Priming has been used as a method to improve seed germination and seedling vigor in either crops or wild plants from different ecosystems. In particular, the seeds of species from ecosystems with a clear seasonality highly respond to this treatment (Martínez-Villegas et al. 2012, Benítez-Rodríguez et al. 2014).
Germination performance improvement could positively impact the success of restoration programs due to a rapid recruitment of seedlings, and ensuring higher survival of plants (González-Zertuche et al. 2000, González-Zertuche et al. 2002). During the priming treatment, seeds hydration is restricted to block radicle protrusion, but allow the progression through the first phases of imbibition. The different types of priming and the seed response result from the different methods applied to restrict water uptake. During osmopriming, seeds are imbibed in a solution of low osmotic potential; meanwhile during hydropriming, seeds are imbibed in water. Natural priming occurs in the seeds exposed to imbibition/drying cycles during their permanence in the soil. In particular, germination performance of the seeds of Buddleja cordata and Opuntia tomentosa is improved by natural priming (González-Zertuche et al. 2002, Olvera-Carrillo et al. 2009).
Buddleja cordata Kunth (Scrophulariaceae) is a wild tree distributed in Mexico and Guatemala, commonly found in disturbed areas, along forest edges and water courses, at elevations of 1,500-3,000 m. Buddleja cordata is a pioneer species that can thrive in poor soils (Norman 2000, Mendoza-Hernández et al. 2010). Several studies have described the B. cordata seed germination response to different priming treatments, showing that primed seeds germinate faster and more synchronously than untreated seeds. Also, the priming treatments improve germination percentage of stored seed lots, which were no different from the germination percentage of freshly shed seed lots (González-Zertuche et al. 2002). Opuntia tomentosa Salm-Dyck (Cactaceae) is a bushy cactus distributed in Mexico and Guatemala at elevations of 2,300-2,800 m that can survive in poor soils and disturbed areas. O. tomentosa exhibits orthodox seeds with hard testa with similar imbibition rates in scarified and non scarified seeds. They achieve higher imbibition rates and germination percentages after been buried 6 months compared to unburied seeds. This germination improvement could be related to the weakening of the funicular cover (Orozco-Segovia et al. 2007), and/or to the induction of early germination processes.
Germination improvement through priming could be a result of the induction of germination metabolism, antioxidant activity and repair processes. The induction of early germination processes by priming has been described in different model and crop plants. These processes include reserve mobilization and primary metabolism activation for energy production processes that occur within the first 8 HAI (Hours after imbibition), and mechanisms involved in stress tolerance in Arabidopsis thaliana seeds (Weitbrecht et al. 2011). Seed storage protein (SSP) mobilization has been observed during osmo- and hydro-priming of Beta vulgaris and Brassica napus seeds (Job et al. 1997, Kubala et al. 2015), and A. thaliana (Gallardo et al. 2001). However, it has been reported that de novo synthesis of SSPs, like the 12S cruciferins, also occur during early germination in A. thaliana seeds, and it has been suggested that these proteins can be involved in oxidative stress relief in the germinating seeds (Galland et al. 2014). The induction of Late Embryogenesis Proteins (LEA) involved in desiccation tolerance during seed maturation and osmopriming has been also described in Brassica napus (Kubala et al. 2015).
The induction and/or mobilization of SSPs by priming in non-model species have not been extensively explored. These processes are particularly important to explain the metabolic mechanisms related to seed priming in the field. In a first report using the non-model plant Wigandia urens, a pioneer species widely distributed in Mexico that grows in disturbed areas, it has been described that natural priming also promotes protein reserve mobilization of vicilin, the principal storage protein that provides the amino acid source during early germination, as well as mobilization or synthesis of a 11S globulin in these seeds (Gamboa-deBuen et al. 2006). Thus, the early mobilization of SSPs or a rapid synthesis of SSPs upon imbibition in response to the priming treatment could help the seed to cope with the different kinds of stress that occur during germination and under prolonged or continuous natural imbibition/drying cycles in the soil.
Several non-model plants have shown a positive response to different priming treatments. Among these plants that could be beneficial in restoration programs, we are interested in determining the effect of natural priming on SSPs mobilization or synthesis in B. cordata and O. tomentosa seeds. Our results will contribute to determine a common process, concerning storage protein function during priming, between seeds from different plant families.
Materials and methods
Study species. Buddleja cordata Kunth (Scrophulariaceae) is a common shrub or tree widespread in center and south of Mexico. This species flowers in summer and sets fruit and sheds seeds in autum-winter (dry season). The seeds lie in the soil for 5-6 months before germination occurs. During this time, the seeds endure the variations in soil water potential produced by the unpredictable and discontinuous precipitations that characterize the early rainy season (González-Zertuche et al. 2001). Seeds of B. cordata were collected in March 2005 in the “Reserva Ecológica del Pedregal San Angel” (REPSA), at 2,200 and 2,700 m asl, in Mexico City. Seeds used as control were stored in paper bags in the laboratory at 23–25 ºC.
Opuntia tomentosa Salm-Dyck (Cactaceae) is a common shrub or tree widespread in Mexico and Guatemala. This species flowers during summer and sets fruit and sheds seeds in autumn (dry season). The seeds lie in the soil for 5-6 months before germination occurs. Seeds of O. tomentosa were collected in November 2006 in the REPSA. Seeds used as control were stored in paper bags in the laboratory at 23–25 ºC.
Natural priming treatment. Natural priming was done as described by González-Zertuche et al. (2001). Briefly, 2 g of seeds from B. cordata or O. tomentosa were enclosed in nylon mesh bags and buried under 10 cm of soil during the dry season for 1 month (April 2006) and 6 months (November 2006-April 2007), respectively. During their permanence in soil, the seeds were exposed to sporadic precipitation, which was less than 25 mm on average per month. After this treatment, control (unburied and stored in the laboratory seeds) and treated seeds were frozen at -70 ºC until used. For O. tomentosa, embryos from treated and control seeds were excised before freezing. For B. cordata, germination improvement by natural priming was observed in treated seeds as previously described (González-Zertuche et al. 2002). Due to small seed size, changes in the seed morphology were used to follow seed imbibition. For O. tomentosa, the germination curves and the relative water content (7.7 ± 0.8 % fresh weight basis) were published in Orozco-Segovia et al. (2007).
Protein purification and identification. The phospho-protein enriched fraction was obtained as previously described (Gamboa-deBuen et al. 2006) using 200 mg of tissue from each sample. The protein samples (50 µg) were run in a 15 % SDS-PAGE and stained with Coomassie blue. The bands were cut and sent for protein identification analysis by liquid choromatography/mass spectrometry/mass spectrometry (LC/MS/MS) to the “Centre Proteomique de l Est du Quebec”. Tryptic digestion was performed according to Shevchenko et al. (1996) and Havlis et al. (2003). Peptide samples were separated by online reversed-phase (RP) nanoscale capillary liquid chromatography (nano/LC) and analyzed by electrospray mass spectrometry (ES/MS/MS).
Database analysis. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.2.0).
Criteria for protein identification. Scaffold software (version Scaffold-01_07_00, proteome Software Inc. Portland Oregon, OR) was used to validate MS/MS-based peptide and protein identification. Peptide identifications were accepted if they could be established at greater than 95 % probability as specified by the Peptide Prophet algorithm (Keller et al. 2002) and contained at least 2 identified peptides. The protein probabilities were assigned using the Protein Prophet algorithm.
Results
Posttranslational regulation is fundamental for the proper mobilization of SSPs during seed imbibition and early germination. Proteomic analysis of seed germination using chromatography approaches to isolate the phosphorylated protein fraction has been successful in identifying some of the most abundant SSPs (Wan et al. 2007). In order to determine SSPs mobilization during priming, we obtained the phosphorylated fraction from primed and control seeds from Buddleja cordata and Opuntia tomentosa. Protein patterns of the phospho-protein enriched fraction from B. cordata control and natural primed seeds are observed in Figure 1. There was an important increase in the 31 and 32 kDa proteins in treated seeds (Figure 1, lane 3). These proteins were identified by LC/MS/MS as two different 11S globulin proteins (Table 1). Nine peptides that cover 20 % amino acid sequence of the 11S globulin isoform 4 from Sesamum indicum were identified for the 31 KDa. For the 32 kDa band, ten peptides were identified and covered 11 % of the 11S globulin sequence from S. indicum (Tai et al. 2001).

Figure 1 Phosphoprotein-enriched fraction of control and primed seeds from Buddleja cordata. A 12 % SDS-PAGE analysis was performed using 50 µg of protein per sample, from the phosphoprotein-enriched fraction of control (Lane 2) and primed seeds (Lane 3). The identification of the bands only present in lane 3 was done using LC/MS/MS.
Table 1 Amino acid sequences of the two 11s globulin-type SSPs from Sesamum indicum, which correspond to the best match to the different peptides identified by LC/MS/MS. 1A) 11S globulin isoform 4 (Q2XSW6). The identified peptides from the 31 kDa band are shown in bold. 1B) 11S globulin (AAK15087). The identified peptides from the 32 kDa band are shown in bold.
| 1A. | |||||
|---|---|---|---|---|---|
| MAKLFLSLLT | FLLLFSLSFA | LRGSTWQQGQ | CRISRINAQE | PTRRIQAEGG | VSEFWDHNSD |
| EFQCAGVSIH | RHRLQARALM | LPAYHNAPIL | AYVQQGRGMY | GVMISGCPET | FESSQQQFEE |
| GRGAQRFRDR | HQKIGQFREG | DILAFPAGAA | HWAYNNGDQE | LVIVVLQDNA | NNANQLDPNP |
| RSFFLAGNPA | GRGQEQQEYA | PQLGRKRGQH | QFGNVFRGFD | VQILSEVFGV | DEQAARSLQG |
| ENDERGHIIT | VARGLQVISP | PLQREEYGRQ | EEEPYYGRRD | NGLEETICSA | KLRENIDKPS |
| RADIYNPRAG | RFSTINSLTL | PILSFLQLSA | ARGVLYRNGI | MAPHWCVNAH | SVIYVTRGES |
| DMQIVSHNGQ | AVFDGRVREG | QVVVVPQNFA | VVKRAGEQGC | EWVEFNTNDN | ALINTLSGRT |
| SALRGLPADV | IANAYQISRE | EAQRLKYSRR | ETMMFSGSFR | SSRERVASA | |
| 1B. | |||||
| MALTSLLSFF | IVVTLLIRGL | SAQLAGEQDF | YWQDLQSQQQ | HKLQARTDCR | VERLTAQEPT |
| IRFESEAGLT | EFWDRNNQQF | ECAGVAAVRN | VIQPRGLLLP | HYNNAPQLLY | VVRGRGIQGT |
| VIPGCAETFE | RDTQPRQDRR | RRFMDRHQKV | RQFRQGDILA | LPAGLTLWFY | NNGGEPLITV |
| ALLDTGNAAN | QLDQTFRHFF | LAGNPQGGRQ | SYFGRPQTEK | QQGETKNIFN | GFDDEILADA |
| FGVDVQTARR | LKGQDDLRGR | IVRAERLDIV | LPGEEEEERW | ERDPYSGANG | LEETLCTAKL |
| RENLDEPARA | DVYNPHGGRI | SSLNSLTLPV | LSWLRLSAEK | GVLYRNGLVA | PHWNLNAHSI |
| IYITRGSGRF | QVVGHTGRSV | FDGVVREGQL | IIVPQNYVVA | KRASQDEGLE | WISFKTNDNA |
| MTSQLAGRLS | AIRAMPEEVV | MTAYQVSRDE | ARRLKYNREE | SRVFSSTSRY | SWPRSSRPMS |
| YMPKPFEYVL | DVIKSMM |
Table 2 Amino acid sequences of the two 12S cruciferin SSPs and a LEA protein from Arabidopsis thaliana, which correspond to the best match to the different peptides identified by LC/MS/MS. 2A) 12S cruciferin (Q96318). The identified peptides are shown in bold. 2B) 12S cruciferin (CRA1, P15455). The identified peptides are shown in bold. 3B) LEA protein group 3 (Q96246).
| 2A. | |||||
|---|---|---|---|---|---|
| MVKLSNLLVA | TFGVLLVLNG | CLARQSLGVP | PQLQNECNLD | NLDVLQATET | IKSEAGQIEY |
| WDHNHPQLRC | VGVSVARYVI | EQGGLYLPTF | FTSPKISYVV | QGTGISGRVV | PGCAETFMDS |
| QPMQGQQQGQ | PWQGRQGQQG | QPWEGQGQQG | QQGRQGQPWE | GQGQQGQQGR | QGQQGQPWEG |
| QGQQGQQGFR | DMHQKVEHVR | RGDVFANTPG | SAHWIYNSGE | QPLVIIALLD | IANYQNQLDR |
| NPRVFHLAGN | NQQGGFGGSQ | QQQEQKNLWS | GFDAQVIAQA | LKIDVQLAQQ | LQNQQDSRGN |
| IVRVKGPFQV | VRPPLRQPYE | SEEWRHPRSP | QGNGLEETIC | SMRSHENIDD | PARADVYKPS |
| LGRVTSVNSY | TLPILEYVRL | SATRGVLQGN | AMVLPKYNMN | ANEILYCTGG | QGRIQVVNDN |
| GQNVLDQQVQ | KGQLVVIPQG | FAYVVQSHGN | KFEWISFKTN | ENAMISTLAG | RTSLLRALPL |
| EVISNGFQIS | PEEARKIKFN | TLETTLTRAA | GRQQQQLIEE | IVEA | |
| 2B. | |||||
| MARVSSLLSF | CLTLLILFHG | YAAQQGQQGQ | QFPNECQLDQ | LNALEPSHVL | KSEAGRIEVW |
| DHHAPQLRCS | GVSFARYIIE | SKGLYLPSFF | NTAKLSFVAK | GRGLMGKVIP | GCAETFQDSS |
| EFQPRFEGQG | QSQRFRDMHQ | KVEHIRSGDT | IATTPGVAQW | FYNDGQEPLV | IVSVFDLASH |
| QNQLDRNPRP | FYLAGNNPQG | QVWLQGREQQ | PQKNIFNGFG | PEVIAQALKI | DLQTAQQLQN |
| QDDNRGNIVR | VQGPFGVIRP | PLRGQRPQEE | EEEEGRHGRH | GNGLEETICS | ARCTDNLDDP |
| SRADVYKPQL | GYISTLNSYD | LPILRFIRLS | ALRGSIRQNA | MVLPQWNANA | NAILYVTDGE |
| AQIQIVNDNG | NRVFDGQVSQ | GQLIAVPQGF | SVVKRATSNR | FQWVEFKTNA | NAQINTLAGR |
| TSVLRGLPLE | VITNGFQISP | EEARRVKFNT | LETTLTHSSG | PASYGRPRVA | AA |
| 2C. | |||||
| MASDKQKAER | AEVAARLAAE | DLHDINKSGG | ADVTMYKVTE | RTTEHPPEQD | RPGVIGSVFR |
| AVQGTYEHAR | DAVVGKTHEA | AESTKEGAQI | ASEKAVGAKD | ATVEKAKETA | DYTAEKVGEY |
| KDYTVDKAKE | AKDTTAEKAK | ETANYTADKA | VEAKDKTAEK | IGEYKDYAVD | KAVEAKDKTA |
| EKAKETSNYT | ADKAKEAKDK | TAEKVGEYKD | YTVDKAVEAR | DYTAEKAIEA | KDKTAEKTGE |
| YKDYTVEKAT | EGKDVTVSKL | GELKDSAVET | AKRAMGFLSG | KTEEAKGKAV | ETKDTAKENM |
| EKAGEVTRQK | MEEMRLEGKE | LKEEAGAKAQ | EASQKTREST | ESGAQKAEET | KDSPAVRGNE |
| AKGTIFGALG | NVTEAIKSKL | TMPSDIVEET | RAAREHGGTG | RTVVEVKVED | SKPGKVATSL |
| KASDQMTGQT | FNDVGRMDDD | ARKDKGKL |
The protein patterns of the phospho-protein enriched fraction from O. tomentosa control and natural primed seeds are observed in Figure 2. There was an important increase in the 35 kDa band in treated seeds (Figure 2, lane 3). From this band, three different proteins were identified by LC/MS/MS. Two SSPs that match with two isoforms of the 12S globulin cruciferin from A. thaliana (AtCRU, Li et al. 2007) and one LEA protein (Table 1, Yang et al. 1997). The first match corresponded to AtCRU3 (At4g28520) with eight peptides that cover 44 % of the amino acid sequence, the second match corresponded to AtCRU1 (At5g44120) with 4 peptides that cover 24 % of the amino acid sequence and the third one match with a LEA group 3 protein (At2g36640) with four peptides that cover 11 % of the amino acid sequence.
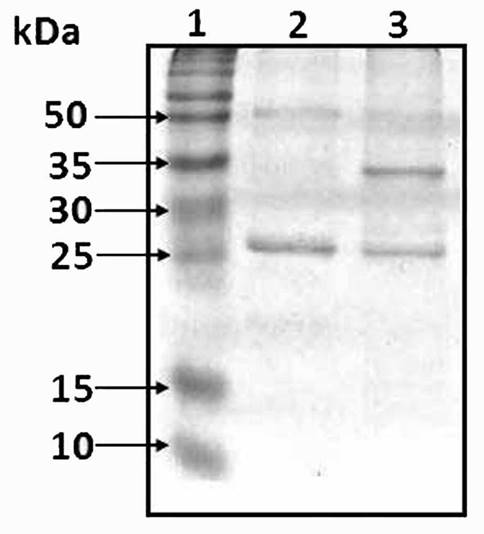
Figure 2 Phosphoprotein-enriched fraction of control and primed seeds from Opuntia tomentosa. A 12 % SDS-PAGE analysis was performed using 50 µg of protein per sample, from the phosphoprotein-enriched fraction of control (Lane 2) and primed seeds (Lane 3). The identification of the bands only present in lane 3 was done using LC/MS/MS.
Discussion
Our results suggest that natural priming also promotes globulin mobilization or synthesis in Buddleja cordata and Opuntia tomentosa seeds, and for O. tomentosa, synthesis of a LEA protein that could be involved in stress tolerance. The two processes are possibly related to the improvement of seed germination performance to natural priming as it was previously described for Wigandia urens (González-Zertuche et al. 2001, Gamboa-deBuen et al. 2006).
SSPs mobilization is one of the most important events during germination, with globulins being the most widely distributed group of SSPs present in both monocots and dicots. SSPs function as an amino acid source during germination and in response to priming, a process that has been described for crop and model plants. In particular, globulins are accumulated in the mature seed and are activated for reserve mobilization during the phase II of imbibition. Globulins are proteins that are continuously phosphorylated during seed maturation and germination. It has been proposed that phosphorylation participates either in globulin processing, assembly, or activation (Wan et al. 2007). The phospho-protein enriched fractions from treated B. cordata and O. tomentosa seeds include 11S or 12S globulins, respectively, that are not present in control seeds. These results suggest that globulin activation via phosphorylation is occurring during natural priming to promote reserve mobilization. For Triticum aestivum seeds, it has been described that phosphorylation of the globulin 3 occurred at twelve HAI in order to activate it for mobilization (Dong et al. 2015).
Priming also promoted globulin proteins accumulation in Brassica napus seeds. However, it has been suggested that this accumulation could be a result of the de novo synthesis of globulins (Kubala et al. 2015). The synthesis of globulins during early germination has been described during imbibition process (0-16 HAI), and it has been proposed that these neosynthesized globulins could function as free radical scavengers during seed imbibition (Galland et al. 2014).
The desiccation tolerance trait of the dry seed is acquired during embryogenesis, when late-embryogenesis-abundant (LEA) proteins are highly accumulated. In general, LEA proteins decrease gradually during priming and germination, as it has been described for globulins. However, osmopriming treatment promotes the synthesis of several LEAs in Medicago truncatula and B. napus seeds (Buitink et al. 2006). Our results suggest that natural priming can induce new synthesis of a LEA protein in O. tomentosa seeds so this protein could help to maintain desiccation tolerance in seeds up to radicle protrusion, as it was previously described for rape seeds (Kubala et al. 2015).
In the natural habitat of B. cordata and O. tomentosa, the soil moisture alteration by random rains promotes a natural priming process in these soil-buried seeds. In this condition, globulin mobilization could be involved in primary metabolism activation. Moreover, new synthesized LEA and globulins can be involved in protecting the seeds from stress during the hydration-dehydration cycles. In conclusion, during their permanence in soil in seasonal habitats, seeds are sensing soil moisture changes due to sporadic rains and displaying a metabolic response to cope with stressful conditions result from these changes in water availability previous to the establishment of the rainy season.











 nueva página del texto (beta)
nueva página del texto (beta)


