The domestication process of wild plants has usually produced heritable changes in morphological and physiological characteristics (Pickersgill 2007, 2013, Rival & McKey 2008, Chacón-Sánchez 2009, McKey et al. 2012, Gepts & Papa 2003). Cereals are the best example, with a longer domestication time. Throughout domestication, cereals have lost their capacity of seed dispersal and modified their plant architecture in order to increase yield and production (Gepts & Papa 2003, Pickersgill 2007, 2013, Mondolot et al. 2008, Chacón-Sánchez 2009, Bautista-Lozada et al. 2012, He 2014), but selection of specific traits has reduced the genetic diversity of the domesticated varieties (Chacón-Sánchez 2009, Rauf et al. 2010, Bautista-Lozada et al. 2012).
Compared to cereals, the domestication process of cacao (Theobroma cacao), a native tree of the Amazonian rainforest (Cheesman 1944) is more recent. Traditionally, three domesticated varieties of cacao are recognized: Criollo, Forastero and Trinitario. Domestication of the Criollo variety began 4,000 years ago in southern Mexico (Powis et al. 2011, Vázquez-Ovando et al. 2012). The onset of the process for the Forastero variety is dated in 1700 in Brazil (Ji et al. 2012). The Trinitario variety, a hybrid between the aforementioned varieties Criollo and Forastero, began to be cultivated in Trinidad and Tobago after 1727 (Bekele 2004).
The cacao fruit is highly valuable in the market, since chocolate is obtained from its seeds. The mature fruit is a berry, commonly known as cacao-pod, with an oblong-oval shape. The mature cacao-pod pericarp, constituted by exocarp and endocarp, is composed of cellulose, hemi-cellulose, lignin, water and mineral salts in a laminated arrangement (Daud et al. 2013). In mature fruits, the proportion of the components of the cacao-pod pericarp is unknown. Likewise, the composition of the “cacao-pod pericarp” of immature fruits, or “cherelle” (0 to 5 weeks after fruit mooring) and juvenile (6 to 10 weeks after fruit mooring) stages is unknown (Sánchez-Mora & Garcés-Fiallos 2012, ten Hoopen et al. 2012).
Like other plant organs, the cacao fruit is susceptible to infection caused by Oomycetes such as Phytophthora and fungi like Moniliophthora (Motamayor et al. 2008). In the last decade, in the Americas, the production of cacao fruits has declined between 80 and 100 % due to the infection caused by Moniliophthora roreri, a specific pathogen of the fruit (Phillips-Mora & Wilkinson 2007, Phillips-Mora & Cerda 2009). The susceptibility to M. roreri, causal agent of moniliasis or frosty pod rot (FPR) disease, depends on the cacao variety and fruit age. In this sense, Phillips-Mora et al. (2005), Rodríguez (2006) and Phillips-Mora & Wilkinson (2007) reported that young fruits are more susceptible to infection by M. roreri than mature fruit. Likewise, the initial evaluations of the incidence of FRP in cacao plantations in Chiapas, Mexico, carried out by our working group suggest that the medium zone of the fruit is the most susceptible to M. roreri infection. Therefore, the aim of this study was to determine the susceptibility of Criollo, Forastero and Trinitario cocoa fruit varieties to FRP caused by M. roreri, and to assess if susceptibility correlates with its domestication age.
Materials and methods
Study site, symptoms and incidence of FPR. This study was carried out at a cacao plantation in Chiapas, México (14.940090 N and -92.171321 W, 411 m a.s.l.), where the Criollo(C), Forastero (F) and Trinitario (T) varieties are grown. The symptoms of FRP (Phillips-Mora et al. 2005) were evaluated from fruit setting to harvest in 20 trees of each variety (60 trees in total) from February to June and from July to November 2013 (two full harvest periods).Every week, the numbers of healthy and infected fruits were recorded. The location of the symptoms on the surface of the infected fruits was also documented. The fruits were divided equidistantly, in peduncle, middle and apical zones. The incidence of disease per age (week) and cacao variety was calculated using the formula:
I (%) = (IF/TF) × 100
where I = incidence, IF = number of infected fruits and TF = total fruits.During the study, 390, 605 and 475 fruits of the Criollo, Trinitario, and Forastero varieties, respectively, were collected (average of 9.8, 15.1 and 11.9 fruits. Tree-1·year-1).
Fruits free of FPR symptoms. Twenty fruits of every cacao variety were washed with sterile saline solution and kept in a cellophane bag to protect them from the possible arrival of Moniliophthora roreri conidia.
Physicochemical studies of healthy mature fruits. For the physicochemical study of the pericarp, healthy fruits at the ages of 4, 8, and 12 weeks without FPR symptoms, were sampled. The content of lignin, peroxidase activity, moisture, hardness and phenolic compounds were measured in the middle area of the pericarp, where the highest frequency of FRP symptoms has been observed.
Preparation of pericarp for histochemical determination of lignin. To determine the content of lignin in the pericarp, we sampled five fruits of each of the three varieties of cacao, and five cubes of tissue were obtained (1.5 × 1.5 × 1.5 cm) from the middle area of the pericarp (5 cubes.fruit-1; 25 cubes.variety-1). The cubes were initially dehydrated for 72 h, in a mixture of formaldehyde-ethyl alcohol-glacial acetic acid (2:10:1 v:v FAA mixture). Subsequently, the samples were placed in aqueous solutions of ethyl alcohol of 10, 25, 50, 75 and 96 % v:v (6 h in each solution). The cubes were placed in solutions of 10, 25, 50, 75 and 100 % of xylol (3h in each solution). Finally, the cubes were embedded in paraffin (McCormickR) and ten cuts of 5 µm thickness (Microtome Microm HM 315) were obtained and each cut placed on a slide.
Staining used for the identification of lignin in the pericarp. The identification of lignin was carried out following the method proposed by Sánchez-García et al. (2010). Each excised sample was incubated for five minutes with 40 µL of 30 % HCl and 80 µL of phloroglucinol (1 % w:v in 70 % ethyl alcohol). The excess of reagents was removed with water and the resulting product was observed and photographed under an optical microscope (Carl Zeiss Axioscope) (Carl Zeiss Microlmaging camera model AxioCam MRc) for further analysis. The red-violet color was indicative of the presence of lignin (Figure 1) and the color intensity was proportional to its concentrations (Almaraz-Buendía 2011).

Figure 1 Lignin accumulation (red) in the pericarp tissue of the cacao fruit at 40x. CM: mucilaginous cavity, CE: epidermis.
Content of lignin in the pericarp of the different cacao varieties. To determine the content of lignin in the pericarp of the different cacao varieties, the intensity value (In) of the red (R), green (G) and blue (B) color was determined at each microphotograph using the Zen program, version 2011. The In parameter takes values between 0 and 250. Thus, the combination 0.0.0 (R,G,B) represents the black color and the combination 250.250.250 represents the white color; The combinations 250.0.0; 0.250.0 and 0.0.250 represent the red, green and blue colors respectively (Triana et al. 2013). The determination was made in six optical fields (1,300 × 1,300 pixels) of each microphotograph, representing 78.5 % of the cut (9.810 655.4 mm2 photo-1; 58,863,9323 mm2 analyzed of 749, 859, 010.2 mm2 in total).
Peroxidase activity (Pox) in the pericarp of fruits. The Pox activity in fruit pericarp was determined using the Kar & Mishra (1976) method with some modifications. Briefly, 100 mg of tissue, were ground in liquid Nitrogen using a pestle and mortar. The mixture was homogenized with one mL of phosphate buffer (100 mM at pH 6.8) and centrifuged at 11,600 rpm. The supernatant was diluted (1:20) with distilled water. To 360 µL of the dilution, 922 µL of H2O and 90 µL of phosphate buffer were added and strongly shaken. The mixture was incubated at 30 °C for 10 min, after adding 360 µL of pyrogallol and 18 µL of H2O2 and the absorbance was determined at 420 nm (Genesys 20 Spectrophotometer Thermo Scientific Model 4001/4). The Pox activity was reported in nkatals g dry weight of tissue-1, taking into account that the oxidation product of pyrogallol has a molar extinction coefficient (E) of 2.47 mM-1cm-1.
Determination of moisture in the pericarp. To determine the moisture in the pericarp of the fruits, 10 g of tissue, from the middle zone, were placed in a porcelain capsule. The samples were placed at 70 ºC until constant weight was achieved. The moisture was determined using the formula:
Moisture (%) = [(Wi-Wf) Wi-1] ×100.
Where, Wi: initial weight of the sample, Wf: final weight of the sample. The determination was performed three times.
Determination of the pericarp hardness. The hardness of the pericarp of the different varieties of cacao was determined as the force required to penetrate it (N cm2) using a penetrometer (Fruit firmness tester, Touroni, Mod. 53205, Italy). The determination was conducted in triplicate in the middle zone of each fruit.
Total phenol content. Five fruits were collected per age and variety, in order to analyze total phenol content. The phenols extraction was made using the method proposed by Restrepo et al. (2009). We took 5 g of tissues of pericarp, which were ground in a mortar and added 10 mL metanol-water (50:50 v/v). Phenol content was measured by the Feng et al. (2010) method, using the Folin Ciocalteu reagent. To 10 µl sample, we added 500 µL of Folin-Ciocalteu’s reagent diluted 1/10 with water, to which 400 µL of sodium carbonate (Na2CO3)was added. Subsequently, the mixture was in total darkness for 15 min for reaction. Absorbance at 765 nm was then recorded against a blank with a mixture of methanol:water (1:1). A calibration curve was performed to estimate polyphenol content with a solution of gallic acid in methanol:water solution. Results of total polyphenol content are expressed as mg equivalents of gallic acid per 1 g of sample.
Statistical data analysis. The FPR incidence data were arcsine-transformed prior to an analysis of variance (ANOVA). The data of the physicochemical properties of the pericarp were subjected to the multivariate analysis of variance (MANOVA), canonical discriminant analysis (CDA) and principal component analysis (PCA). All statistical analyses were performed using the InfoStat software (v. 2015).
Results
Incidence of FPR. In the fruits of the Criollo variety, the first symptoms of FRP were observed two weeks after fruit mooring; in the Trinitario and Forastero varieties after three weeks (Figure 2). The disease reached maximum values between the fourth and fifth week after fruit mooring (average incidence was 8.70 ± 1.07 %, 6.20 ± 0.90 % and 7.19 ± 0.43 % for the Criollo, Forastero, and Trinitario varieties, respectively), remaining in this condition for five weeks. The differences were statistically significant (F1, 2 = 185.18, p < 0.0001). After week 10, the incidence of FPR decreased, reaching a value of 0 in week 14. During the study period, the percentage of fruits with FPR was 82.4, 63.6 and 51.9 % for the Criollo, Trinitario, and Forastero varieties, respectively. The dynamics of the incidence of FPR between the two production periods evaluated did not show significant differences (F2, 1 = 0.0, p = 0.9999).
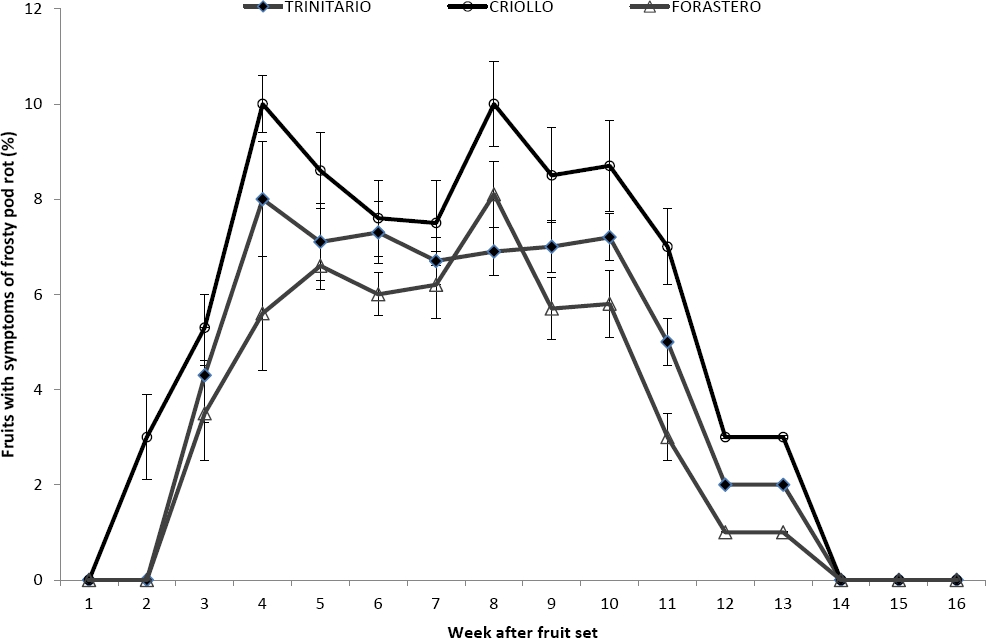
Figure 2 Incidence of Frosty pod rot in the cacao fruits of the Criollo, Forastero and Trinitario varieties along nine months (February-November 2013).Zero represents fruit setting.
Frequency of FPR in the different zones of the cacao fruit. Considering all the fruits of the Criollo variety with symptoms of FPR, 62.7 % were registered in the middle zone; in the Trinitario and Forastero varieties the frequency was registered at 63.5 % and 64.5 %, respectively. In the apex and peduncle zones of the Criollo, Trinitario, and Forastero varieties, the frequencies were 26.6, 26.6 and 25.9 % and 10.6, 9.9 and 9.1 %, respectively. Among the fruit areas, the difference was significant (F3,2 = 92.77, p < 0.0001).
Relative lignin content, peroxidase (Pox) activity, phenols content, moisture and hardness in the pericarp.Table 1 shows the values of In (for lignin), Pox, phenols, moisture and hardness of the middle zone of healthy fruits of the three cacao varieties. The values of all the variables were higher at the age of 12 weeks than at the 4 weeks. The MANOVA (Table 2) showed significant differences between varieties, age of fruit development and their interaction.
Table 1 Mean value of physicochemical properties in the middle zone of the pericarp of the three cacao varieties used in this study. Relative content of lignin (In), peroxidase activity (Pox), phenols (P), moisture (H) and hardness (D).
| Cacao variety | Fruit Age (weeks) | In | POX (nkatals.g-1) | P (mg AG.g-1) | H (%) | D (N cm2) |
|---|---|---|---|---|---|---|
| Criollo | 4 | 77.6±5.5 | 1.22±0.02 | 0.71±0.03 | 55.12±1.7 | 116.2±4.9 |
| 8 | 118±1.7 | 1.33±0.02 | 0.89±0.08 | 68.66±1.0 | 114.8±2.5 | |
| 12 | 126.6±3.3 | 2.11±0.04 | 1.13±0.05 | 75.10±0.8 | 148.8±2.3 | |
| Trinitario | 4 | 100.6±6.6 | 1.28±0.02 | 0.82±0.04 | 53.73±2.1 | 113.2±3.1 |
| 8 | 132±2.1 | 1.39±0.03 | 0.83±0.05 | 67.40±2.0 | 118.6±2.3 | |
| 12 | 142.6±4.1 | 3.55±0.06 | 1.22±0.02 | 72.27±1.3 | 173.7±3.7 | |
| Forastero | 4 | 110.3±7.2 | 1.49±0.02 | 0.91±0.04 | 55.06±1.1 | 112.8±5.0 |
| 8 | 148±1.9 | 1.48±0.03 | 1.07±0.04 | 65.44±0.8 | 121.2±2.1 | |
| 12 | 169±6.2 | 4.49±0.08 | 1.35±0.05 | 73.51±1.1 | 178.7±4.8 |
Table 2 MANOVA test of physicochemical properties in the middle zone of fruits at different ages and cacao varieties.
| Test Stat | Aprox. F | Num. Df | Den Df | Pr (>F) | |
|---|---|---|---|---|---|
| Age | 1.93 | 184.91 | 10 | 66 | <0.0001 |
| Variety | 1.31 | 12.6 | 10 | 66 | <0.0001 |
| Variety: Age | 1.6 | 4.64 | 20 | 140 | <0.0001 |
The CDA of the data, showed that 97.3 % of the variance was explained by the first canonical function and the remainder by the second function. The lignin content was the most important variable for the discrimination of the groups on the canonical axis 1 (Table 3A) so that the individuals with the highest lignin content appear in the right side of the scatter plot in the discriminant space (Figure 3). The discriminant dispersion centroids (Table 3B) showed that the group of individuals of the Criollo variety opposes the groups of the Trinitario and Forastero varieties in the canonical axis 1. This means that the differences in lignin (In) distinguish the Criollo variety fruits from those of the Forastero and Trinitario varieties. On the other hand, canonical axis 2 explains little variation between groups. Finally, the cross-classification table (Table 5) shows that all individuals initially assigned to the Criollo, Trinitario, and Forastero varieties were well classified using the discriminant function (0 % error).
Table 3 Canonical discriminant analysis A) Discriminant function on each variable age, Pox, phenols, moisture, hardness and lignin for relationship between cacao varieties in middle zone. B) Dispersion centroids of each cacao variety in discriminant space, and C) Cross classification of the three cacao varieties.
| (A) | |||||
| Variable | Can 1 | Can 2 | |||
| Age | -5.17 | 0.43 | |||
| Pox | 0.25 | 1.03 | |||
| Phenols | 1.27 | 3.43 | |||
| Moisture | -1.12 | 0.26 | |||
| Hardness | 1.58 | -3.61 | |||
| Lignin | 3.61 | -1.67 | |||
| (B) | |||||
| Variable | Axis 1 | Axis 2 | |||
| Criollo | -4.21 | 0.37 | |||
| Forastero | 4.05 | 0.42 | |||
| Trinitario | 0.17 | -0.79 | |||
| (C) | |||||
| Variable | Cri | For | Tri | Total | Error |
| Criollo | 15 | 0 | 0 | 15 | 0 |
| Forastero | 0 | 15 | 0 | 15 | 0 |
| Trinitario | 0 | 0 | 15 | 15 | 0 |
| Total | 15 | 15 | 15 | 45 | 0 |
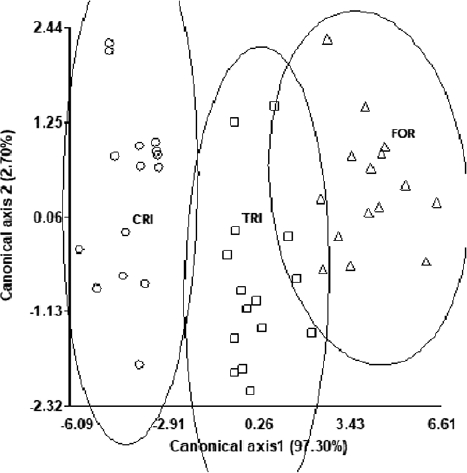
Figure 3 Canonical discriminant analysis for cacao varieties in middle zone. Variety: CRI = Criollo, TRI = Trinitario, FOR = Forastero.
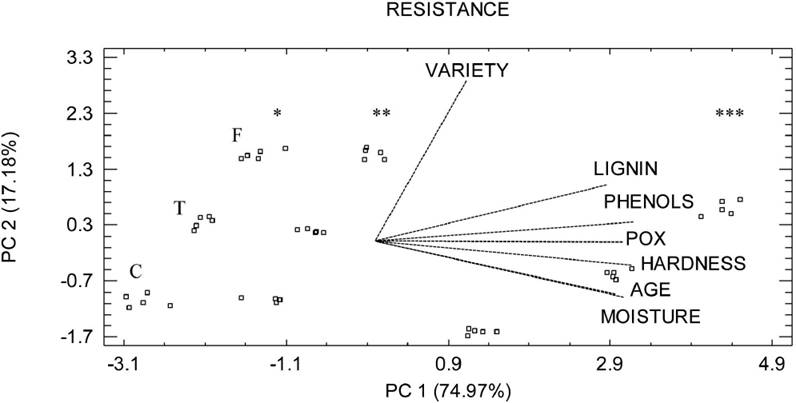
On the other hand, the PCA of the data, showed that the first two components explained 92.15 % of the variance. In PC 1 all variables had a positive coefficient, highlighting the variable “variety” as the lowest value. Due to the above, the higher the value of the variable the greater the resistance to FPR. Likewise, in PC 2 the variables “moisture” and “time of development of the fruit (age)” had the highest negative coefficients, whereas the variable “variety” had the highest positive value. The lower moisture and age, the lower the resistance of fruits to FPR. Forastero variety was more resistant than Trinitario and Criollo (Figure 4).
Discussion
In the present study we found that the Criollo variety, which is the most domesticated (Motamayor et al. 2008, Powis et al. 2011), was also the most susceptible to cocoa frosty pod rot.
Phillips-Mora et al. (2005) and Phillips-Mora & Wilkinson (2007) reported the FPR disease symptoms observed after eight weeks of the arrival of conidia. Here, we detected disease symptoms in the second week after fruit setting in the Criollo variety, and in the third week in the Trinitario and Forastero varieties (Figure 2). This pattern may be due to the arrival of conidia during floral anthesis, as previously reported by Omolaja et al. (2009) and Frimpong-Anin et al. (2014). Another possibility is that the strain of Moniliophthora roreri present in the area is very aggressive (Sánchez & Garcés 2012). Yet, this question warrants further research. Delay of the onset of symptoms in the Forastero and Trinitario, or disease progression in Criollo varieties, is related to the physicochemical parameters of the pericarp of the fruit (Nyadanu et al. 2013, Takam et al. 2013).
The incidence of FPR, between the second and tenth week after fruit setting (Figure 2) coincides with reports by Leandro-Muñoz (2011) and Sánchez & Garcés (2012). This development period (to second and tenth week) is depends on the longitudinal growth path of the fruit (ten Hoopen et al. 2012, Sheng & Maisin 2012), when cells are in mitotic activity and lack cellular differentiation. During that initial phase of fruit development, the orientation of the lignin fibers (Agrios 2005, Lira 2007, Keegstra 2010, Fanwoua et al. 2013), the lack of polymers consolidation in the cell wall, and the exudate produced (Filonow 2002, Zelinger et al. 2006, Rodríguez-López et al. 2013), can promote conidia germination of Moniliophthora roreri on the fruit surface.
On the other hand, decrease in FPR after the tenth week of fruit development (Figure 2) may be associated with changes in the composition of the pericarp resulting from an increment in cell size and a decrement in the rate of duplication (Agrios 2005, Lira 2007, Keegstra 2010, Fanwoua et al. 2013, Rodríguez-López et al. 2013). These changes coincide directly with the transition from the immature to the juvenile stage of the fruit (Martijn ten Hooper et al. 2012, Sheng & Maisin 2012). The above phenomenon is similar to the one reported by Ando et al. (2009), who found that mature fruits of Cucumis sativus are more resistant to infection by Phytophthora capsici than the immature fruits. Moreover, the absence of FPR symptoms in fruits after thirteen weeks of development supports the hypothesis that the physicochemical characteristics of the pericarp do not allow the development of Moniliophthora roreri.
Incidence of FPR seems to be related to both fruit age and the physicochemical properties of the epidermis of the fruit pod husk, and the most probably to molecules involved in plant defense (Ando et al. 2009, Weng & Chapple 2010, Zeng et al. 2014).
Furthermore, it has been documented that the pericarp develops from the cells of the ovary wall (Rangel-Fajardo et al. 2012), that the size of the fruit is defined by the number of cells that form the pericarp (Okello et al. 2015), and that the final volume of the fruit is biophysically limited by the extensibility of the epidermis (Bertin 2005). So, we believe that the Pox activity, concentration of lignin and phenols in the pericarp of the cacao fruit during the immature and juvenile stages are similar to the one found in mature fruits. Consequently, the greater susceptibility to RPF observed in the equatorial region of immature and juvenile fruits, may be the result of the differential distribution of physicochemical properties. Likewise, the lower amount of lignin, phenols and Pox activity in the equatorial region of the fruits may be the result of both the rate of cell division (Evans et al. 1978) and the area, which are higher in this region than in the peduncle and the apex (Bekele et al. 2006).
The observed differences in epicarp physicochemical characteristics of the three cacao varieties (cf. Table 1, Figure 3 and 4), and in their FPR susceptibility, may be associated with its life history (domestication). In this sense, the Criollo variety has been grown in the Soconusco region of Chiapas, for more than 4,000 years (Vázquez-Ovando et al. 2012). Cheesman (1944) proposes that the seeds of the Mexican Criollo variety were initially selected, in the upper Amazonas, due to their low astigmatism and clear color. The transference of these seeds to the west of the Andes mountain range, and their subsequent transport to the Soconusco region, also contributed to the selection (Motamayor et al. 2002, 2003). On the other hand, the Forastero variety has 400 years of domestication (Ji et al. 2012), and the Trinitario variety (a cross between of the Criollo variety with the Forastero variety) was obtained in the island of Trinidad and Tobago 300 years ago (Bekele 2004), although its introduction to continental America occurred 100 years ago (Motamayor et al. 2003).
Considering that during the process of cacao domestication, man has played an important role in the selection of seed (Chacón 2009, Raya-Pérez et al. 2010), that cacao has cross-pollination, and that on average, a cacao tree has a productive life of 40 years (Somarriba-Chávez et al. 2013), we inferred that: 1) at least 100 selection processes have taken place for the Criollo variety, 13 for the Forasterovariety, and three for Trinitario variety, 2) as a result of the lack of wild specimens in the domestication region of the Criollo variety, the existing population presents a genetically low diversity (Motamayor et al. 2002, 2003, Vázquez-Ovando et al. 2014), and the Trinitario variety can be considered as an improved Criollo variety. This is particularly supported by co-clustering in the clades generated from the analyses of lignin content and phenols (cf. Table 3, Figures 3 and 4).
Although the domestication process has improved the quality and quantity of fruit and seed, and has allowed a sustainable production of cacao all along the year, it has also affected susceptibility to frosty pod rot. Our study shows that the Criollo variety, which has been under domestication for the longest time, and appreciated by its fruits with high-quality sensory characteristics, such as sweet pulp, less bitter beans, and attractive aroma, flavor, and taste (Smith 1999, Vázquez-Ovando et al. 2015), is also the most susceptible to Moniliophthoraroreri.
Considering that the finest cacao is obtained from the Criollo variety due to its highest sensory quality (Vázquez-Ovando et al. 2012), there is a need for research on enhancing the resistance of cacao to this fungal pathogen while keeping the organoleptic and commercial qualities of the seed (Vázquez-Ovando et al. 2012, 2014), and as a first step we suggest using material from wild Criollo populations and from the Forastero and Trinitario varieties in future breeding and selection programs.











 nueva página del texto (beta)
nueva página del texto (beta)