.78 | 0.68 | 0.04 | 0.29 | 2.48 | 0.18 | 0.44 | 0.12 | 1.22 | 0.74 (0.89) |
0.59 (0.56) |
||
| GAL (µg/ml) | 0.31 | 0.21 | 0.17 | 0.12 | 0.11 | 0.15 | 0.10 | 0.12 | 0.13 | 0.17 (0.07) |
0.12 (0.01) |
* | * |
| CLO (µg/ml) | 0.15 | 0.06 | 0.03 | 0.01 | 0.16 | 0.04 | 0.03 | 0.03 | 0.03 | 0.075 (0.064) |
0.03 (0) |
* | |
| CAF (µg/ml) | 0.00 | 0.00 | 3.29 | 0.00 | 0.88 | 0.00 | 0.03 | 0.08 | 0.04 | 0.69 (1.31) |
0.05 (0.026) |
||
| ELA (µg/ml) | 49.71 | 25.66 | 0.00 | 5.20 | 11.18 | 18.62 | 2.56 | 4.26 | 3.33 | 18.39 (17.87) |
3.38 (0.85) |
* | |
| PCO (µg/ml) | 0.86 | 0.49 | 0.00 | 0.12 | 0.08 | 0.32 | 0.76 | 0.23 | 0.15 | 0.31 (0.32) |
0.38 (0.33) |
* | |
| FER (µg/ml) | 1.17 | 0.46 | 0.77 | 0.00 | 0.24 | 0.41 | 0.46 | 0.17 | 0.09 | 0.508 (0.411) |
0.24 (0.19) |
* | |
| CAT (µg/ml) | 0.08 | 0.02 | 0.10 | 0.01 | 0.13 | 0.01 | 0.02 | 0.01 | 0.02 | 0.058 (0.051) |
0.02 (0.005) |
* | |
| VAI (µg/ml) | 0.00 | 0.01 | 0.00 | 1.59 | 0.26 | 0.10 | 0.03 | 0.01 | 0.01 | 0.32 (0.62) |
0.02 (0.01) |
* | |
| API (µg/ml) | 1.02 | 0.76 | 3.14 | 0.63 | 0.17 | 0.29 | 0.80 | 0.34 | 0.19 | 1.001 (1.09) |
0.44 (0.31) |
* | |
| KAE (µg/ml) | 0.04 | 0.06 | 0.57 | 7.20 | 5.51 | 0.99 | 0.17 | 0.32 | 0.69 | 2.39 (3.13) |
0.39 (0.26) |
* | |
| AMI (UA/ml) | -276.00 | -360.00 | -432.00 | -216.00 | -168.00 | -536.00 | -656.00 | -398.00 | -208.00 | -331.33 (138.48) |
-420.67 (224.85) |
||
| PRO (µg/ml) | 0.40 | 0.39 | 0.45 | 0.40 | 0.61 | 0.62 | 0.39 | 0.31 | 0.56 | 0.47 (0.10) |
0.42 (0.12) |
||
In addition, seven phenolic acids (caffeic, chlorogenic, ellagic, gallic, ferulic, sinapic, and p-coumaric acids), an aldehyde (vainillin) and five flavonoids (rutin, (+)-catechin, quercetin, apigenin, and kaempferol) were determined by HPLC (Table 3). Sample PV1, P. vulgaris “negro” from Mexican Plateau, has the highest content of gallic, chlorogenic, ellagic, p-coumaric and ferulic acids and apigenin (53.22 µg/ml in total). Sample PV5, P. vulgaris negro from Sierra Gorda, possessed the highest concentration of chlorogenic acid, vainillin, and kaempferol (5.93 µg/ml in total). The highest content of caffeic acid and rutin was found in the seed coat extract of sample PV3, P. vulgaris “bayo” (3.29 µg/ml), and sample PV4, P. vulgaris “mantequilla” (0,035 µg/ml), respectively. Even rutin and quercitin were detected in some samples but in most of them this substances were undetectable. Sinapic acid was not detected in the assayed samples. Protein content was high in samples PV5 (0.61 µg/ml) and PV6 (0.62 µg/ml), both representing P. vulgaris from Sierra Gorda. Amylase inhibitors values were on average lower for P. vulgaris and 20 % higher in P. coccineus. The lowest content was recorded in the sample PV5 (-168 UA/ml) and the highest in PC1 (-656 UA/ml).
Correlation analysis between samples and species (P. vulgaris and P. coccineus). Differences were not statistically significant between species. However, the secondary metabolite concentration was higher in P. vulgaris than in P. coccineus, with the exception of p-coumaric acid. In contrast, when analyzing by origin most of the values are higher in Mexican Plateau seeds, with the exceptions of anthocianins, vainillin and amylase inhibitors.
Statistically, differences in of chemical characteristics were found in gallic, chlorogenic and ellagic acids, cathechin, vainillin and kaempferol, finding that all of them were higher in P. vulgaris. The chemical characteristics that had differences were gallic, p-coumaric and ferulic acids and apigenin. All of them were higher in the Mexican Plateau samples.
Pairs of chemical variables between which a correlation was detected when analyzed by species were ferulic acid- p-coumaric acid, tannins and apigenin, quercitin-gallic acid, chlorogenic acid, total phenolic compounds in cotyledon and amylase inhibitors (Figure 3). The significant model that maximized differences between species (Wilks λ = 0.20, 3 df, P = 0.03) included three variables: ferulic and gallic acids, anthocianins, kaempferol and total phenolic content in seed coat. The variables that revealed differences between the samples of different origins were catechin-chlorogenic and caffeic acids, whereas in the seed coat they were anthocianins and total phenolic content (Figure 4).
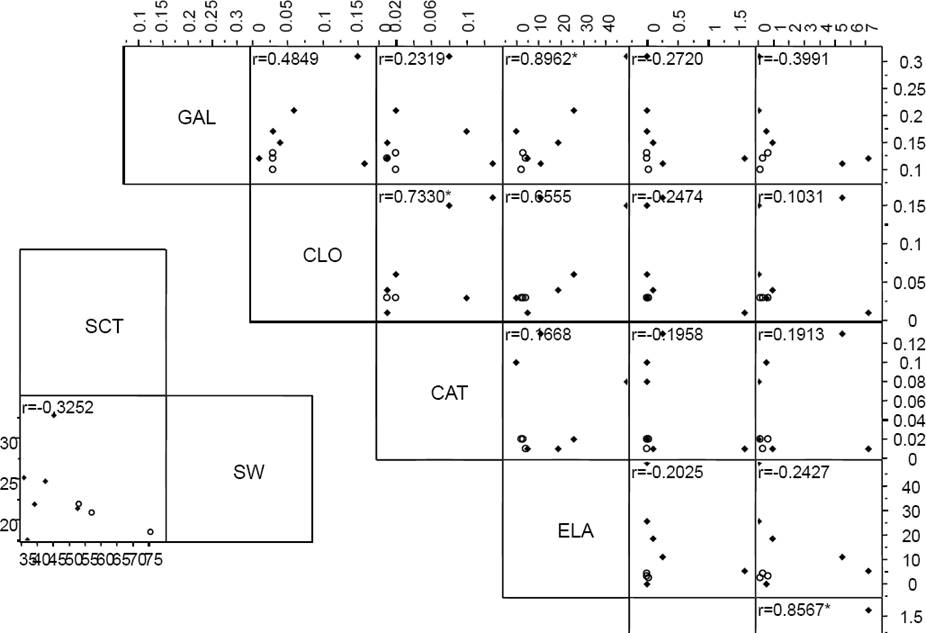
Figure 3 Multivariate analysis by species. GAL (gallic acid), CLO (chlorogenic acid), CAT (catechin), ELA (ellagic acid), VAI (vainillin), KAE (kaempferol).* means significant pairwise correlation.
Discussion
Several factors affect the amount secondary metabolites in the seeds, including species, site of cultivation, storage and environmental conditions during growth (Luthria & Pastor-Corrales 2006). The selection of varieties is made according to the regional environmental conditions, consumer preferences and the objective of cultivating a particular variety. The concentrations of phenolic compounds in the seed coat and cotyledon was higher than those obtained by Luthria & Pastor-Corrales (2006). They reported content ranges between 0.019 and 0.048 µg GAE/ml in 15 varieties of P. vulgaris. In comparison to the results of Peña et al. (2012) and Mejía et al. (2003), which observed tannin concentrations higher than a hundred times, i.e., between 0.01 and 0.05 µg (+)- Cat/ml., whereas in this study the concentrations were lower. Also, the amounts of anthocyanins are much lower than the values reported by Puertas-Mejía et al. (2013) and Espinosa-Alonso et al. (2006), who reported quantities of 13.3 and 25.5, and 3.4 µg eq. cyanidin-3-glucoside/ml, respectively, in the common bean. These differences could be caused by the storage, drying and extraction methods, coupled with the intrinsic variability in the samples (Dai & Mumper 2010, Puertas-Mejía et al. 2013).
The phenolic acid content in negro, pinto and bayo seeds of P. vulgaris were similar to those reported by Díaz-Batalla (2006) in terms of vanillin and quercetin. In contrast, the amounts of p-coumaric and ferulic acids, and kaempferol were higher. Comparing the data from black bean P. vulgaris with thev values reported by Ramírez-Jiménez et al. (2014), the contents of gallic and chlorogenic acids as well as catechin and rutin were similar. Caffeic, p-coumaric and ferulic acid values were higher, and the rutin, ferulic acid and quercetin values lower. Luthria & Pastor-Corrales (2006) studied 15 varieties of common bean and they founded great variability in their secondary metabolites. Their reported concentrations of caffeic, p-coumaric and ferulic acid were lower than the values reported here; however, sinapic acid was detected in their study. Moreover, protein contents reported here are lower compared with the one obtained by Luthria & Pastor-Corrales (2006) (15.94 mg / ml).
The findings were high quantities of caffeic and gallic acids, kaempferol, rutin and cathechin in the cultivated bean P. vulgaris. We expected these findings because of the secondary metabolites antioxidant, antimutagenic, antiviral and antimicrobe functions, anti-inflammatory properties, as well as the ability to regulate triglicerids metabolism and prevent degenerative illnesses (Cardador-Martínez et al. 2002, Mejía et al. 2003). In the case of gallic and caffeic acids and cathechin and kaempferol this goal was achieved.
The metabolites found in higher concentrations were ellagic acid, kaempferol and apigennin. These have antioxidant and antimutagenic properties, help in insulin liberation and reduce cardiovascular illnesses (Ramírez-Jiménez et al. 2014). When analyzing by species P. coccineus has the highest concentration of p-coumaric acid. When analyzing by origin, anthocianins, vainillin and amylase inhibitors were highest for samples from Sierra Gorda. Those metabolites mainly provide the fragrance to attract or deter pests and to reduce starch digestibility in insects (Mbogo et al. 2009). The concentration of secondary metabolites is highly diverse and could be affected not only by species type and domestication process, but also by storage, drying techniques and environmental conditions in crop fields, thus increasing the variability within each species and sample (Luthria & Pastor-Corrales 2006). According to our data, the gallic, ellagic, ferulic and p-coumaric acids and vainillin are the metabolites that differ most. For future research we widely suggest sampling in a gradient of origin that lends to identify the effects of this factor in the metabolites analyzed.











 nueva página del texto (beta)
nueva página del texto (beta)



