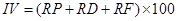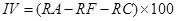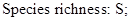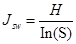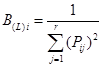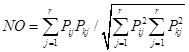The composition and structure of a community provides the basis for understanding the processes and functions of ecosystems, and is important to discuss the classification and succession of a community (Qu et al. 1984). Therefore, knowledge of species composition and community structure is vital to clarify population characteristics, species regeneration, and the formation and stability of communities and the rules that govern their succession, which can reveal the ecological mechanisms of a community.
Ecological niches are an important tool to evaluate inter-and intra-specific relationships and the status of populations in a community (Grinnell 1917, Alley 1982, Zhao et al. 2004, Li et al. 2006, Wang et al. 2008). Essentially, a niche is the functional unit of a species in a specific environment at a certain scale, including its relation to the environment and the rules that govern them. Therefore, the niche of a plant population exhibits the species attributes and quantitatively reveals the correlation between a species and its habitat (Hurlbert 1978, Aplet & Vitosek 1994, Leibold 1995). Niche theory provides the basis to explore interspecies correlation, the structure and succession of a community, biodiversity, and species evolution (Zhang 2011). The breadth and overlap of niches show the adaptability of species to the environment (Colwell & Futuyma 1971, Wissinger 1992), and are used for biodiversity conservation and to assess endangered species (Li et al. 2006, Wang et al. 2008). Studies on the niches of dominant populations in endangered plant communities clarify the interrelation between endangered and associated species, which is vital for the conservation and management of endangered plants.
Tetracentron sinense Oliv., is the only deciduous tree in the family Tetracentraceae (Fu & Bruce, 2001) and is mainly distributed in the mountains of central and southwest China. Fossils of T. sinense originate from the Cenozoic Eocene, indicating that it is a relict species with an ancient origin. This is important for the study of ancient flora systems, and the origin and evolution of angiosperms. T. sinense has been over-harvested due to its use in medicine, ornaments, and furniture resulting in poor regeneration of natural populations (Wang et al. 2006). It has been listed as a national second-grade-protected endangered plant in China (Fu & Chin 1992). Recently, the population ecology (Tang et al. 2013), reproductive biology (Gan et al. 2012, Gan et al. 2013), seed and seedling ecology (Zhou 2007, Luo et al. 2010, Cao et al. 2012, Han et al. 2015, Li et al. 2015), and genetic diversity (Sun et al. 2014, Li et al. 2016, Han et al. 2017) of T. sinense has been studied to examine the factors influencing natural population regeneration. To date, little is known about the structural characteristics or niches of dominant tree populations in T. sinense communities.
This study examined the structural characteristics and niches of dominant tree populations in a Tetracentron sinense community in Meigu Dafengding Nature Reserve in southwest China. The aims of were (1) to reveal the status of T. sinense populations in the community, (2) to discuss the interspecific relationships between T. sinense and its dominant associated species, and (3) to forecast the development trend of the populations and communities. This information is particularly useful for developing strategies for the conservation and management of T. sinense.
Materials and methods
Study area. The study was conducted in Meigu Dafengding Nature Reserve (102º 52’–103º 20’ N, 28º 30’–28º 50’ E), which has an area of 50,655.0 hm2, and is located toward the southeast of transverse mountains in the Qinghai-Tibet Plateau, southwest of Sichuan Basin, Sichuan Province, China. The average annual temperature was 9.6 °C, the total annual rainfall was 1.100 mm, and the annual average relative humidity was 80 %. The highest temperature was in July. Vegetation in the reserve was evergreen and deciduous, broad-leaved mixed forest, where T. sinense was dominant, and always scattered or mixed with Acer spp., Viburnum spp., and Betula albosinensis Burk.
Field survey and sampling. Based on a thorough investigation of T. sinense distribution in the reserve, 6 sites of 20 × 20 m were selected as large plots for canopy sampling (Table 1). Each sample plot was divided into grids of 5 × 5 m, from which 16 alternate squares were selected as medium plots for shrub sampling.
Table 1 The baseline conditions of Tetracentron sinense communities
| Sites | Slope degree (°) | Bareness degree of rock (%) | Altitude (m) | Slope aspect | Canopy density (%) | Type of community |
|---|---|---|---|---|---|---|
| Q1 | 35 | 2 | 2,182 | NW | 65 | T. sinense + Padus brunnescens - Ribes nigrum |
| Q2 | 45 | 0.5 | 2,153 | SE | 25 | T. sinense + Acer franchetii - Viburnum betulifolium |
| Q3 | 25 | 0.5 | 2,273 | NW | 45 | T. sinense - V. betulifolium |
| Q4 | 5 | 8 | 2,377 | NW | 60 | T. sinense + Cercidiphyllum japonicum - V. betulifolium |
| Q5 | 5 | 18 | 2,371 | SE | 55 | T. sinense - V. betulifolium |
| Q6 | 5 | 0.5 | 2,261 | SE | 20 | T. sinense - Rhododendron sp. |
The forest was divided into 3 strata, viz. canopy (height: > 4 m), shrub (height: 1–4 m), and ground (height: < 1 m). Six large quadrats (size: 20 × 20 m) were used for sampling all plants in the canopy stratum, and 4 medium (size: 5 × 5 m) or small (size: 1 × 1 m) plots were selected at the 4 corners of each large quadrat respectively, and 1 medium or small plots were also selected at the center, and then the medium and small plots were used for vegetation surveys in shrub and ground strata, respectively.
To assess the tree layer structure, height and diameter-at-breast-height of each tree (height: > 4 m) was measured in all large plots. In shrub (height: 1–4 m) and ground (height: <1 m) strata, the height, coverage, and number of clumps of all shrubs or herbaceous species, respectively, were measured.
Young trees of 1–4 m were treated as saplings, and tree species < 1 m as seedlings; they were counted and measured in all respective plots. All plants were identified to species level, and voucher specimens were deposited in the Herbarium of China West Normal University.
Importance value. Importance value is the common name of the “advantage degree index” of the community. Dominant species at each strata and community types are determined by the importance value, including Tree species:
Shrub and herb species:
where IV is the importance value, RP is the relative dominance, RD is the relative density, RF is the relative frequency, RA is the relative abundance, and RC is the relative coverage (Zhang 2011).
Species diversity. Species diversity was determined using the following indexes (Pielou 1969, Magurran 1988, Sun et al. 2002, Zhang 2004).
Shannon-Weiner index was used to quantify the uncertainty in predicting the species identity of an individual:
Simpson index was used to measure the degree of concentration when individuals are classified into types:
Pielou evenness index was used to measure how close in numbers each species in an environment is:
where S is the total number of species in sample plots, Pi = Ni/N, Ni is the individual number of i species, and N is the total individual samples in each sample site.
The difference in species diversity among different strata at all sites was analyzed using one-way ANOVA in IBM SPSS Statistics data version 21.0. When the homogeneity of variance was normal, Duncan’’s test for multiple comparisons was used, and Dunnett’s comparison T3 was used when homogeneity of variance was violated. All figures were created using OriginPro 9.0.
Niche breadth of dominant tree populations. The horizontal niche breadth of the dominant tree population in the T. sinense community was estimated using the Shannon-Wiener index (Shannon 1948) and Levins index (Levins 1968).
Shannon-Wiener index:
Levins index:
where B(SW) i is the Shannon-Wiener niche breadth of population i, B(L)I is the Levins niche breadth of population i with the field value [1/r, 1], Pij is the proportion of total application resources accounted for by the application resource j number of population i, and Pij = nij / Ni; r is the resource digits, namely sample plot number. Greater values of Bi indicate wider niche breadths, indicating greater total resource use by the species, and stronger competitiveness.
The vertical niche breadth was calculated using Levins index as for the horizontal niche breadth. Two meters was used as one height-class (resource unit), and then the individual number of each tree species at different height classes was counted, where r is the number of height classes, Pij is the individual number proportion of tree species i in the resource location j, and the other variables were the same as those for the horizontal niche breadth.
Niche overlap of the dominant tree population. The niche overlap of the dominant tree populations in T. sinense communities was estimated using the following formula:
where NO is the niche overlap value, and Pij and Pkj are the dominance degree of i and k species, respectively, in resource location j.
Results
Floristic composition. Eighty species of vascular plants from 67 genera and 41 families were recorded in T. sinense communities (Table 2). Among these, Pteridophyta accounted for 12.19 %, 8.95 %, and 7.5 % of the total families, genera, and species, respectively. One species of Gymnosperm accounted for 2.44 %, 1.49 %, and 1.25 % of the total families, genera, and species, respectively. Monocotyledons accounted for 7.32 %, 11.94 %, and 11.25 % of the total families, genera, and species, respectively. Dicotyledons accounted for 78.05 %, 77.61 %, and 80 % of the total families, genera, and species, respectively.
Table 2 Vascular plants in Tetracentron sinense communities
| Family | Number of genera | Number of species | Family | Number of genera | Number of species |
|---|---|---|---|---|---|
| Rosaceae | 7 | 8 | Aquifoliaceae | 1 | 1 |
| Caprifoliaceae | 4 | 4 | Acanthaceae | 1 | 1 |
| Liliaceae | 4 | 4 | Amaranthaceae | 1 | 1 |
| Urticaceae | 3 | 4 | Rubiaceae | 1 | 1 |
| Gramineae | 3 | 3 | Oxalidaceae | 1 | 1 |
| Saxifragaceae | 3 | 3 | Convolvulaceae | 1 | 1 |
| Labiatae | 3 | 3 | Balsaminaceae | 1 | 1 |
| Umbelliferae | 3 | 3 | Caryophyllaceae | 1 | 1 |
| Polygonaceae | 2 | 4 | Berberidaceae | 1 | 1 |
| Betulaceae | 2 | 2 | Araliaceae | 1 | 1 |
| Ranunculaceae | 2 | 2 | Vitaceae | 1 | 1 |
| Athyriaceae | 2 | 2 | Aristolochiaceae | 1 | 1 |
| Aceraceae | 1 | 4 | Crassulaceae | 1 | 1 |
| Juglandaceae | 1 | 4 | Violaceae | 1 | 1 |
| Ericaceae | 1 | 3 | Plantaginaceae | 1 | 1 |
| Cyperaceae | 1 | 2 | Taxaceae | 1 | 1 |
| Cercidiphyllaceae | 1 | 1 | Osmundaceae | 1 | 1 |
| Tetracentraceae | 1 | 1 | Dicksoniaceae | 1 | 1 |
| Nyssaceae | 1 | 1 | Adiantaceae | 1 | 1 |
| Lauraceae | 1 | 1 | Pteridiaceae | 1 | 1 |
| Rutaceae | 1 | 1 |
Seven families (including Rosaceae, Caprifoliaceae, Liliaceae, Urticaceae, Polygonaceae, Aceraceae, and Juglandaceae, and 22 genera and 32 species) with more than 4 species accounted for 17.07 %, 32.84 %, and 40 % of total families, genera, and species, respectively. Additionally, 5 families (including Gramineae, Saxifragaceae, Labiatae, Umbelliferae, and Ericaceae) with 3 species accounted for 12.19 %, 19.40 %, and 18.75 % of total families, genera, and species, respectively. Twenty-nine families with only 1–2 species accounted for 70.73 %, 89.55 %, and 36.25 % of the total families, genera, and species, respectively.
According to Wu (1991), 61 genera of seed plants in T. sinense communities were classified into 9 areal-types. Among these, there were 9 Cosmopolitan genera, accounting for 14.75 % of total genera; 12 tropical-type genera s (including Pantropic, Old World Tropics, and Tropical Asia), accounting for 19.67 % of total genera; 20 North Temperate genera, accounting for 32.79 % of total genera; and 7 disjunct North Temperate & South Temperate genera, accounting for 11.47 %. The genus with N. Temperate character was the largest, accounted for 44.26 % of total genera.
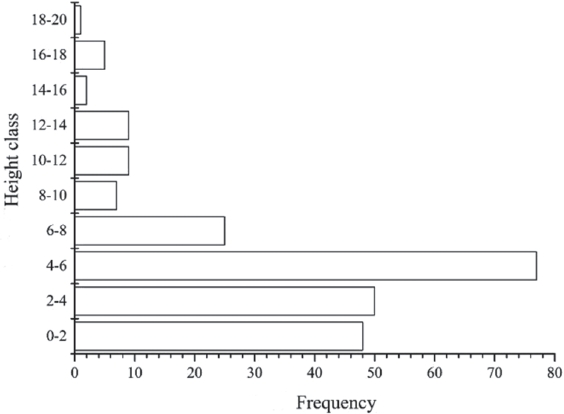
Vertical structure in Tetracentron sinense communities. The vertical structure of T. sinense communities was divided into 3 layers: trees, shrubs, and herbs. The tree layer was also divided into 3 sublayers: the upper (height: 14–20 m), the middle (height: 8–14 m), and the lower (height: 4–8 m) (Figure 1). Species richness was the highest in the lower sublayer, which mainly had Pterocarya and Acer spp. The main species in the middle sublayer was T. sinense, with T. sinense and Cercidiphyllum japonicum Sieb. et Zucc., dominating the upper sublayer. Species in the shrub layer (height: 1–4 m) were mainly Rhododendron, Rubus, Viburnum, and Fargesia. Species richness was highest in the herb layer, with the dominant species Potentilla contigua Soják, together with Girardinia diversifolia subsp. suborbiculata (C. J. Chen) C. J. Chen & Friis, Carpesium abrotanoides L., Fragaria orientalis Lozinski., Duchesnea indica (Andr.) Focke, Lecanthus peduncularis (Wall. ex Royle) Wedd., and Clinopodium gracile (Benth.) Matsum.
Species diversity in Tetracentron sinense communities. The species richness and Pielou evenness indexes were significantly different among the 3 vertical layers (Table 3). The highest species richness was observed in the herb layer, and the lowest in the tree layer. Conversely, the highest evenness index was observed in the tree layer, and the minimum in the shrub layer. The diversity indexes (Shannon-Wiener and Simpson) were not significantly different among the 3 layers; the highest appeared in the herb layer and the lowest in the shrub layer.
Table 3 Species diversity in each stratum of Tetracentron sinense communities
| Layer | Species richness S | Shannon-Weiner index H‘ | Simpson index D | Pielou evenness index Jsw |
|---|---|---|---|---|
| Tree | 5.50±0.885b | 1.3891±0.160a | 0.6837±0.046a | 0.8427±0.015a |
| Shrub | 8.50±2.045b | 1.1164±0.409a | 0.4462±0.165a | 0.4829±0.154 b |
| Herb | 22.50±2.110a | 1.9702±0.139a | 0.7400±0.043a | 0.6378±0.043 ab |
Importance values in dominant tree populations. There were 17 species in the tree layer of T. sinense communities; the sum of importance values of the former 6 species (including T. sinense, Acer franchetii Pax, A. elegantulum W.P.Fang & P.L.Liu, Padus brunnescens T.T.Yu & T.C.Ku, Cercidiphyllum japonicum, and Pterocarya stenoptera C.DC.) accounted for 71.1 %, indicating that these 6 species were the dominant tree species in the communities. The importance value of T. sinense was the highest, and much higher than the other tree species (Table 4). Thus, T. sinense dominated as the constructive species of the communities.
Table 4 Importance values of dominant tree species
| Dominant species | Relative density | Relative frequency | Relative dominance | Importance value |
|---|---|---|---|---|
| Tetracentron sinense | 0.342 | 1.000 | 0.521 | 186.257 |
| Acer franchetii | 0.125 | 0.500 | 0.014 | 63.900 |
| Padus brunnescens | 0.108 | 0.333 | 0.018 | 45.921 |
| Cercidiphyllum japonicum | 0.100 | 0.500 | 0.216 | 81.565 |
| Acer elegantulum | 0.092 | 0.833 | 0.048 | 97.285 |
| Pterocarya stenoptera | 0.067 | 0.500 | 0.015 | 58.128 |
Niche breadth of dominant tree populations. There was a significant difference in the niche breadths of different species in T. sinense communities (Table 5). The Shannon-Wiener index (B(SW)i) showed that the greatest horizontal niche breadth was for T. sinense, followed by Acer pictum subsp. mono, Pterocarya stenoptera, A. franchetii, Cercidiphyllum japonicum, and Padus brunnescens, which was consistent with the Levins index (B(L)i). The importance value was significantly, positively correlated with the horizontal niche breadth (Shannon-Wiener index: r2 = 0.882, P < 0.05; Levins index: r2 = 0.961, P < 0.01).
Table 5 Horizontal and vertical niche breadths of dominant tree species in Tetracentron sinense communities
| Species | Horizontal niche breadth | Vertical niche breadth | ||||
|---|---|---|---|---|---|---|
| B(SW)i | Ordination | B(L)i | Ordination | B(L)i | Ordination | |
| Tetracentron sinense | 1.714 | 1 | 5.141 | 1 | 4.556 | 1 |
| Acer franchetii | 0.970 | 4 | 2.419 | 4 | 1.142 | 6 |
| Padus brunnescens | 0.429 | 6 | 1.352 | 6 | 1.610 | 5 |
| Cercidiphyllum japonicum | 0.960 | 5 | 2.323 | 5 | 4.500 | 2 |
| Acer elegantulum | 1.414 | 2 | 3.457 | 2 | 2.373 | 4 |
| Pterocarya stenoptera | 0.974 | 3 | 2.462 | 3 | 2.462 | 3 |
The greatest vertical niche breadth in the dominant tree populations was T. sinense, followed by Cercidiphyllum japonicum, Pterocarya stenoptera, Acer pictum subsp. mono, Padus brunnescens, and A. franchetii (Table 5). There was no correlation between importance values and vertical niche breadths.
Niche overlap of dominant tree populations. Horizontal niche overlaps among dominant tree populations were relatively higher in T. sinense communities, and niche overlap values greater than 0.5 were observed between 9 pairs of dominant tree populations, accounting for 60 % of all niche pairs (Table 6).
Table 6 Horizontal niche overlap of dominant tree species
| Species | Tetracentron sinense | Acer franchetii | Padus brunnescens | Cercidiphyllum japonicum | Acer elegantulum | Pterocarya stenoptera |
|---|---|---|---|---|---|---|
| Tetracentron sinense | 1.000 | 0.430 | 0.312 | 0.618 | 0.636 | 0.651 |
| Acer franchetii | 1.000 | 0.659 | 0.132 | 0.473 | 0.569 | |
| Padus brunnescens | 1.000 | 0.250 | 0.832 | |||
| Cercidiphyllum japonicum | 1.000 | 0.580 | 0.423 | |||
| Acer elegantulum | 1.000 | 0.762 | ||||
| Pterocarya stenoptera | 1.000 |
For T. sinense, the greatest horizontal niche overlap appeared in Pterocarya stenoptera, followed by Acer pictum subsp. mono, Cercidiphyllum japonicum, A. franchetii, and Padus brunnescens. Therefore, the niche overlap values of the former 3 species were relatively high (values all greater than 0.6).
The values of vertical niche overlap among dominant tree populations in T. sinense communities were greater than 0.5, indicating the greater similarity of vertical space utilization in dominant tree populations (Table 7). The overlap values between T. sinense and its associated trees were all greater than 0.7.
Table 7 Vertical niche overlap of dominant tree species
| Species | Tetracentron sinense | Acer franchetii | Padus brunnescens | Cercidiphyllum japonicum | Acer elegantulum | Pterocarya stenoptera |
|---|---|---|---|---|---|---|
| Tetracentron sinense | 1.000 | 0.760 | 0.823 | 0.801 | 0.867 | 0.888 |
| Acer franchetii | 1.000 | 0.987 | 0.567 | 0.748 | 0.824 | |
| Padus brunnescens | 1.000 | 0.621 | 0.834 | 0.900 | ||
| Cercidiphyllum japonicum | 1.000 | 0.743 | 0.728 | |||
| Acer elegantulum | 1.000 | 0.989 | ||||
| Pterocarya stenoptera | 1.000 |
Discussion
Composition and structure of Tetracentron sinense communities. Our data showed that the plant species in T. sinense communities were abundant, but more concentrated in some families and genera, and most families had only a few or a single species. Additionally, the composition of families and genera was also dispersed, and the concentration of genera was relatively small. These results indicated that T. sinense communities need to be further differentiated. Based on the geographical distribution of the seed plant genera, there were more North Temperate genera in T. sinense communities. The Cosmopolitan genera comprised a greater proportion, reflecting the secondary character of the vegetation (Zhang & Song 2001), which was attributed to serious disturbance in T. sinense communities so that these communities were generally secondary stage forests.
The structural patterns of plant communities usually differ due to tree species numbers and spatial distribution, which results in different vertical species diversity (Fan 2000). Generally, species diversity and evenness is greater in more mature than immature communities (Peng 1996). In T. sinense communities, the vertical structure was relatively complex, and was divided into 3 layers: trees, shrubs, and herbs. The species richness, diversity, and evenness indexes of different layers in the vertical structure were relatively low. Thus, T. sinense communities were immature and unstable communities, and still in an early stage of succession.
The diversity and evenness indexes were lowest in the shrub layer of the vertical structure, and the diversity index in the herb layer and the evenness index in the tree layer was the highest. This may be related to the degree of microhabitat differentiation. In T. sinense communities, the degree of differentiation of the herb layer microhabitat was relatively high, and the degree of rock bareness was low, resulting in greater density and species richness of herb plants. In addition, the relatively stable tree species in the tree layer contributed to the greater evenness index. The microhabitat differentiation degree of the shrub layer was larger; seedlings and saplings of trees, especially T. sinense, were rarely surveyed in the shrub layer, resulting in a lower species richness and evenness index.
Importance values and niche breadths of dominant tree species. Importance value is a comprehensive quantitative index of a species that characterizes the position, advantages, and role of the species in the community. The niche breadth of a species is an important tool for measuring the degree of species utilization of environmental resources (Weider 1993, Wang et al. 2008). Horizontal niche breadth reflects the ability of different species to occupy and utilize resources in horizontal space. The importance value and horizontal niche breadth of T. sinense populations were greatest in T. sinense communities. This indicated that T. sinense populations had the strongest ability to use resources in horizontal space, and occupied a dominant position in the communities. Vertical niche breadth indicates the ability of different species to utilize ecological resources dominated by light factors with the change in height (Wang et al. 2008). The vertical niche breadth of T. sinense populations was also the highest in the communities, and showed that individuals of T. sinense with different heights were more evenly distributed in each resource niche, and took greater advantage of light in the communities. This showed that T. sinense was the dominant species, which had an advantage of using environmental resources, and played an important role in the stability and composition of the communities.
The niche breadths of dominant tree species also indicated that the utilization ability of different tree species in horizontal and vertical space was different. For small trees, the horizontal ecological resources were fully utilized, while the vertical resource utilization was not. For example, the horizontal niche of Acer pictum subsp. mono ranked second, while its vertical niche ranked fourth. For tall trees, the vertical ecological resources were fully utilized, while the horizontal ecological resources were not. For example, the vertical niche of Cercidiphyllum japonicum ranked second, but its horizontal niche ranked fifth. These results showed that each species in T. sinense communities had different abilities to utilize resources in horizontal and vertical space, thus forming its own ecological niche. This was also the basis for their coexistence in the communities.
Niche overlap of dominant tree species. Niche overlap can reflect utilization of the same resources by different species, and reflect the similarity in demand for some ecological factors (Colwell & Futuyma 1971, Zhang et al. 2005). In this study, the highest niche overlap was observed between the dominant tree species, indicating that interspecies relationships in the communities were relatively complex, and the degree of demand for ecological resources between the dominant tree species was similar. Therefore, the horizontal niche overlaps between T. sinense and its associated species (including Pterocarya stenoptera, Acer pictum subsp. mono, and Cercidiphyllum japonicum) were relatively high compared to other species, demonstrating the higher similarity of habitat demand between T. sinense and these species. Additionally, the vertical niche overlaps between T. sinense and its associated tree species were all greater than 0.7, showing stronger interspecific competition for light-related resources.
Generally, greater niche overlap exists between species with similar habitat requirements, but does not necessarily indicate intense competition (Li et al. 2006). Bengtsson et al. (1994) concluded that spatial and temporal heterogeneity of environmental conditions should be considered when assessing the role of ecological niche differentiation on competition and coexistence of species. According to our field surveys, two tree species (T. sinense and Cercidiphyllum japonicum) both grew in sheltered and wet places, which indicated a similar habitat demand. The value of niche overlap between these two species was also high (0.618 or 0.801 in horizontal or vertical space, respectively). The results showed that the two species shared similar ecological resources as the co-dominant species when the resource was abundant (Colwell & Futuyma 1971). However, when resources were relatively scarce, relative competition would occur between these two species.
Previous studies suggested that plant species from the same genus usually have similar bio-ecological characteristics, and their demand for environmental resources differ in order to coexist in a small habitat, with smaller niche overlap (Chen & Zhou 1995). This phenomenon was also observed in our study, as the extent of niche overlap between Acer sterculiaceum subsp. franchetii and A. pictum subsp. mono was relatively low (0.473), and remarkably lower than that between other species.
Implications for conservation. Our study showed that seedlings and saplings of T. sinense were scarce in the communities investigated, and the population regeneration of T. sinense was poor. Gan et al. (2009) found that the seed set of T. sinense individuals was high and that there were no factors influencing the population regeneration during seed production. Field investigations suggest that T. sinense is a light-demanding plant; its seeds need light in order to germinate and the shade-tolerance of seedlings and saplings is inferior to other species (Zhou 2007). Therefore, to facilitate the natural regeneration of T. sinense populations, moderate anthropogenic disturbance should be conducted in T. sinense communities to form forest gaps, which can aid seed germination and seedling establishment.
Our data suggested that T. sinense had an advantage of resource utilization in the communities; however, strong interspecific competition for light-related resources existed between T. sinense and its associated species. Thus, manual interventions based on the vertical niche overlap between T. sinense and its associated species should be conducted to reduce the competition for light, which will aid the conservation of endangered species.
In summary, we investigated the structural characteristics and dominant tree population niches in T. sinense communities. T. sinense communities were immature and unstable, and still in a certain stage of succession. T. sinense was the constructive species in the communities. A higher similarity of habitat demand existed between T. sinense and its associated tree species (Pterocarya stenoptera, Acer pictum subsp. mono, and Cercidiphyllum japonicum) in horizontal space. The intense competition for light was apparent between T. sinense and its associated trees. Moderate anthropogenic disturbance should be conducted to form forest gaps and reduce the competition for light between T. sinense and its associated species to aid the conservation and recovery of endangered species.











 nova página do texto(beta)
nova página do texto(beta)