An emerging problem associated with the indiscriminate use of antibiotics is selection pressure, which results in bacteria with high levels of resistance (Fischbach & Walsh 2009). Therapeutic options are becoming limited, a serious problem that urgently needs to be adresssed (Roy et al. 2011). Among new anti-virulence strategies to combat resistant bacteria, inhibition of bacterial quorum sensing systems is the most frequently proposed and studied (Muñoz-Cazares et al. 2017).
Bacterial communication, or quorum sensing (QS), is a regulatory mechanism that depends on population density and promotes multicellular behaviour of bacteria. It is carried out by quorum sensing systems (QSS), which consist in the production, diffusion, detection and responses to chemical signaling molecules known as autoinducers that play a fundamental role in the expression of some phenotypes in terms of their pigments, bioluminescense, siderophores and, in the case of bacterial pathogens, the production of virulence factors and biofilm formation (de Kievit 2009, Stauff & Bassler 2011, Koh et al. 2013), which is the new target for antimicrobial chemotherapy (Zhang & Dong 2004, Adonizio et al. 2006). Unlike antibiotics, quorum sensing system inhibition (QSS-I) represses the expression of virulence factors and biofilms without affecting bacterial viability (Rasmussen & Givskov 2006, Fischbach & Walsh 2009). As a result, it is postulated that the bacterium does not develop resistance and the immune system can eliminate the infection (Roy et al. 2011).
New investigations have focused on discovering agents derived from synthetic and natural products to handle bacterial pathogenesis by means of QSS-I (Pan & Ren 2009). In Mexico around 4,000-5,000 species of plants have medicinal properties which are frequently used to treat disorders (Espinosa et al. 2008, Valdivia-Correa et al. 2016). The bark of “pochote” or “pochotl” trees is widely used in therapeutic applications. The name “pochote” is used in the traditional nomenclature to refer several species of the genus Ceiba distributed in different regions of Mexico (Canales et al. 2005, Pennington & Sarukhán 2005, Avendaño et al. 2006). Ceiba pentandra (L.) Gaertn. and Ceiba aesculifolia (Kunth) Britten & Baker f. (Malvaceae) are the best-known in the national territory.
Although C. pentandra is native to Central America, it has been introduced to various regions of the world (Gibbs & Semir 2003, SEMARNAT 2013) and extracts from its seeds, leaves, bark and fruits have been reported to have antibacterial activity (Anosike et al. 2012, Osuntokun & Adeoye 2017, Parulekar 2017). Also, compounds such as naphtaquinones, sesquiterpenoids, isoflavones, steroids, fatty acids and different glucosides in the extracts have been isolated (Noreen et al. 1998, Ngounou et al. 2000, Ueda et al. 2002, Kishore et al. 2003, Refaat et al. 2013), but only sesquiterpene lactones from the root bark showed bactericidal activity (Rao et al. 1993).
Ceiba aesculifolia is native to the Mexican tropical dry forest (Niembro et al. 2010) in central Mexico. Its bark is used to cure skin infections and wounds (Canales et al. 2005, Orozco et al. 2013, Franco et al. 2016). Methanolic extracts from the bark and fibers of mature fruits showed bactericidal activity. In these extracts phenolic compounds such as coumarins, flavonoids and phenylpropanoids were found to be the major components, together with isoflavones, sterols, terpenes and fatty acids (Orozco et al. 2013, Franco et al. 2016).
Commercial distribution of the Ceiba bark has not been documented. The bark can be found in traditional medicine stands in native markets, sold in pieces as “pochote”, with no differentiation between species. It is, however, important to differentiate species and their medicinal properties. Thus, methods of identification using anatomical characteristics to distinguish species of medicinal importance are needed to support pharmacognosy studies (Rivera-Arce et al. 2007, Rosas-Acevedo et al. 2011). In this study, we evaluated the effectiveness of C. aesculifolia and C. pentandra barks to inhibit QSS in Chromobacterium violaceum and Pseudomonas aeruginosa to propose them as possible source of anti-virulence metabolites. In addition, an anatomical study was made to support identification of the species.
Materials and methods
Plant material. Stem bark of Ceiba aesculifolia was collected in the municipality of Tierra Blanca, state of Veracruz, Mexico (18° 34.189’ N and 096°22.690’W). For C. pentandra, samples were obtained in the municipality of Acatlán, state of Oaxaca, Mexico (18° 32.677’ N and 096° 36.336’ W). Botanical identification was carried out by Dr. Antonio Guízar Nolasco (DICIFO/UACh), and two voucher specimens were deposited at the Herbarium of the Universidad Autóno- ma Chapingo (CHAP), registration numbers C. pentandra 66,486 and C. aesculifolia 66, 487.
Extract preparation and fractionation. The air-dried and powdered bark (1.0 kg) of the two Ceiba species was sequentially extracted with hexane (Hex), dichloromethane (D), and methanol (MeOH) (J.T. Baker®). The solvent was evaporated under low pressure, yielding CpHex 3.37 g, CpD 5.22 g, CaHex 2.42 g and CaD 4.32g of crude extracts. The hexane and dichloromethane extracts were subjected to a vacuum column chromatography using silica gel 60 (70-230 mesh, Merck®) and eluted with different mixtures of Hex-Ethyl acetate (EtOAc) and EtOAc:MeOH (J.T. Baker®), resulting in eight fractions of CaHex, 12 fractions from CaD, CpHex six fractions and 12 final fractions in CpD. The obtained fractions were concentrated and analyzed by thin layer chromatography (TLC). TLC analyses were performed according to conventional techniques, using 0.25 mm thick aluminum plates (60 F254 Merck®). The plates were visualized under UV light (254 nm) and subsequently developed with 2 % vanillin-10 % H2SO4 in ethanol.
Phytochemical screening. Active fractions were examined for the presence of common classes of secondary plant metabolites by TLC with various reagents to detect alkaloids, flavonoids, phenols, tannins, terpenes, triterpenes and steroids, following the methods described by Harborne (1984).
Anti-quorum sensing activity in C. violaceum. Two biomonitor strains were used. ATCC553 is a wild type strain that synthesizes violacein, a QS purple pigment, whose production is regulated by the C4 and C6 homoserine lactones autoinducer molecules (AHL). The other strain, CV026, is a mini Tn5 mutant-indicator derived from the wild type CV31532 strain; it is unable to synthesize its own AHL but retains the ability to respond against exogenous AHL.
The effect of extracts and fractions on the QS controlled production of violacein was determined using the wild-type ATCC553 strain, while the potential toxic effects on growth was monitored using the non-pigmented CV026 strain. To determine whether violacein was inhibited and bacterial growth was affected, a multi-well system assay was conducted. Cultures were adjusted to an optical density of 600 nm = 0.05 (105 CFU/mL-1) (Multiskan Spectrum) and seeded (200 µL) in each well of 96-well microtiter polystyrene plates (Corning®). Extracts and fractions were dissolved in dimethyl sulfoxide (DMSO) and 5 µL added to the cultures to final concentrations of 100 and 200 µg/mL. For all the assays at least three independent cultures were included.
Plates were incubated at 25 °C with constant shaking at 120 rpm for 48 h. DMSO and LB medium were used as negative controls and anacardic acid mixture (AA) 100 µg/mL as positive control (Castillo-Juárez et al. 2013). The violacein obtained after drying the culture medium was resuspended in 200 µL of DMSO and the absorbance was measured at 590 nm. To calculate the percentage of inhibition, absorbance of the negative controls was considered 100 % violacein production. Bacterial growth was determined by absorbance of the cultures at 600 nm. Inhibition percentage was calculated by subtracting the absorbance of the extracts and fractions from that of the cultures. The value obtained in LB medium controls was considered 100 % growth.
Anti-quorum sensing activity in Pseudomonas aeruginosa. To test the expression of virulence factors, a PA14 wild type was used. For all experiments, precultures were initiated aerobically from single colonies in LB broth at 37 °C, shaking at 200 rpm for 20 h. To evaluate QSS-I, overnight cultures were again inoculated in LB broth at initial turbidity of OD600 ~0.05 (UV-1800, Shimadzu). Extracts and fractions were then dissolved in DMSO and 5-10 µL added to 5 mL of the cultures with final concentrations of 128 to 500 µg/mL and incubated for 7 h. Bacterial growth was measured every two hours at 600 nm. DMSO was used as negative control, and the production of all the virulence factors was normalized by growth (absorbance 600 nm). After incubation time, cells were centrifuged and the supernatant was used to determine expression of QS-controlled virulence factors. For extracts, at least three independent cultures were included, while for fractions one assay was done with two replicates.
Pyocyanin production was determined spectrophotometrically after extraction from the cultures with chloroform and a further extraction with hydrochloric acid 0.2 N. The pyocyanin concentration was estimated from the peak to an optical density at 520 nm with a millimolar extinction coefficient of 2.46 mM-1 cm-1 (O’Malley et al. 2004). The pyoverdine present in the supernatants was assayed spectrophotometrically by absorbance at 407 nm, diluting the supernatants 1:10 in distilled H2O (Ren et al. 2005a).
Alkaline protease activity was detected spectrophotometrically by the Hide-remazol blue assay, the absorbance was measured at 595 nm (Howe & Iglewski 1984). Quantification of elastolytic activity in the supernatants was determined by elastin-congo red (ECR) SIGMA assay, according to a previously reported procedure (Maeda et al. 2012).
Histological methods. Segments of the inner and outer bark (3 × 2 cm) of four C. aesculifolia and C. pentandra individuals were obtained at a height of 1.30 m from the main stem. Subsequently, the segments were softened in a solution of ethyl alcohol, glycerin and water (GAA, 1:2:3) for a period of 30 days. For the microtechnical procedure transverse, tangential and radial sections (20-30 µm) were made, using a sliding microtome. In the case of tangential sections, serial cuts were made from the bark to the vascular cambium. Sections were stained with safranin-fast green to be mounted in synthetic resin (Ruzin 1999). Ceiba bark was described anatomically following the recommendations of Trockenbrodt (1990) and Angyalossy et al. (2016). Images were obtained using the analyzer elements NIS-BR 2.33 (Nikon corporation 1991-2006). The general images were prepared using a Lucida camera at 1X on a Nikon Labophot-2 microscope.
Statistical analyses. The results are presented as the average and standard deviation of at least three independent experiments. For extracts and fractions against violacein production in C. violaceum the Student´s t test was used, while fractions gainst P. aeruginosa virulence factors were analyzed by One-way with Bonferroni test. These analyses were done in IBM-SPSS 22v software.
Results
Inhibitory activity of the extracts in production of QSS-regulated C. violaceum and P. aeruginosa phenotypes. QSS-I was observed mainly in the extracts CaD, CpHex and CpD, which inhibited violacein production 60 %, while non-significative effects on CV026 strain growth were found (Figure 1). CaMeOH, CpMeOH and CaHex extracts reduced pigment production but showed significant effect on CV026 strain growth (Figure 1). In these assays, a AA mixture was employed as a positive control since a previous study reported that this compound inhibits the production of violacein (Castillo-Juárez et al. 2013). Only extracts from C. aesculifolia at the highest dose (384 µg/mL) significantly inhibited P. aeruginosa virulence factors. CaHex decreased elastolytic activity (26.5 %) and CaD inhibited pyocyanin, pyoverdin and elastolyic activity up to 30 %, without affecting bacterial growth (data not shown).
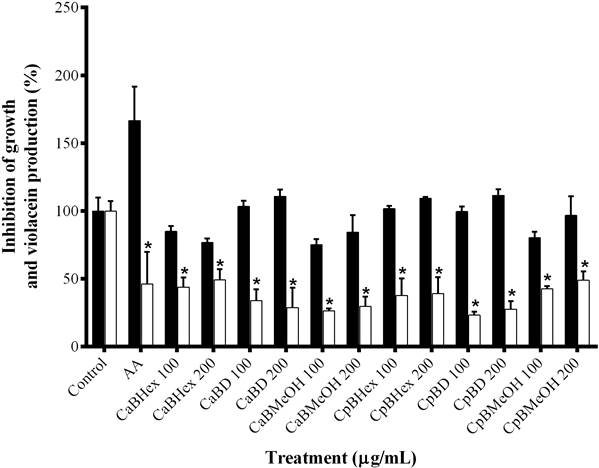
Figure 1 Inhibition of violacein production by C. aesculifolia and C. pentandra bark extracts. Black bars represent bacterial growth (mutant CV026) and white bars represent violacein production (CV12472 WT). AA = anacardic acid mixture. Hex = hexane, D = dichloromethane, MeOH = methanol. *P < 0.05 by Student´s t-test compared with control.
Effect of the fractions on production of QSS-regulated C. violaceum and P. aeruginosa phenotypes. Only fractions with a yield sufficient for conducting the assays were selected. The fractions with significant QSS-I, as well as the yield and system of elution, are shown in Table 1. The fractions exhibited different activities stimulating or inhibiting virulence; ICaHex, VICpHex, ICpHEX and IIICpD were the most active fractions against the pyocyanin and alkaline protease activity of P. aeruginosa. Interestingly, whereas these fractions inhibit virulence factors in this bacterium, C. violaceum stimulates production of violacein or shows a discrete inhibitory effect.
Table 1 Effect of the fractions of Ceiba aesculifolia and Ceiba pentandra on violacein production in Chromobacterium violaceum and two virulence factors of Pseudomonas aeruginosa.
| Specie | Extract | Fraction/ Solvent |
Yield (mg) |
Inhibition of QS (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
C. violaceum (200 µg/mL) |
P. aeruginosa (500 µg/mL) |
|||||||||||
| VN | sd | GW | PY | sd | AP | sd | ||||||
| Ceiba aesculifolia | H | I | 9H:1EA | 485 | +3 | 26.1 | +3 | 57.4** | 1.7 | 79.5** | 7.7 | |
| II | 8H:2EA | 352 | 42* | 7.9 | -7 | +31.9 | 8.7 | +14.2** | 27.2 | |||
| III | 6H:4EA | 282 | 45* | 7.6 | -3 | 18.8 | 7.8 | 38.6 | 18.9 | |||
| IV | 3H:7EA | 129 | 60* | 12.8 | -16 | 12.8 | 1.1 | 5.86 | 6.19 | |||
| V | 9A:1M | 8.6 | 69* | 8.7 | +15 | +15.2 | 2.8 | 3.9 | 4.6 | |||
| D | VI | 9H:1EA | 115 | +40* | 30.4 | -15 | 59.7** | 1.3 | 75.3** | 0.1 | ||
| VII | 1EA | 54 | 22* | 4.5 | +23 | +23.8 | 11.2 | +41.3** | 46 | |||
|
Ceiba pentandra |
H | I | 8H:2EA | 520 | 34* | 8.9 | +5 | 59.6** | 1.9 | 88.3** | 11.6 | |
| II | 6H:4EA | 310 | 46 | 9.5 | +15 | 20.2 | 14.8 | 2.13 | 26.4 | |||
| D | III | 8EA:2M | 370 | 24* | 6.1 | +20 | 51.9** | 2.7 | 78.8** | 0.39 | ||
H = hexane. D= dichrolometane. EA = ethyl acetate. M = methanol. VN = violacein. GW = growth. PY = pyocyanin. AP = alkaline protease. sd = standar deviation.Yield = mg for every Kg. * P < 0.05 by Student’s t-test. ** P < 0.05 by Bonferroni test.
Dose-response QSS-I effect of fractions on the alkaline protease activity of P. aeruginosa. The dose-response effect of the active fractions ICaHex, VICaD, ICpHex and IIICpD on alkaline protease activity and growth of P. aeruginosa was analyzed, and the effect was observed only for ICpHex and ICaBHex (Figure 2). It should be noted that the fractions at higher doses of 250 µg/mL presented problems of solubility in the cultures, a phenomenon that may be responsible for the unclear dose-response effect in some fractions.
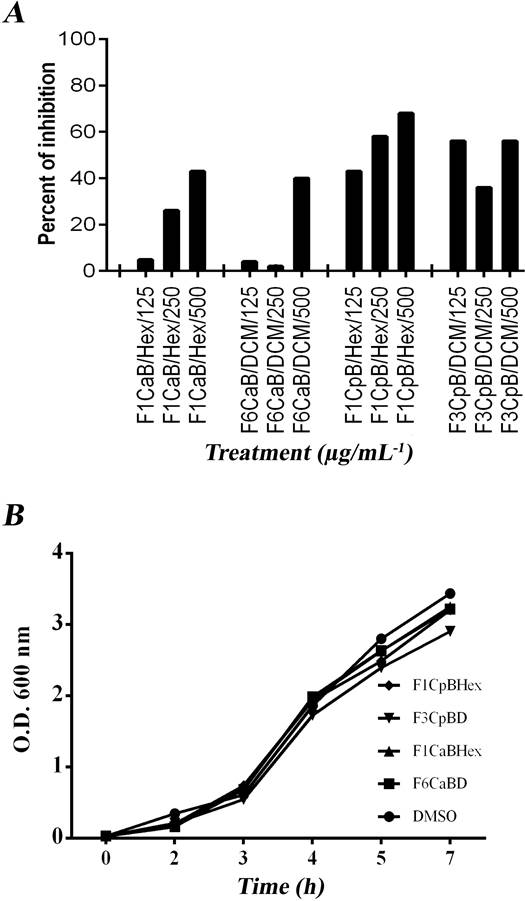
Figure 2 Dose-response effects of C. aesculifolia and C. pentandra fractions on production of QSS-controlled virulence factors. A) Growth. B) Alkaline protease activity. Data show the average of at least three independent experiments.
Principal groups of metabolites present in the active fractions. The active fractions were screened for the presence of common classes of plant secondary metabolites. The four fractions analyzed are composed mainly of terpene-type metabolites (Table 2). Triterpene and steroidal type compounds were detected in ICaHex, VICaD and ICpHex, whereas flavonoids were found in VICaD and 1CpHex.
Table 2 Results of phytochemical screening of active fractions of Ceiba aesculifolia and Ceiba pentandra.
| Metabolites | Test/reagent | Fraction/Result | |||
|---|---|---|---|---|---|
| ICaHex | VICaD | ICpHex | IIICpD | ||
| Terpenoids | 2 % Vanillin/ 10 % H2SO4 ethanol | positive | positive | positive | negative |
| Flavonoids | 1 % NP /5 % PEG | negative | positive | positive | negative |
| Alkaloids | Dragendorff | negative | negative | negative | negative |
| Steroids and triterpenoids | Liebermann-Burchard | positive | positive | positive | positive |
| Tannins | FeCl3/Folin-Ciocalteu | negative | negative | negative | negative |
| Phenols | FeCl3/Folin-Ciocalteu | negative | negative | negative | negative |
Ca = Ceiba aesculifolia. Cp = Ceiba pentandra. Hex = Hexane. D = Dichloromethane.
Anatomical differences between C. aesculifolia and C. pentandra barks. Clear differences between the Ceiba barks (Figure 3) were seen in cross-section. In C. aesculifolia, the prickles are stratified (stratified phellem): 2-3 layers of cells with clear lumens and thin walls alternate with numerous layers of thicker-walled cells (Figure 3B). This stratification is repeated (Figure 3A) up to more than five times in some prickles. In C. pentandra, the prickles are smaller and form a homogeneous tissue (nonstratified phellem) composed of numerous cell layers of phellem elongated radially (Figure 3F,G). Furthermore, C. pentandra shows some of multiseriate rays strongly dilated near the vascular cambium (wedge-shaped) alternating with irregularly dilated rays (Figure 3F). In contrast, C. aesculifolia has multiseriate rays that are longer and irregularly dilated toward the periphery (Figure 3A). Interspersed among the rays, there are evident groups of sclereid cells, which are large and tangentially elongated, in the nonconducting phloem of C. pentandra, while those of C. aesculifolia are irregularly rounded, smaller and close to periderm (Figure 3C). In the conducting phloem, narrow fiber bands are evident (Figure 3I, J), prismatic crystals are numerous and druses scarce in C. pentandra (Figure 3I), while in that of C. aesculifolia druses are more numerous (Figure 3E).
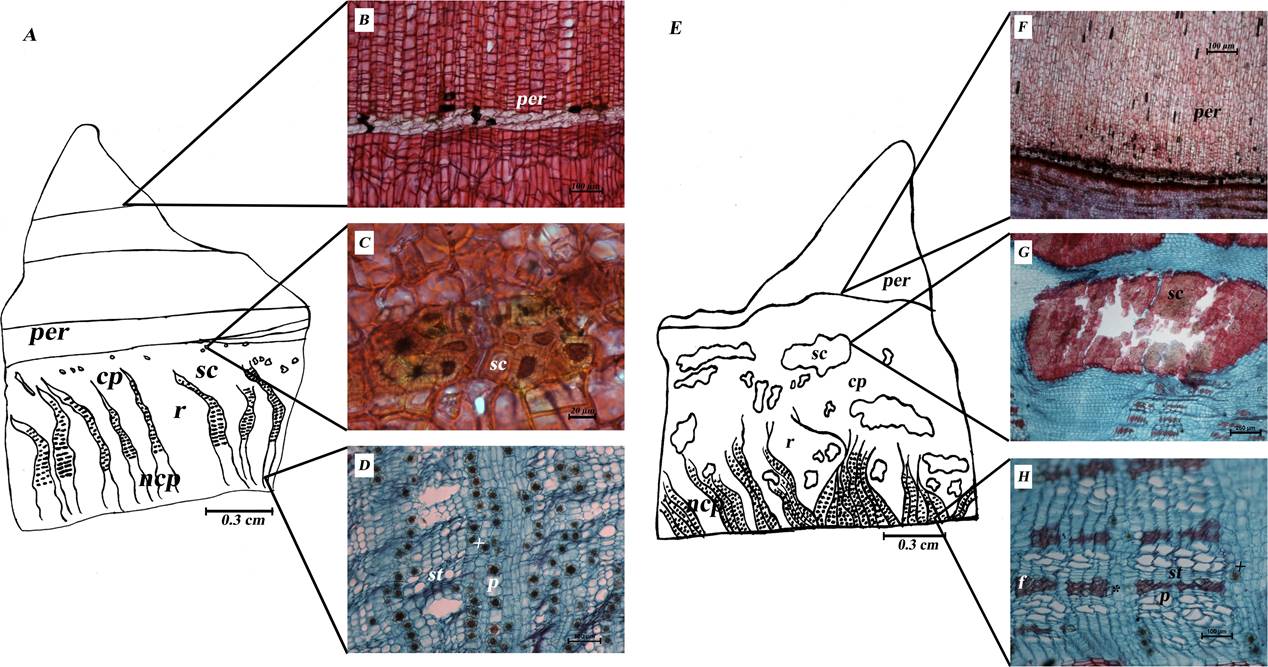
Figure 3 Sections of Ceiba barks: A-J) C. aesculifolia transverse sections: B) Stratification of prickle phellem, C) sclereid groups, D) conducting phloem, E) Druses in conducting phloem. F-J) C. pentandra transverse sections: G) nonstratified prickle phellem, H) sclereid groups, I,J) conducting phloem. Abbreviations: cp = conducting phloem; f = fibers; np= nonconducting phloem; per = periderm; r = ray; st = sieve tube; sc = stone cells; + = druses; * = prismatic crystal.
Discussion
The bacteria-plant interaction is a phenomenon that has enabled plants to perfect evolutionary strategies, which include production of metabolites to regulate bacterial QSS (Nazzaro et al. 2013). Ceiba aesculifolia and C. pentandra exhibit this type of strategy. Their barks showed QSS regulatory activity, indicating the presence of inhibitor and promoter metabolites. Differences in the activity recorded in the two biological systems used may be ralated to the complexity of the QSS of bacterial species.The two bacterial species used in our study reflect different complexities of their QSS. C. violaceum is an aquatic bacterium that can infect humans and cause abscesses and bacteraemia (Stauff & Bassler 2011). The wild-type strain and biosensor mutants of this bacterium are widely used in the study of QSS-I by natural products (Steindler & Venturi 2007) since its purple pigment violacein production is controlled by a single QSS. On the other hand, to date, in P. aeruginosa three QS interrelated systems that regulate production of virulence factors such as pyocyanin, pyoverdine, alkaline protease and elastolytic activity have been reported (Christensen et al. 2007, Gellatly & Hancock 2013). This bacterium is an opportunistic pathogen that is a major health problem worldwide, responsible for 10 % of nosocomial infections (Antunes et al. 2010, Castillo-Juarez et al. 2015, García-Contreras 2016) and classified as a pathogen of critical prority by the World Health Organization (WHO 2017).
Our results indicate that the diversity of metabolites in the two Ceiba species is complex. The bark extract exhibited overall QSS-I effect. However, when extracts were fractionated, different effects were found. Several fractions stimulated while others inhibited virulence factors. Moreover, some had a slight effect on strain growth. Regulatory behavior over QSS (positive or negative) may be related to changes in concentration of metabolites as well as to selectivity of the molecules over each QSS. It is necessary to investigate the effect of purified and identified molecules from the extracts to define their selectivity and antagonistic effects on other bacterial QSS, as well as their mechanisms of action.
Although our study did not identify the molecules involved, we identified the major groups of metabolites in the active fractions against the P. aeruginosa. Our analysis revealed that they were composed mainly of terpenes, triterpenes and sterols. The active fractions may be an important source of new inhibitor metabolites, potentially expanding the repertoire of QSS-I molecules.
This result is important because there are few reports of this type of metabolites as QSS-I.
However, pentacyclic triterpenes derivatives (oleanane, corosolic, asiatic and ursane) have shown anti-biofilm and anti-virulence activity against E. coli, S. aureus, P. aeruginosa and V. harveyi were reported (Eldrige 2005, Ren et al. 2005b, Hu et al. 2006, Garo et al. 2007, Gilabert et al. 2015). Also, sterols from species of the genus Dalbergia showed inhibitory activity against virulence factors of P. aeruginosa (Rasamiravaka et al. 2013).
Our results suggest the presence of bactericidal molecules in the extracts or fractions, that may mask QSS-I activity. A representative example was the C. aesculifolia extract, which reduced the violacein production, but affected bacterial viability. However, presence of bactericidal molecules could complement QSS-I molecules to provide a more potent anti-virulence effect, as demonstrated when asiatic and corosolic acid increased susceptibility of P. aeruginosa biofilm to tobramycin (Garo et al. 2007). Other reports also showed that others QSS-I molecules from natural sources can potentiate the effect of antibiotics against pathogenic bacteria (Rasmussen & Givskov 2006, Pan & Ren 2009), favoring the use of lower doses and avoiding indiscriminate use of broad-spectrum antibiotics (Bjarnsholt & Givskov 2007).
As we have seen, the QSS-I activity of the fractions of the extracts of the different Ceiba species were not the same, and thus it is important to distinguish between the species. In this sense, the anatomical characteristics of bark may be helpful in species identification (SEMARNAT 2013). Some general traits of bark anatomy of the Ceiba genus has been mentioned by Roth (1981), but species-specific traits have not been studies until now. The two species described here follow the general pattern of the genus, but we did find differences. The phellem characteristics of the prickles, ray dilation close to vascular cambium, fibers evident in conducting phloem, as well as the position, size and form of sclereid groups, are the most noticeable bark features that distinguish the two species anatomically. We suggest further anatomical studies to provide information related to the recognition of species and location of possible active principles within the plant tissues.
In this study we determined that extracts and fractions obtained from the two species of Ceiba are sources of phytochemicals with the ability to regulate positively or negatively the bacterial QSS evaluated. The active fractions are rich in terpenes and sterols, QSS-I metabolites which have been poorly studied in contrast with other groups. Future work will focus on isolation and identification of these metabolites.











 text new page (beta)
text new page (beta)


