The marine ecosystem is a community of living and non-living organisms, including plants, animals, rocks, sediment, and seawater, with severe competition, cooperation, and regulation occurring between them. For competition, some seaweeds produce allelopathic substances—biochemicals that influence the growth, survival, and reproduction of other organisms—that facilitate growth of the producing organism (Whittaker & Feeny 1971). Allelochemicals are either beneficial (positive allelopathy) or detrimental (negative allelopathy) to the target organisms and play an important role in plant defense against herbivory (Stamp 2003). Allelochemicals that suppress or eliminate competing plant species have received special attention due to their potential as natural herbicides in agriculture (Vyvyan 2002). This focus has shifted attention to alternative seaweed control technologies, such as antifouling or algicidal agent development based on selective natural products. For example, the coralline alga Lithophyllum yessoense produces an algal spore lytic C17 fatty acid (Luyen et al. 2009), and the red seaweed Ceramium rubrum has anti-germination activity in Sargassum muticum, Enteromorpha intestinalis, and Ulva lactuca (Hellio et al. 2002). The brown seaweed Dictyota dichotoma inhibits the growth of the harmful algal blooming dinoflagellate Ostreopsis cf. ovata in co-cultures, cultured medium filtrate, and dry powder assays (Accoroni et al. 2015). The green seaweed U. lactuca inhibits the growth of several harmful algal bloom species via allelopathy (Tang & Gobler 2011). For the development of environmentally friendly algicidal or antifouling products, natural compounds from plant and animal sources are the best replacements for traditional harmful metals. Many seaweeds, and other invertebrates, are relatively free from fouling organisms, as they produce a diverse array of secondary metabolites with antibacterial (Hellio et al. 2001), antialgal (Hellio et al. 2002), and antifungal (Kubanek et al. 2003) activities (Almeida & Vasconcelos 2015).
The red seaweed Porphyra suborbiculata Kjellman is a common wild seaweed that uses a discoidal holdfast to grow on rocks in the higher intertidal zone (Aye-Mon-Sein et al. 2003). Monospores (blade archeospores) from juvenile blades can be produced year-round by adjusting culture conditions in the laboratory. Most monospores germinate to produce new juvenile blades, which themselves produce monospores under axenic culture conditions (Choi et al. 2002, 2005). Thus, monospores of P. suborbiculata are used as a bioassay for rhizoid and blade formation. To search for natural algicidal or anti-seaweed fouling products, we prepared 18 common seaweed extracts and screened for suppression of rhizoid and blade formation using the convenient monospore assay in the laboratory scale. Lead extracts were further tested against other seaweed species spores and optimized for treatment concentions, and the effects during culturing with the most potent repressor, Hizikia fusiformis (Harvey) Okamura, were also measured.
Materials and methods
Collection and extraction of seaweed. Seaweed thalli collected from common 18 different species on the coast of Korea from October 2012 to July 2015 were dried for 3-7 days at room temperature. Thalli were then ground to a powder for 5 min using a coffee grinder. For each 20 g sample, 1 l methanol was used for extraction at room temperature for 24 h. For a stock solution of each methanol-soluble fraction, 1 ml dimethyl sulfoxide (DMSO) was added to every 40 mg dried extract.
Spore collection. To obtain monospores, juvenile blades of Porphyra suborbiculata, collected from Cheongsapo (35° 9’ 46.47” N, 129° 11’ 43.76” E), Busan, Korea, were sonicated (28 kHz) twice for 1 min in autoclaved seawater, and immersed in 1 % betadine for 2 min to eliminate epiphytes (Jin et al. 1997). For each 24-well plate, five excised tissue pieces (each 5 × 5 mm2) were cultured in 1 ml Provasoli’s enriched seawater (PES) (Provasoli 1968). The blades were incubated at 18 °C with 40 µmol m-2 s-1 light intensity on a 12 h light: 12 h dark cycle to obtain monospores. Spores from Ulva pertusa, Undaria pinnatifida, and Ecklonia cava were obtained from each matured thallus that had been washed five times in autoclaved seawater, sonicated twice for 1 min, and dried by pressing between sheets of paper towel. Thalli were pretreated at 4 °C for 1 day to maximize spore release (Fletcher 1989). Spore release was induced by placing the thalli in PES at 20 °C with 80 µmol m-2 s-1 light intensity for 1 day.
Monospore culture and bioassay. Spore germination assays (for rhizoid and blade formation) were performed by adding approximately 100-200 spores to a 200 µl aliquot of PES in a 96-well plate, which was placed in the dark at 18 °C for 1 day. After non-settled spores were removed by centrifugation (1,500 rpm, 15 min) in an inverted position, 200 µl fresh PES was added to each well with 1 µl extract (from a 10-fold diluted stock solution; 20 µg ml-1 final concentration). DMSO inhibited spore germination by a minimum of 0.5 % (data not shown). Spore cultures were placed at 18 °C and 80 µmol m-2 s-1 light intensity on a 12L:12D cycle for 1 week to facilitate spore development (Cho et al. 2001). After 1 week, rhizoid formation (number of spores that produced rhizoids per total spores tested), number of rhizoids per rhizoid-holding spore, rhizoid length, blade formation (number of spores that produced blades per total spores tested), and blade length were measured using a microscope (200×). The relative rate (%) of rhizoid or blade formation was determined by the following formula: (S/T) × 100, where S = number of spores that produced rhizoids or blades, and T = total spores tested.
Diatom attachment. The microalgae Navicula annexa KMMCC-902 and Nitzschia pungens KMMCC-803 were obtained from the Korean Marine Microalgae Culture Center. Diatoms were cultured in F/2 media at 20 °C with 50 µmol m-2 s-1 light intensity on a 12L:12D cycle with shaking at 50 rpm (Guillard & Ryther 1962). The effect of H. fusiformis extract on diatom attachment was investigated in a 12-well plate containing 3.75 ml autoclaved seawater and 1 ml diatom cell suspension (~1 × 104 cells ml-1). A glass slide (2.5 × 1.5 cm2) was immersed in a well and 25 µl stock extract was added to a final 100 µg ml-1 culture and mixed. The plate was incubated in a growth chamber under fluorescent light (50 µmol m-2 s-1) for 12 h. The cell suspension was then discarded, and loosely attached cells were washed with 5 ml sterile seawater. The attached diatom cells were counted using a microscope (200×).
Statistical analysis. Various concentrations of the Hizikia fusiformis extract were added to the monospore culture to determine suppression activity. The suppression dose 100 (SD100) is expressed as the concentration of H. fusiformis extract required to prevent monospores from rhizoid and blade production after 7 days of culture. The suppression dose 50 (SD50) is the concentration of H. fusiformis extract required to limit rhizoid and blade production in 50 % of monospores after 7 days. The experiments were repeated at least three times. Mean differences between extract and control assays were compared using Student’s t-test.
Results
To search for allelopathic agents in seaweed, common seaweed extracts were tested for their ability to suppress rhizoid and blade production in a Porphyra suborbiculata monospore assay. The 18 seaweed species tested included green seaweed (Codium fragile, Monostroma nitidum, Ulva linza, Ulva pertusa), brown seaweed (Ecklonia cava, Eisenia bicyclis, Hizikia fusiformis, Ishige sinicola, Saccharina japonica, Sargassum fulvellum, Sargassum hemiphyllum, Sargassum hornei, Sargassum thunbergii, Scytosiphon lomentaria, Undaria pinnatifida), and red seaweed (Chondrus ocellatus, Corallina pilulifera, Pachymeniopsis elliptica). Among the seaweed extracts tested at 20 µg ml-1, H. fusiformis, U. pertusa, S. hemiphyllum, and M. nitidum exhibited more than 50 % inhibition of rhizoid formation compared with the control PES (Table 1). Hizikia fusiformis had the strongest suppression, with 8 % of monospores producing only rhizoids compared with 41 % with PES (P < 0.05). Rhizoid numbers per rhizoid-holding spore ranged from 1.0 to 1.6. In H. fusiformis, S. hornei, S. thunbergii, and P. elliptica, rhizoid length was suppressed significantly, to less than half. Hizikia fusiformis had the strongest inhibition, with average rhizoid lengths of 8.7 µm compared with 19.5 µm for the control (P < 0.05). Regarding germinated spores, H. fusiformis, E. cava, and I. sinicola extracts reduced blade formation to less than half. Hizikia fusiformis suppressed blade formation the most, with 1 % of monospores germinating to juvenile blades in 1 week compared with 14 % for the control (P < 0.05). Hizikia fusiformis extracts reduced blade growth significantly, to an average of 0.7 µm compared with 7.1 µm for the control (P < 0.05). Thus, the H. fusiformis extract was selected for further evaluation.
Table 1 Comparison of the ability of seaweed extracts (20 µg ml-1) to inhibit Porphyra suborbiculata monospores’ rhizoid and blade production. aSeaweed number 1, Chondrus ocellatus; 2, Codium fragile; 3, Corallina pilulifera; 4, Ecklonia cava; 5, Eisenia bicyclis; 6, Enteromorpha linza; 7, Hizikia fusiformis; 8, Ishige sinicola; 9, Monostroma nitidum; 10, Pachymeniopsis elliptica; 11, Saccharina japonica; 12, Sargassum fulvellum; 13, Sargassum hemiphyllum; 14, Sargassum horneri; 15, Sargassum thunbergii; 16, Scytosiphon lomentaria; 17, Ulva pertusa; 18, Undaria pinnatifida; PES, Provasoli’s enriched seawater. bValues are expressed as means ± SE (n > 3). *P < 0.05 vs. PES control.
| Seaweed numbera |
Rhizoid formation (%)b |
No. of rhizoids / rhizoid-holding sporeb |
Rhizoid length (µm)b |
Blade formation (%)b |
Blade length (µm)b |
|---|---|---|---|---|---|
| 1 | 30 ± 15 | 1.0 ± 0.0 | 19.9 ± 6.8 | 8 ± 2 | 1.7 ± 0.5 |
| 2 | 50 ± 6 | 1.1 ± 0.0 | 19.9 ± 5.9 | 11 ± 1 | 8.9 ± 1.2 |
| 3 | 34 ± 6 | 1.3 ± 0.1 | 18.4 ± 1.2 | 7 ± 2 | 7.9 ± 1.4 |
| 4 | 49 ± 8 | 1.6 ± 0.2 | 26.8 ± 3.2 | 5 ± 5 | 2.1 ± 2.1 |
| 5 | 35 ± 3 | 1.0 ± 0.0 | 16.4 ± 1.2 | 11 ± 3 | 7.7 ± 1.9 |
| 6 | 25 ± 9 | 1.0 ± 0.0 | 11.9 ± 2.6* | 15 ± 11 | 3.5 ± 1.9 |
| 7 | 8 ± 3* | 1.0 ± 0.0 | 8.7 ± 3.2* | 1 ± 1* | 0.7 ± 0.7* |
| 8 | 42 ± 9 | 1.2 ± 0.4 | 20.0 ± 6.7 | 6 ± 1* | 13.7 ± 1.2 |
| 9 | 16 ± 1* | 1.1 ± 0.0 | 20.0 ± 6.7 | 16 ± 5 | 8.1 ± 1.4 |
| 10 | 26 ± 4* | 1.0 ± 0.0 | 9.7 ± 5.9* | 16 ± 3 | 8.9 ± 1.7 |
| 11 | 24 ± 7* | 1.1 ± 0.1 | 19.9 ± 6.7 | 10 ± 4 | 6.6 ± 1.5 |
| 12 | 35 ± 1 | 1.2 ± 0.1 | 16.7 ± 0.1 | 8 ± 3 | 10.3 ± 0.5 |
| 13 | 13 ± 4* | 1.0 ± 0.2 | 19.8 ± 4.1 | 11 ± 4 | 8.2 ± 2.5 |
| 14 | 43 ± 1 | 1.1 ± 0.0 | 8.9 ± 1.8* | 15 ± 2 | 9.2 ± 0.6 |
| 15 | 36 ± 4 | 1.0 ± 0.0 | 9.4 ± 0.2* | 20 ± 3 | 5.4 ± 1.5 |
| 16 | 43 ± 4 | 1.0 ± 0.2 | 17.3 ± 11.1 | 9 ± 5 | 1.2 ± 0.8 |
| 17 | 12 ± 4* | 1.3 ± 0.0 | 20.0 ± 6.7 | 17 ± 0 | 7.9 ± 1.7 |
| 18 | 35 ± 16 | 1.0 ± 0.0 | 18.3 ± 4.9 | 7 ± 3 | 7.3 ± 1.2 |
| PES | 41 ± 5 | 1.1 ± 0.0 | 19.5 ± 2.1 | 14 ± 4 | 7.1 ± 0.4 |
Various concentrations of the H. fusiformis extract were added to the monospore culture to determine suppression activity. The suppression dose 100 (SD100) and suppression dose 50 (SD50) values reflecting the amount of H. fusiformis extract required to suppress rhizoid formation (in terms of number of spores with rhizoids per total spores tested) were 114 and 15 µg ml-1, respectively, in the monospore culture (Figure 1A). The SD100 and SD50 values of the extract required to suppress rhizoid number were 20 and 2.4 µg ml-1, respectively (Figure 1B). Most suppressed rhizoids grew at least 6 µm in 7 days, and the SD100 and SD50 values reflecting the suppression of rhizoid length were 88 and 13 µg ml-1, respectively (Figure 1C). The SD100 and SD50 values for suppression of blade formation (number of spores with blades per total spores tested) were 145 and 6 µg ml-1, respectively, where blades grew an average of 6 µm in 1 week (Figure 1D). The SD100 and SD50 values for blade length suppression were 100 and 11 µg ml-1, respectively (Figure 1E). Next, growth of P. suborbiculata monospores was observed upon treatment with 100 µg ml-1 (approximate SD100 against all parameters) H. fusiformis extract for 10 days. The H. fusiformis extract almost completely suppressed rhizoid formation (Figure 2A), rhizoid numbers per rhizoid-holding spore (Figure 2B), rhizoid length (Figure 2C), blade formation (Figure 2D), and blade length (Figure 2E) in the monospore assay. However, after 10 days of culture, monospores began to increase rhizoid formation, rhizoid numbers, and rhizoid length.
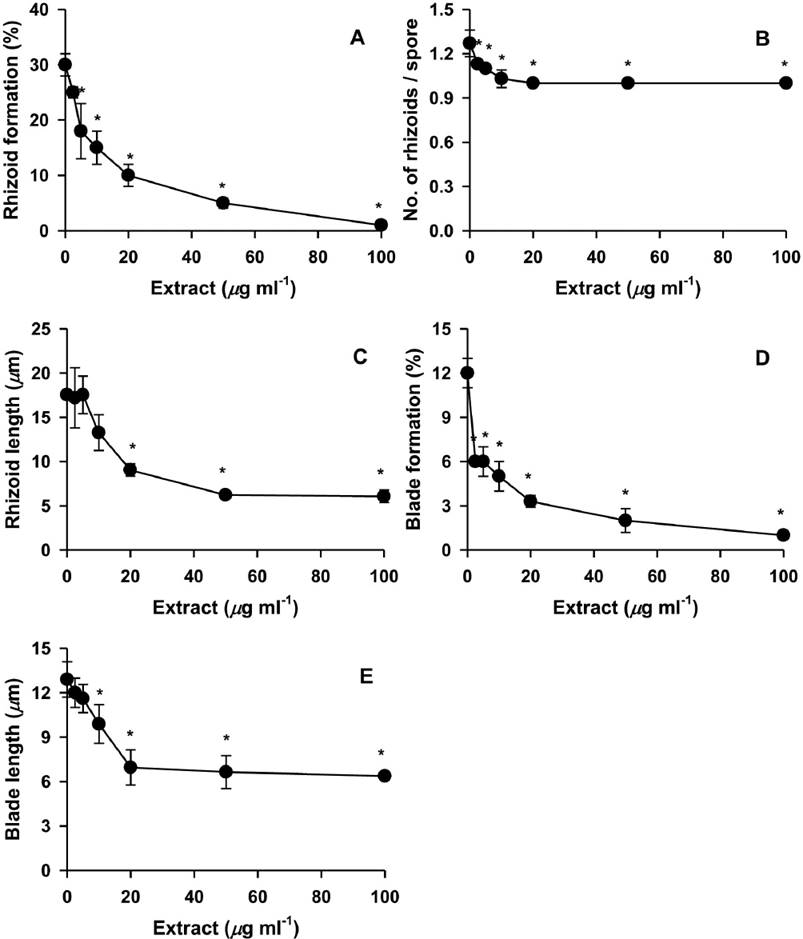
Figure 1 Effects of different concentrations of Hizikia fusiformis extract on the production of rhizoids and blades from Porphyra suborbiculata monospores. Suppression activities were measured using rhizoid formation (% of spores with rhizoids / total spores tested; A), number of rhizoids / rhizoid-holding spore (B), rhizoid length (C), blade formation (% of spores with blades / total spores tested; D), and blade length (E). *P < 0.05 vs. PES control.

Figure 2 Effects of Hizikia fusiformis extract on the production of rhizoids and blades from Porphyra suborbiculata monospores. Suppression was measured by rhizoid formation (% of spores with rhizoids / total spores tested; A), number of rhizoids / rhizoid-holding spore B), rhizoid length C), blade formation (% of spores with blades / total spores tested; D), and blade length E). ●, H. fusiformis extract (100 µg ml-1). ●, PES control. *P < 0.05 vs. control.
The suppression effects of the H. fusiformis extract were also evaluated using spores from other common seaweed species, including Ulva pertusa, Undaria pinnatifida, and Ecklonia cava. The extract significantly suppressed the rhizoid formation of U. pertusa, U. pinnatifida, and E. cava spores. This extract caused suppression to 7 % (relative % against each control; 4/54 spores), 33 % (13/39 spores), and 3 % (1/36 spores), respectively, which were comparable to 3 % from P. suborbiculata spores (Table 2). Hizikia fusiformis extract also reduced rhizoid length (51 %), blade formation (25 %), and blade length (80 %) of U. pertusa, compared with control. The extract also suppressed rhizoid numbers (63 %), rhizoid length (67 %), and blade formation (4 %) from E. cava spores. Taken together, the H. fusiformis extract suppressed rhizoid and blade production from leafy green (U. pertusa), brown (U. pinnatifida and E. cava), and red (P. suborbiculata) seaweed spores. The methanol extract of H. fusiformis also inhibited the attachment of the fouling diatoms Navicula annexa and N. pungens by 86 % and 38 %, respectively (Table 3). The crude extract (100 µg ml-1; approximate SD100 against seaweed parameters) significantly suppressed settlement of N. pungens.
Table 2 Effects of Hizikia fusiformis extract on the production of rhizoids and blades from Ulva pertusa, Undaria pinnatifida, Ecklonia cava, and Porphyra suborbiculata spores. Spores were cultured in the extract (100 µg ml-1) for 1 week.
| Rhizoid formation (%) |
No. of rhizoids / rhizoid-holding spore |
Rhizoid length (µm) |
Blade formation (%) |
Blade length (µm) |
|
|---|---|---|---|---|---|
| Spores of U. pertusa | 4 ± 0* | 1.6 ± 0.2 | 11.8 ± 2.4* | 5 ± 2* | 22.8 ± 1.7* |
| in the extract | |||||
| Control | 54 ± 3 | 2.0 ± 0.1 | 23.0 ± 0.7 | 20 ± 0 | 28.6 ± 0.7 |
| Spores of U. pinnatifida | 13 ± 0* | 1.3 ± 0.1 | 5.3 ± 0.7 | 0 ± 0* | 19.9 ± 0.8 |
| in the extract | |||||
| Control | 39 ± 3 | 1.5 ± 0.1 | 6.6 ± 0.6 | 4 ± 0 | 21.4 ± 0.6 |
| Spores of E. cava | 1 ± 1* | 1.0 ± 0.0* | 2.9 ± 0.4* | 2 ± 0* | 15.0 ± 1.8 |
| in the extract | |||||
| Control | 36 ± 3 | 1.6 ± 0.0 | 4.3 ± 0.1 | 55 ± 5 | 16.3 ± 1.1 |
| Spores of P. suborbiculata | 1 ± 0* | 0.5 ± 0.0* | 3.0 ± 0.3* | 1 ± 0* | 3.2 ± 0.1* |
| in the extract | |||||
| Control | 30 ± 2 | 1.3 ± 0.1 | 17.5 ± 0.3 | 12 ± 1 | 12.2 ± 0.6 |
Table 3 Effects of Hizikia fusiformis extract on the settlement of diatoms Navicula annexa and Nitzschia pungens. Diatoms were cultured in extract (100 µg ml-1) for 12 h. Values are expressed as means ± SE (n > 3). *P < 0.05 vs. control.
| No. of settled diatoms | Relative value against control |
|
|---|---|---|
| Navicula annexa | 379 ± 111 | 86 % |
| Control | 443 ± 76 | |
| Nitzschia pungens | 303 ± 67* | 38 % |
| Control | 790 ± 43 |
Discussion
In marine ecosystems, some seaweeds are covered heavily by epiphytes, while others in the same habitat have defense mechanisms that prevent colonization. Such epiphyte-free seaweeds may synthesize secondary metabolites that protect against epibiont colonization (Águila-Ramírez et al. 2012). To search for such allelopathic agents in seaweed, common seaweed species extracts were tested for their ability to suppress rhizoid and blade production in a monospore assay. The monospores are easily maintained under laboratory conditions and reproduces throughout the year (Choi et al. 2005). They germinate to produce new juvenile blades, which can then produce additional monospores about every 20 days. It allows the monospores to be conveniently used as a target organism in a preliminary screening system for detecting allelopathic or antifouling compounds in the seawater condition. Even though this monospore assay system has a limit that is not done under natural marine environment, the bioassay provides a valuable early indication of the allelopathic or antifouling action efficacy prior to field testing.
Among the seaweed species tested, Hizikia fusiformis showed the strongest suppression activities. H. fusiformis is an edible and abundant aquaculturable brown seaweed. The amount of H. fusiformis produced by farming in 2013 amounted to 13,000 t (wet weight), with an additional 2,000 t (wet weight) collected from natural populations in Korea (Korea Fisheries Association 2014). In our previous study (Choi et al. 2005), methanol extracts of the seaweed at high concentration (200 µg ml-1) lysed monospores of Porphyra yezoensis. Additionally, the methanol extract of H. fusiformis also suppressed tissue growth (49 %), spore settlement (59 %), and zygote formation (49 %) of the green seaweed Enteromorpha prolifera (Cho et al. 2001). The H. fusiformis extract almost completely suppressed rhizoid formation, rhizoid numbers, rhizoid length, blade formation, and blade length at the early stages of the P. suborbiculata monospore assay. However, after 10 days of culture, monospores began to increase rhizoid formation, rhizoid numbers, and rhizoid length. Thus, epigenetic adaptation of rhizoids resulting in the induction of alternative pathways, enzymes, or detoxification may overcome the extract treatment (Gonzales & Widholm 1985). The suppression effects of the H. fusiformis extract were also demonstrated against rhizoid formation, rhizoid numbers, rhizoid length, blade formation, and blade length from leafy green (Ulva pertusa), brown (Undaria pinnatifida and E. cava), and red (P. suborbiculata) seaweed spores. Mature U. pertusa has a wide blade and forms extensive mats in shallow coastal waters due to its fast rate of growth and high reproductive capacity (Han et al. 2003). This seaweed is a main component of green tides and fouling coverage (Sidharthan et al. 2004), and contributes to higher trophic levels. Undaria pinnatifida is an invasive species, competing with native kelp species occupying shallow sublittoral and infralittoral zones (Farrell & Fletcher 2006). Ecklonia cava is a marine forest species commonly used to develop artificial seaweed forests (Hayashida 1984). Thus, by suppressing fast-growing and massive leafy seaweeds, H. fusiformis or its extract may provide negative allelophatic activity against diverse fouling seaweeds. In the early stages of biofouling, single-cell diatoms sense the surface, settle, and form a biofilm (Costerton et al. 1995). The inhibition of biofilm is assumed to lead to the inhibition of adhesion and subsequent fouling stages (Hellio et al. 2001). The methanol extract of H. fusiformis inhibited the attachment of the fouling diatoms N. annexa and N. pungens, and possibly contains allelochemicals that suppress or eliminate soft marine foulants. Such allelochemicals may act as a selective natural herbicide for seaweed control technologies, including antifouling or algicidal agent development. The suppressing compound from H. fusiformis was soluble in methanol, chloroform, acetonitrile, and dimethylsulfoxide. Isolation of the active compound presumed as a moderately polar phenolic compound is now in progress.











 nova página do texto(beta)
nova página do texto(beta)


