Approximately one-third of the land worldwide are dedicated to the growth of crops (FAO 2006). However, the quality and fertility of the soils, including the microbial biodiversity and its capacity to buffer pathogens and pollution have decreased (Doran & Parkin 1994, Karlen et al. 1997, Gianinazzi et al. 2010) with a consequent reduction in productivity. The dominant strategy to increase soil quality and agroecosystems productivity is centered in the use of chemical or inorganic fertilizers (Bhardwaj et al. 2014). Hence, it is a priority to investigate, develop and transfer innovative methods for a sustainable agriculture considering social, economic and ecological factors (Goodland & Daly 1996, Hünnemeyer et al. 1997). An alternative that is gaining terrain is the use of biofertilizers allegedly more compatible with the environment. Hence, the use of biofertilizers formulated with microorganisms such as fungi and bacteria are a key alternative for sustainable agriculture. Accordingly, understanding the processes that occur belowground is a major challenge to install in earnest sustainable agricultural at least at the regional scales to catapult productivity while being concordant with conservation actions, the restoration or rehabilitation of disturbed ecosystems and agroecosystems (Álvarez-Sánchez & Peña 2009).
Arbuscular mycorrhizal (AM) fungi (Phylum Glomeromycota) are a key functional group of the soil biota, their hyphae proliferate in the root cortex and grow into the soil matrix where they facilitate the uptake of water and mineral soil nutrients (phosphorus, nitrogen and micronutrients) by their host plants (Lee & George 2005, Smith & Read 2008). Due to the improved nutrient acquisition, mycorrhizal symbiosis often increases plant growth, health and fitness. In consequence, mycorrhizal plants are better suited to tolerate biotic (e.g., herbivores, pathogens, Wehner et al. 2010) and abiotic (e.g., drought, salinity, heavy metals, Miransari 2010) stresses than non-mycorrhizal plants. Therefore, it is relevant to understand the symbiosis between plants and AM fungi to develop a truly sustainable agriculture.
Some studies have documented that crops are positively affected by mycorrhizal interactions (reviewed in Naher et al. 2013). However, mechanistic and functional understandings of these interactions are poorly understood still since several factors such as the environmental conditions (soil nutrients availability and climatic variables), soil biogeochemistry, the symbiont’s identities and the origin of the inoculant are known to affect the outcome of the mycorrhizal symbiosis (Berruti et al. 2016). For instance, high concentrations of chemical fertilizers may favor plant genotypes that are less tolerant, or capable of associating with microbial symbionts (Kiers et al. 2007), also suppress AM colonization, and mycorrhizal functions such as the efficiency of the mycorrhizal to uptake nutrients from the soil (Thonar et al. 2011), affecting negatively the mass of extraradical mycelium and the abundance and diversity of fungal propagules in the soil (Oehl et al. 2003, 2010).
Recently, Berruti et al. (2016) found that out of 127 studies 15 % have been done with commercial inocula and the majority have used one fungal species. Despite the apparent standardization that may be thought across these studies a major drawback is that no information is available on how the spores were propagated leaving a black box regarding the genetic diversity and geographic origin of the inoculum used. Also, Berruti et al. (2016) found that overall, the plants growing with native inocula were bigger and had better protection against pathogens and high root colonization compared to plants supplemented with commercial mycorrhizal inocula (Berruti et al. 2016, Emam 2016).
In Mexico, the use of mycorrhizal inocula is relatively recent, and few studies have evaluated their effects on the crops (Montaño et al. 2012). In the present study, we compared the infectivity and effectivity of different commercial mycorrhizal inocula and AM fungi spores extracted from native natural ecosystems on the performance of seedlings of Zea mays under greenhouse conditions. Given that several inocula do not promote mycorrhizal colonization and neither enhance plant biomass (Corkidi et al. 2004), we hypothesized that seedlings inoculated with native fungi would have higher biomass and AM colonization inside the roots than seedlings inoculated with commercial inocula, and inoculated plants will grow bigger than non-mycorrhizal plants.
Materials and methods
Study species. Zea mays L. (Poaceae) is native to the uplands of Mexico, was domesticated by indigenous people, and is a major cereal grain crop now cultivated worldwide in various agroecological environments (Kato-Yamakake et al. 2009, Sánchez-Ortega 2014). Zea mays is an annual or perennial, monocot and monoecious plant (separated pistillate and staminate spikelets in the same plant). Culms grow to 0.5-6 m tall, 1-5 cm thick, monopodial, often branching, lower nodes with prop roots, internodes pith-filled. Maize is a fast-growing plant, its roots are readily colonized by AM fungi (highly mycotrophic) and induces high AM sporulation (Liu & Wang 2003, Yao et al. 2010).
Mycorrhizal inoculum. Commercial inoculum.- We used two commercial mycorrhizal inocula (biofertilizers), bought in a local store of agroindustrial products in Tepic, Nayarit, Mexico. One biofertilizer was in solid presentation (5,500 AM spores kg-1) included Glomus constrictum, G. tortuosum, G. geospurum, Acaulospora scrobiculata, Gigaspora margarita. Accordingly with the manufacturer the recommended application was 1 kg ha-1 suspended in 1 to 3 liters of guanofol™ (bat guano liquid fertilizer, product not included) depending of the crop. The suspension is used to soak the seeds before they are sown. The second biofertilizer was in liquid presentation and included separately 250 ml of the nitrogen fixing bacteria Azospirillum brasilense (1×1011), and 250 ml with a blend of spores of a single species of Glomus sp. (6×103) and Trichoderma sp. (7×109). According to the manufacturer, the content of the two bottles is combined and used to soak sufficient seeds to sow a hectare, although the dose could vary up to three-fold depending on the crop. The inocula chemical parameters were carried out in the Laboratorio de Análisis de suelo, Agua y Plantas of the Unidad Académica de Agricultura, Universidad Autónoma de Nayarit (Table 1).
Table 1 Nutrient parameters analyzed in the two commercial inoculants (liquid and solid). N = nitrogen, P = phosphorus, K = potassium, Ca = calcium, Mg = magnesium, Na = sodium, EC = electric conductivity, OM = organic matter.
| Nutrient | Liquid (mg L-1) | Liquid mix (mg L-1) | Solid (g kg-1) |
|---|---|---|---|
| N | 30.1 | 33.6 | 2.3 |
| P | 8.6 | 5.1 | 0.6 |
| K | 776.0 | 561.0 | 2.1 |
| Ca | 134.0 | 415.0 | 98.6 |
| Mg | 25.0 | 65.0 | 14.2 |
| Na | 49.0 | 243.0 | 0.7 |
| pH | 7.2 | 7.7 | 7.8 |
| EC (ds m-1) | 49.0 | 2.2 | 0.3 |
| OM % | - | - | 6.0 |
Native inoculum.- AM spores were extracted from a tropical cloud forest fragment localized in Cumbres de Huicicila (21°17' 49.1" N, 105° 01' 39" W, at an elevation of 916 m) in the municipality of Compostela, Nayarit. The soil was collected using a metal soil core sampler (2.5 cm of diameter and 25 cm long) in ten plots of 5 × 5 m along a transect (five plots to the right, and five plots to the left, one by one). In each plot we collected 5 soil cores (~800 g) consolidated into a single sample. AM spores were extracted with the wet sieving and decanting method (Gerdemann & Nicolson 1963); using 212, 106 and 38 µm sieves, followed by two centrifugation cycles: first with water at 3,000 rpm for five minutes and then after disposing all the liquid phase we added 70 % sucrose solution to the sediment and it was suspended by vigorous shaking and then centrifuged at 2,500 rpm for two minutes. The spores were stored in 1,000 ml of water to posteriorly be used as inoculum. A total of nine AM morphospecies were identified in the inoculum: Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüßler, Septoglomus constrictum (Trappe) Sieverd., G.A. Silva & Oehl, Claroideoglomus sp., Acalospora sp., and five non-identified species.
Experimental design. We performed an unifactorial experiment with five inoculum levels: 1) liquid, only Glomus sp. and Trichoderma sp., 2) liquid mix, Glomus sp., Trichoderma sp., and Azospirillum brasilense, 3) solid, 4) native AM spores (AM-native), and 5) non-mycorrhizal inoculum (NM-inoculum). Maize seeds were provided by the Laboratorio de Semillas of the Unidad Académica de Agricultura, Universidad Autónoma de Nayarit, Mexico. The seeds were of the yellow variety, and were harvested on January 6, 2016 at the Unidad Académica de Agricultura. Seeds were disinfected with sodium hypochlorite 1 % solution for five minutes and 150 seeds were germinated on February 13, 2016 in a germination tray filled with sterilized commercial soil, to ensure that the seedlings were not colonized by AM fungi. The soil was acquired in a local nursery, and then was heat sterilized at 125 ºC for 1.5 h to kill AM propagules and microorganisms. The soil chemical parameters after sterilization were: organic matter 20.3 %, nitrogen 442 kg ha-1, phosphorus (P) 432.1 mg kg1, and pH 7.7. These values were high in agreement with the Official Mexican Norm PROY-NOM-021-RECNAT-2000 (SEMARNAT 2002). Soil chemical analyses were carried out in the Laboratorio de Análisis de Suelo, Agua y Plantas of the Unidad Académica de Agricultura, Universidad Autónoma de Nayarit.
After 13 days of germination, we selected seedlings of similar size to minimize initial differences in plant biomass; additionally, the initial fresh total biomass was determined at the onset of the experiment for each seedling. Consequently, at the beginning of the experiment the seedlings allocated to each treatment did not differ in their average fresh weight (F 4,120 = 2.061, P = 0.090, mean ± standard error: NM-inoculum 3.534 ± 0.168, AM-native 3.691 ± 0.135, liquid 3.057 ± 0.129, liquid mix 3.240 ± 0.207, and solid 3.445 ± 0.104). Individual pots were filled with 1,300 cm3 of a commercial soil containing heat autoclaved soil (chemical parameters of soil were described above), and 25 seedlings were allocated to each treatment. The commercial mycorrhizal inocula were applied in agreement with the manufacturer instructions, and the spores number applied were an estimate based on the spore concentration mentioned by the manufacturer: 1) liquid, 1 ml containing 24 spores was added on the roots, 2) liquid mix, we used a mix 1:1 of both bottles and 1 ml containing 12 spores was added to the roots, 3) solid, a solution was made with 95.45 g of AM biofertilizer in 1 L of water, and 25 ml (six spores per 1 ml) were added to the roots, 4) AM-native, 7 ml of water containing ~100 spores were added to the roots and 5) NM-inoculum received 1 ml of water without AM spores. All pots received an additional 1 ml of a soil microbial suspension filtered through a 0.8 µm nitrocellulose Millipore filter to partially return the microflora to the sterilized soil. The microbial suspension was obtained from the commercial soil before of the sterilization. In order to know the approximate number of spores inoculated in the AM treatments, we took 10 aliquots of 1 ml per type of AM inoculum and the spores in each ml were counted using a stereomicroscope. In the commercial fertilizer we did not observed spores of AM fungi, but in the native AM inoculum we counted on average 15 spores per milliliter.
The experimental pots were grown in a greenhouse at the Unidad Académica de Agricultura, Universidad Autónoma de Nayarit, and we rotated the pots every week. The plants were watered with tap water every day.
Plant parameters. Given that we were interested only in the initial inoculation and not in the recolonization by the production of new spores, the plants were harvested after 32 days with the objective of estimating the seedling’s biomass and root colonization by AM fungi. Corkidi et al. (2004) observed AM root colonization in seedlings of Zea mays by the commercial mycorrhizal inoculants after four weeks of having been applied. During the harvest, the final fresh total biomass was obtained and the relative growth rate (RGR) was calculated as: ln(final total biomass) - ln(initial total biomass) / 32 days. Afterwards, we separated below- and aboveground biomass and oven-dried in paper bags at 60 °C for 3 days and then, the root/shoot (R/S) ratio was calculated as belowground/aboveground dry biomass.
Fungal parameters. During the harvest, we took 0.5 - 1 g of root sample to assess mycorrhizal colonization before drying the roots. The roots were stained with trypan blue (0.05 %) after clearing by 24 h incubation with 10 % KOH, then the roots were rinsed several times with tap water and were acidified with 10 % HCl for 10 min (Koske & Gamma 1989). We calculated the colonization frequency by AM fungi in 15 root fragments (each ~1.5 cm long) from each seedling. Each root fragment was examined at three equally spaced points under a light microscope at 100x and 400x total magnification, using the cross-hair intersection method (McGonigle et al. 1990). The presence/absence of fungal structures (hyphae, vesicles and arbuscules) was used to calculate the percentage of root colonization by AM fungi as: % of colonization = (sum of positive counts / total number of points observed) × 100.
Statistical analyses. All statistical analyses were performed with the statistical language R (R Core Team 2016). To assess the potential best models with which to analyze each type of variable, we performed a graphical data exploration (Zuur et al. 2010). For all models, we examined whether the residuals were normally distributed and homoscedastic, when necessary, data transformations were used (see below).
To test for differences in initial fresh biomass between the levels of inoculum treatments (liquid, liquid mix, solid, AM-native and NM-inoculum), we used one-way ANOVA. We also used one-way ANOVA to test differences among the inoculum treatments on the RGR, the R/S ratio, total, below-, and aboveground masses. To meet the model assumptions, a rank transformation (method average) was applied to the R/S ratio and belowground biomass. Significant differences among the levels of the inoculum factor (liquid, liquid mix, solid, AM-native and NM-inoculum) were tested with Tukey’s test.
The mycorrhizal colonization was only observed in two mycorrhizal treatments (AM-native and solid treatment), therefore, we did not do any statistical analyses.
Results
Plant parameters. At the end of the experiment, all seedlings survived. For all response variables the ANOVA models showed statistically significant effects of the treatments (RGR: F 4,120 = 13.780, P < 0.001, total biomass: F 4,120 = 23.620, P < 0.001, belowground biomass: F 4,120 = 8.620, P < 0.001, aboveground biomass: F 4,120 = 30.990, P < 0.001, root/shoots ratio: F 4,120 = 2.770, P < 0.030).
The seedlings growing in the NM-inoculum and liquid mycorrhizal treatment showed no significant differences in RGR but these two treatments were significantly different from the other mycorrhizal inoculum treatments (Figure 1a), which did not differ amongst each other. In regard to total biomass, the NM-inoculum seedlings had higher total biomass than mycorrhizal inoculum treatments (Figure 1b). But within mycorrhizal inoculum treatments, the seedlings in the liquid and solid AM inoculum had similar biomass and did not show significant differences (Figure 1b); also, there were no significant differences between AM-native and liquid mix, and between AM-native and solid (Figure 1b).
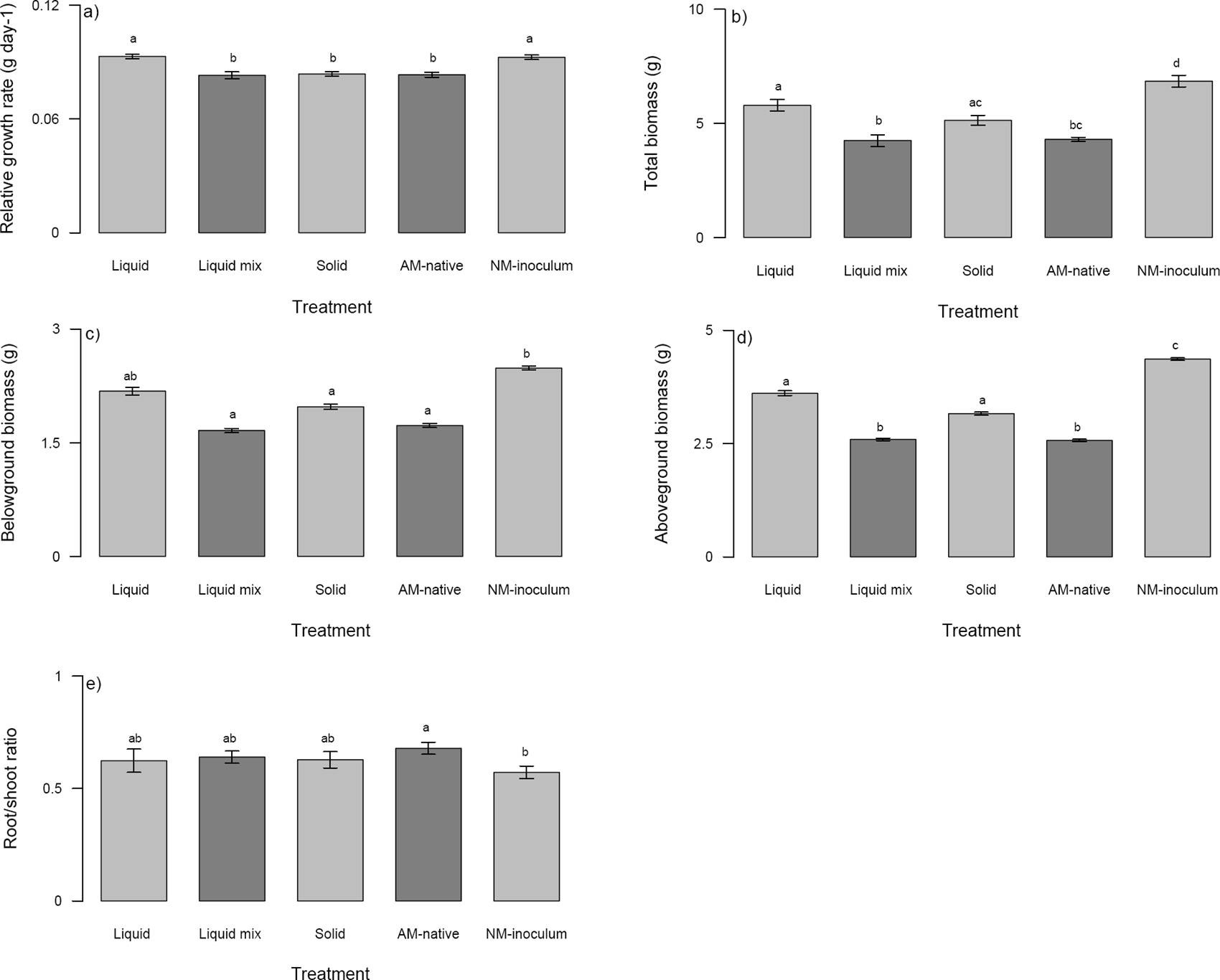
Figure 1 Plant parameters analyzed in Zea mays seedlings growing with different arbuscular mycorrhizal treatments (liquid, liquid mix, solid, AM-native, and NM-inoculum). a) Relative growth rate (g day-1), b) Total biomass (g), c) Belowground biomass (g), d) Aboveground biomass (g), e) Root/shoot ratio. Different letter above the bars indicate statistically significant differences among arbuscular mycorrhizal treatments according to Tukey’ test (P ≤ 0.005). Mean ± standard errors are given without data transformation.
The mycorrhizal inoculum treatments affected the belowground biomass, which was greater in the NM-inoculum and liquid mycorrhizal treatment and did not show significant differences between them (Figure 1c). However, the liquid mycorrhizal inoculum did not show significant differences with the other three mycorrhizal treatments (Figure 1c). Regarding the aboveground biomass, the NM-inoculum had the highest biomass when compared to the other treatments, while the AM-native and liquid mix had the lowest biomass (Figure 1d). The liquid and solid treatments had intermediate values of aboveground biomass (Figure 1d) and there were also no significant differences.
The R/S ratio was also affected by the mycorrhizal inoculum treatments, the NM-inoculum and AM-native treatments were the only treatments that did show significant differences (Figure 1e).
Fungal parameters. At the end of the experiment, all NM plants remained non-mycorrhizal. The only treatments that showed intraradical mycorrhizal colonization were the AM-native and solid treatments. The AM-native inoculum had a greater total mycorrhizal colonization (mean ± standard error) 14.723 ± 1.666 %, compared with the solid treatment 2.311 ± 0.898 %. The fungal structures more frequently observed were the hyphae; and the AM-native treatment had greater percentage of hyphae (14.264 ± 1.542 %), while the solid treatment had lower colonization (2.311 ± 0.898 %). The AM-native treatment was the only mycorrhizal treatment where the arbuscules (specialized haustoria for nutrient exchange between fungi and their host plant) were observed, although their frequency was very low (0.458 ± 0.323 %).
Discussion
In the present study, we compared the effect of commercial and native mycorrhizal inocula on the growth of maize seedlings. Unexpectedly, the seedlings growing in the AM-native and NM-inoculum, in general, had the lower and greater biomass respectively; while the commercial mycorrhizal inoculum had intermediate values of biomass. Therefore, our initial hypothesis was not supported.
Several studies have shown that mycorrhizal inoculation has positive effects on cultivated plants in terms of growth, uptake of mineral nutrients, and tolerance to biotic and abiotic stresses (Naher et al. 2013). Although the opposite pattern has also been documented (e.g., Valentine et al. 2001, Gosling et al. 2013) and this is consistent with our results in terms of biomass. The results found in this study might be explained by three hypotheses. First, high concentrations of phosphorus in the soil. Several studies have shown that high levels of phosphorus can induce a depression in the growth of mycorrhizal plants compared with non-mycorrhizal plants (Phosri et al. 2010, Smith & Smith 2011). For instance, Valentine et al. (2001) observed a growth depression in Cucumis sativus growing in soil with high concentrations of phosphorous, likely due to a reduction in the photosynthetic nitrogen-use efficiency. Also, a negative correlation between high phosphorous concentrations and intraradical mycorrhizal colonization has been documented, for instance, in Carica papaya (Vega-Frutis & Guevara 2009) and Zea mays (Lu et al. 1994, Gosling et al. 2013). Because the commercial soil used in this study had high concentrations of mineral nutrients, this might explain the greater biomass observed in the NM-inoculum and the lower mycorrhizal percentage observed.
Second, edaphic origin of AM-native. Johnson et al. (2010) showed that the AM fungi are locally adapted to its specific soil site, therefore, this could explain the low plant biomass observed in the AM-native, because we used a commercial substrate. However, the AM-native was the only treatment where we observed arbuscules, this could indicate a functional symbiosis compared with the commercial inocula. In this way, Corkidi et al. (2004) found that three of ten commercial inocula did not promote the AM colonization, in our study, likely the commercial inocula used had low density of spores or unviable spores. Another possibility is the functional incompatibility between AM species and host plants (Barea et al. 1991, Cuenca 2009). Although there is no strict host-specificity, there is a host-preference between the symbionts (Kiers et al. 2011). Even though the maize is considerate a mycotrophic species, Gosling et al. (2013) found that maize plants had a preference for certain AM species, it has recently been shown that the “plants can detect, discriminate and reward with more carbohydrates to the best fungal partners” (Kiers et al. 2011, p. 80).
The commercial solid inoculum used in this study had five AM species, three species from the genus Glomus (Glomus constrictum, G. tortuosum, G. geospurum) together with Acaulospora scrobiculata and Gigaspora margarita, the genus Glomus has been found in the roots of maize (Gosling et al. 2013). Therefore, it is likely that both the variety of maize and the species of AM spores used did not form a functional symbiosis. It is also possible that the mycorrhizal infective potential (capacity of AM fungi propagules within the soils to produce mycorrhizal symbiosis, Klironomos & Hart 2002) in the commercial inocula could be low at least in the solid treatment. This is consistent with lower AM colonization in the roots of maize growing with the solid inoculum compared with the native-inoculum. However, the infectivity could also be explained as a function of the experiment time, Corkidi et al. (2004) showed that only two of ten commercial inocula colonized the plants in four weeks, but at the six weeks other four products also colonized the roots of the plants. Therefore, our result might also be explained by the short time of the experiment.
We did not observe any spores and fungal structures in the seedlings inoculated with the liquid inocula, and this agrees with a previous study with Zea mays showing that some commercial inoculants did not promote mycorrhizal colonization (Corkidi et al. 2004). In our study, in general, the biomass was very similar in all inoculum treatments. It has been suggested that the inoculants could contain growth-promoting additives (Corkidi et al. 2004, Garmendia & Mangas 2014). Therefore, the AM spores for the production of biofertilizers should be collected in ecosystems near or around the agroecosystems where these will be applied, given that the AM fungi are locally adapted to the edaphic conditions of their environment (Johnson et al. 2010). In addition, the AM fertilizer should contain the different fungal structures, i.e., spores, extraradical mycelium and root fragments colonized with AM fungi, thus increasing the mycorrhizal infective potential (Klironomos & Hart 2002).
Third, and not exclusive with the other two explanations, is the fact that AM fungi are expensive symbionts and they can consume up to 30 % of the carbohydrates produced by their host plants (Jakobsen & Rosendahl 1990, Drigo et al. 2010). Therefore, the beginning of the symbiosis could be expensive for the maize host, especially when the plant has already enough P in the substrate. Although the colonization is normally observed a few days after inoculation (Gay et al. 1982, Corkidi et al. 2004, Li et al. 2005, Li et al. 2006), as we observed in the AM-native and solid inoculum after one month of the harvest, the establishment of functional nutrient exchange may take longer. The R/S ratio was greater in the AM-native, i.e., the plants allocated more resources to roots and likely to fungi, therefore the aboveground biomass was lower compared with the commercial inoculants. This could explain the greater growth in non-mycorrhizal plants than in mycorrhizal plants. Additionally, there are maize genotypes that have low percentages of colonization because these are more efficient in the uptake of phosphorous and nitrogen than other genotypes (Montaño et al. 2001).
Perspectives. Most biofertilizers are propagated without an acceptable quality control scheme, as no investigation has been conducted on the formulation strategies to find the best combinations of AM species for specific crops. There is no indications storage conditions and little to null indications to the final user on how, when and where apply the biofertilizer (Singh et al. 2014, Weber 2014, Berruti et al. 2016). Also, commercial biofertilizers include one or a limited mix of AM species while it is widely known that in natural ecosystems plants interact with several species of AM fungi (Öpik et al. 2009). As AM fungi are obligate symbionts cannot be cultivated and propagated without their host plants what makes the large-scale production a complex issue because of the extensive infrastructure needed and most importantly the risk of propagating pathogens.
Finally, it is important to indicate that in Mexico as in other countries there are no laws regulating the production and use of AM inocula (Singh et al. 2014), and some AM species declared on the commercial inoculum composition and the number of spores do not match what is shown on the label. Thus, the challenge for Mexico is to reduce the harmful agricultural practices because they affect not only the soil quality, but also its biodiversity and human health. Therefore, there is an interest in knowing and evaluating the diversity of AM fungi not only in agroecosystems but also in natural ecosystems and applying these fungi efficiently as an agroecological alternative (Cuenca et al. 2007, Armenta-Bojórquez et al. 2010). Also, to elucidate the complex interactions between the AM fungi and their host plants and how the biotic and abiotic local conditions affect this symbiosis.











 nueva página del texto (beta)
nueva página del texto (beta)


