Maytenus Molina is one of the largest genera of Celastraceae, found in tropical and subtropical regions, and distributed throughout the Brazilian territory (Lombardi et al. 2014). Many of the chemical components isolated from its species have pharmacological properties (Din et al. 2013, Duarte et al. 2013, Moo-Puc et al. 2014). A good example is M. ilicifolia Mart. ex Reissek, that is mentioned in the Brazilian Pharmacopoeia (ANVISA 2011) to treat gastric ulcer (Santos-Oliveira et al. 2009). Similarly, M. imbricata Mart. ex Reissek, (Silva et al. 2009, Rodrigues et al. 2012), M. aquifolium Mart., (Gonzalez et al. 2001), M. salicifolia Reissek, (Magalhães et al. 2011) and M. truncata Reissek, (Silva et al. 2005a) are used in folk medicine to treat the same stomach illness, and have the same popular name “espinheira-santa” (Niero et al. 2011). For these reasons it is necessary a careful quality control of raw material in the manufacture of drugs using Maytenus species and is required define anatomical key characters for the unambiguous species identification (Jacomassi & Machado 2004, Duarte & Debur 2005, Joffily & Vieira 2005, Nakamura et al. 2013). For five species of Maytenus, Den Hartog & Bass (1978) elected stomatal type and the presence of trichomes and crystals as safe parameters among others epidermal characteristics. Duarte & Debur (2005) proposed epidermal cells containing styloids, cuticular flanges and amphicrival vascular bundles as relevant characters for the diagnosis of M. ilicifolia.
M. imbricata is known for the presence of bioactive compounds (Silva et al. 2005b, 2007, Silva et al. 2009, Rodrigues et al. 2012) nevertheless there is no study to quality control. The goal of this work was to describe the morphological and anatomical features of the leaf and relate them to the environment where the species occurs.
Materials and methods
Fully expanded leaves of M. imbricata were obtained from three individuals in an area of ‘campo rupestre’ (one of the Brazilian Savannah vegetation, 20º 22’ 11.02”S, 43º 30’ 22.81”W) and they represent the morphological variation of the studied population. A voucher specimen (MCTB Messias, 2565) was deposited at the OUPR Herbarium (Universidade Federal de Ouro Preto, Minas Gerais, Brazil). Samples of the median region of the leaf blade and the petiole were fixed in Karnovsky solution (Karnovsky 1965) for 12 hours, dehydrated in a graded ethanol series (Jensen 1962), and cold-embedded (Paiva et al. 2011) in hydroxyethyl-methacrylate resin (Leica Microsystem Inc., Heidenbeg, Germany). Cross sections of the median rib, transverse and paradermal sections of the internerval region, and cross-sections of the margin and the petiole were obtained using a rotary microtome (Atago, Tokyo, Japan), stained with Toluidine blue pH 4.7, rutenium red (O’Brien et al. 1964, modified), basic Fuchsin and Astra blue and mounted in acrylic resin (Itacril, Itaquaquecetuba, Brazil). Phenolic compounds were identified by the red color obtained with basic Fuchsin and bright blue color obtained with Tolulidine blue, and acids polysaccharides with ruthenium red. Images were captured using an AxioCam MRc camera coupled to a AxioVision LE light microscope (Zeiss, Oberkochen, Germany).
Results
Morphology. The leaves are light green, congested, erect, imbricate, coriaceous and glabrous, and are coated on both sides by a conspicuous wax layer. Leaf shape varies from orbicular to oval, the base is slightly cordate and the apex is obtuse-emarginate. The margins are revolute, crenate, and have teeth. The petiole is 0.1 to 0.2 cm long, and the leaf blade is 1.5 to 3 cm long and 1 to 3 cm wide with a protruding central nerve on the adaxial side. Secondary nerves are evident at the leaf surface but are not protruding (Figure 1A). The venation is craspedodromous, and the marginal teeth contain multicellular secretory structures named colleters (Figure 1B-C).
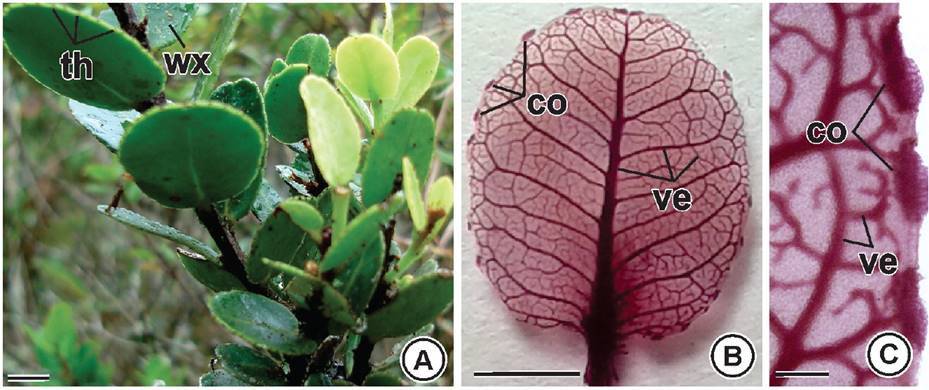
Figure 1 Leaf morphology of Maytenus imbricata Mart. ex Reiss. A) General view of the plant showing the rounded marginal teeth and epicuticular wax. B) Venation craspedodromous. C) Colleters on marginal teeth. co: colleters; th: teeth; ve: vein; wx: waxy. Bars: A-C = 1cm.
Anatomy. At the midrib region the epidermal cells have elongated anticlinal wall and the vascular system has the shape of a closed loop in cross-section, and is surrounded by a conspicuous sheath of gelatinous sheath (Figure 2A). At the internerve region the cells of the adaxial face of the epidermis have thick external periclinal wall, and straight anticlinal wall with strips on its innermost region (Figure 2B-C). The mesophyll is dorsiventral with palisade parenchyma composed of two layers of transversely elongated cells, and spongy parenchyma composed of globoid cells compactly arranged facing the abaxial surface (Figure 2D-E). The presence of phenols is observed throughout the whole leaf tissues (Figure 2A-E).

Figure 2 Leaf anatomy of Maytenus imbricata Mart. ex Reiss. A) Midrib region. Vascular system with a closed-loop shape (cross section). B-C) Cells of the adaxial epidermis with strips on the base of the straight anticlinal walls (cross section on B, and paradermic section - external view on C). D-E) Internerve region. Hypostomatic leaf (arrows). Dorsiventral mesophyll (cross section on D and paradermic-oblique section on E). ab: abaxial epidermis; ad: adaxial epidermis; cl: collenchyma; cu: cuticle; gf: gelatinous fibers; ph: phloem; pp: palisade parenchyma; sr: strips; sp: spongy parenchyma; sw: straigth anticlinal wall; tw: thick external periclinal wall; vs: vascular system; xy: xylem. Bars: A = 200 µm; B-E = 50 µm.
The vascular bundles in secondary veins are collateral and gelatinous fibers are on both sides (Figure 3A). In cross section, subsidiary cells have pear-shaped and narrow diameter on the leaf surface, and are internally voluminous (Figure 3B). The leaf is hypostomatic displaying a higher number of ciclocytic stomata on the adaxial face (Figures 2D, 3B-C). The external walls of the ordinary cells of the abaxial epidermis are thick and show large pits such as pores (Figure 3B, D).

Figure 3 Leaf anatomy of Maytenus imbricata Mart. ex Reiss. A) Collateral vascular bundle with gelatinous fibers on both sides (cross section). B) Subsidiary pear-shaped cells with narrow diameter on the leaf surface, and voluminous internally (cross section). C) Ciclocytic stomata (paradermic section - internal view). D) Thick external wall of the ordinary cells of the abaxial epidermis with large pits as pores (cross section). gf: gelatinous fibers; ph: phloem; po: pore; sb: substomatal chamber; sc: subsidiary cell; sp: spongy parenchyma; st: stomata; tw: thick wall; xy: xylem. Bars = 50 µm.
In leaf margin, the epidermal cells have thick cuticle and thick pectic walls. The mesophyll is homogeneous and involves a bicolateral vascular bundle with a prominent gelatinous fibers cap on one side (Figure 4A). The petiole outline is rounded with two lateral ribs on the adaxial side and is covered by epidermal cells with thick cuticle and thick pectic walls. The outer cortex is constituted by cells with periclinal divisions and the inner cortex has sclereids clusters. The vascular system is closed loop, and it is wrapped by a discontinuous sheath of gelatinous fibers and displays cambial activity. The secondary xylem is well developed and encircle a parenchymatous pith (Figure 4B).

Figure 4 Leaf anatomy of Maytenus imbricata Mart. ex Reiss. A) Leaf margin: epidermal cells with thick cuticle and pectic walls, homogeneous mesophyll and a bicolateral bundle with gelatinous fibers on both sides. B) Petiole: ribs on the adaxial side, epidermal cells with thick cuticle and thick pectic walls, outer cortex cells with periclinal divisions, inner cortex with clusters of sclereids, vascular system closed loop, sheath of gelatinous fibers, cambial activity, secondary xylem and parenchymatous pith. cu: cuticle; ep: epidermis; gf: gelatinous fibers; hm: homogenous mesophyll; ph: phloem; pi: pith; ri: ribs; sc: sclereids; tw: thick wall; xy: xylem. Bars: A = 50 µm; B = 250 µm.
Discussion
The structural features present in plant organs are useful tools for quality control in the manufacture of herbal medicines. The leaf margin teeth are an important feature in the identification of Maytenus species (Joffily & Vieira 2005, Lombardi & Groppo 2014). The thorns and scars present on the end of the teeth indicate the drop of the colleters observed at the primordial leaf margin. Some anatomical characters in leaves occur in response to environmental conditions (Voltolini & Santos 2011, Sack & Scoffoni 2013). Colleters secrete resin or mucilage to protect the stem apex against desiccation, which is favored by the low humidity of the air during the dry season in Brazilian Savannah (Mercadante-Simões & Paiva 2013).
Epidermal traits are particularly useful for quality control purpose, because they are well preserved even in pulverized material. In this work ciclocytic stomata were found in M. imbricata whereas anomoctic stomata were reported for M. aquifolia and M. ilicifolia (Jacomassi & Machado 2004). Acicular crystals on both sides of the epidermis were recorded for M. ardisiaefolia, M. brasiliensis, M. communis and M. obtusifolia (Joffily & Vieira 2005) but not observed in M. imbricata. The presence of epicuticular wax, thick cuticle and thick periclinal wall on the epidermis of M. imbricata has also been recorded in other species of Maytenus (Duarte & Debur 2005, Joffily & Vieira 2005). The thickening of the epidermal cell walls of M. imbricata seems to be related to the restriction of water loss by transpiration. Also, the reduced area of exposure of the subsidiary cell on the leaf surface minimizes water loss under high solar radiation conditions (Voltolini & Santos 2011).
The presence of pores in the wall of epidermal cells and the occurrence of guard cells arranged in rosette and subsidiary cells positioned below the surface in M. imbricata have been reported for other species of Celastraceae (Metcalfe & Chalk 1957). However, strips on the epidermal cell wall are reported for the first time for Maytenus and their role in the control of water traffic in apoplast is well recognized (Moreira & Isaias 2008, Yang et al. 2011). The ‘Phi thickening’ on the cell walls was also related to the water traffic control (Fernandez-Garcia et al. 2009, Idris & Collings 2015). On the other hand, the occurrence of large pits such as pore on the wall in M. imbricata may favor the deposition of deterrent compounds throughout the whole leaf surface protecting against herbivory and microorganisms infection (Yeats & Rose 2013).
Cells containing phenolic compounds are common in Maytenus species (Gupta & Sharma 2012) and have also been reported for other genera of Celastraceae (Mercadante-Simões et al. 2014). The secondary metabolites are responsible for the relation of the plant with the biotic and abiotic environment. Bioactive compounds accumulation can be induced by the severe water constraint conditions in the Brazilian Savannah (Di-Ferdinando et al. 2014).
The major branching of the craspedodromous venation in M. imbricata is related to the need of an adequate water supply. The vein length for area has a strong correlation with environmental aridity and higher values were found to sun plants when compared to shade plants (Sack & Scoffoni 2013). In our work a sheath of gelatinous fibers was found in M. imbricata. These characters were not observed in other species of Maytenus but a vascular bundle with a sclerenchymatic sheath at the margin were reported for M. aquifolia and M. ilicifolia (Jacomassi & Machado 2004) and perivascular fibers near the vascular system were observed in M. ardisiaefolia, M. brasiliensis, M. communis and M. obtusifolia (Joffily & Vieira 2005). The conspicuous sheath of gelatinous fibers surrounding the vascular bundles of M. imbricata contributes to the better use of water absorbed by the xylem. The gelatinous fibers have thickened walls rich in acid polysaccharides allowing water retention (Melo et al. 2011, Sonsin et al. 2012).
Conclusions
The structural features on medicinal plants are useful for their unequivocal identification, particularly when they are in small pieces to be used in herbal medicine production. The key characters to M. imbricata are strips on the epidermal cell anticlinal wall, reported here for the first time for the genus, and conspicuous gelatinous fibers sheath. These structural characters are related to the dry Brazilian Savanna conditions.











 nueva página del texto (beta)
nueva página del texto (beta)


