Adaptation to a specific environment is an evolutionary process and natural plant communities provide exclusive opportunity to study mechanism of adaptation based on structure and function (Ahmad et al. 2016). Adaptive components may fix in plant species, and even in populations of a same species as a result of long-term evolutionary history within a particular set of environments (Anderson et al. 2011). Salt tolerance mechanism of plants is relatively difficult to quantify because of varied plant responses and complex nature of salt stress. The use of Typha domingensis Pers. as phytoremediation under salt stress condition has been found to be very much effective to alleviate salt induced damages (Khandare & Govindwar 2015).
Plants growing in contaminated water provide a model for studying salt mechanisms of accumulation and tolerance in plants (Hegazy et al. 2011). Differential response to salinity differs greatly among plant species depending on the capability to gather noxious ion (Flowers & Clomer 2008), levels of stress (Hameed et al. 2014) and environmental conditions (Ahmad et al. 2016). Salt tolerant plants are well-known for their ability to tolerate the high stress of salinity (Reddy et al. 2008), and are characterized by the presence of large air spaces and radiate chlorenchyma in leaf (Rad & Sonboli 2008).
Salinity is a noticeable stress which stimulate many physiological and biochemical reactions in plants and disturb almost all functioning of plant cell (Megdiche et al. 2007) especially growth, leaf area and ultimate yield of the plants. Dehydration, ionic toxicity, nutritional deficiency and hormonal imbalance are major mechanisms affected by salt stress (Farouk 2011). Saline environment causes: i) reduction in the water potential, ii) reduce the root efficiency for the absorption of water and nutrient, and iii) caused unequal distribution of ions, and thus shows the toxicity symptoms in plants (Meloni et al. 2003).
Salinity tolerance takes place by two ways: by prevent the entry of the salt in plants or by minimize the concentration of the salt in the cytoplasm (Munns 2002). To protect the plants from osmotic stress, plants accumulate high amount of proline found in plants and other organisms (Bartels & Sunkar 2005). It is widely accepted that under high salinity conditions, most halophytes can compartmentalize the Na+ in vacuoles and these ions can utilize as osmoticum (Glenn & Brown 1999).
Modifications of anatomical structures is an adaptive mechanism for the plant species (Grigore & Toma 2007). Different changes in shoot anatomy of plants during growth have been observed at earlier stages (Hilal et al. 1998, Maggio et al. 2007). Important anatomical characteristics related to salt tolerance are the sclerification in leaf parenchyma and other tissues (Dolatabadian et al. 2011), large aerenchyma (Abulfatih 2003), and presence of thin walled and large bulliform cell (Grigore & Toma 2011), under unfavorable environment.
Salt tolerant plants are used to accumulate metals from contaminated water and carry out the phytoremediation process. Typha domingensis is competent to accumulate the metal ions preferentially from wastewater than from sediments and has the potential to tolerate the high salinity. It was hypothesized that differently adapted ecotypes of T. domingensis may have different structural and biochemical response to various levels of salt stress. The present study was therefore aimed to evaluate the effect of salt stress on some foliar anatomical and biochemical attributes.
Materials and Methods
Plant material. Typha domingensis is a dominant species of aquatic wetlands, which is also known for its adaptability to saline and polluted waters. Six ecotypes were evaluated under salt stress to investigate the anatomical and biochemical response against the salt stress. Ecotypes were first established in the Botanic Garden Research Area, University of Agriculture, Faisalabad for six months in non-aerated standing water. The vegetative buds with three mature tillers were detached from each mature plant and were grown in plastic tubs (20 liters) in non-aerated flooded water conditions. Plastic tubs were half filled with a mixture of clayey-loam and sand in 1:1 ratio, and then filled with 10 L water. Gatwala (Faisalabad) ecotype was collected along the bank of freshwater canal. Knotti and Jahlar ecotypes were collected from hyper saline wetlands of Knotti Garden and Jahlar Lake in the Salt Range. Treemu ecotype was collected from the bank of River Chenab near Treemu headworks. Other ecotypes Sahianwala and Sheikhupura were collected from saline-waterlogged area near Faisalabad and industrial polluted wetland respectively (Plate 1 and 2). All sites showed significant difference in their physico-chemical characteristics (Table 1).

Plate 1 Plate 1. Ecotypes of Typha domingensis from a) River bank of Treemu Headworks, b) Jhang, c) Canal bank of Gatwala, d) Canal water of Faisalabad, e) Salt affected wetland of sheikhupura, f) Industrial-polluted waters of Sheikhupura.

Plate 2 Ecotypes of Typha domingensis from a) Hyper-saline Jahlar Lake, b) The Salt Range, Chakwal, c) Hyper-saline wetland Knotti Garden, d) the Salt Range, Chakwal, e) Saline-waterlogged wetland Sahianwala, f) Normal Faisalabad.
Table 1 Soil physico-chemical characteristics of the habitats of different Typha domingensis ecotypes.
| Habitat | Jahlar | Sheikhupura | Sahianwala |
|---|---|---|---|
| Plant species | Typha domingensis | ||
| Coordinates | 31° 17’ 04.27N | 31° 17’ 24.49N | 31° 40’ 12.92N |
| 72° 24’ 45.17E | 40° 06’ 19.82E | 73° 12’ 22.81E | |
| Elevation (m) | 2,739 | 673 | 629 |
| Saturation percentage | 35.55 | 28.84 | 41.17 |
| pH | 7.49 | 8.81 | 6.98 |
| ECe (dSm-1) | 16.57 | 2.54 | 18.85 |
| Na+ (mg kg-1) | 2,848.07 | 84.58 | 4,448.38 |
| Cl- (mg kg-1) | 1,629.18 | 325.67 | 2,128.34 |
| K+ (mg kg-1) | 247.63 | 45.28 | 149.81 |
| Ni2+ (mg kg-1) | 14.67 | 39.38 | 21.54 |
| Habitat | Gatwala | Treemu | Knotti |
| Plant species | Typha domingensis | ||
| Coordinates | 31° 73’ 30.10N | 31° 72’ 23.46N | 32° 72’ 52.80N |
| 28° 12’ 46.17E | 08° 08’ 47.72E | 32° 08’ 55.31E | |
| Elevation (m) | 637 | 477 | 3021 |
| Saturation percentage | 27.58 | 24.16 | 38.19 |
| pH | 9.15 | 6.94 | 8.48 |
| ECe (dSm-1) | 3.12 | 2.52 | 35.47 |
| Na+ (mg kg-1) | 72.38 | 337.71 | 5,627.18 |
| Cl- (mg kg-1) | 434.38 | 341.57 | 2,529.13 |
| K+ (mg kg-1) | 59.59 | 63.78 | 378.56 |
| Ni2+ (mg kg-1) | 18.28 | 14.32 | 12.09 |
Experimentation. All the ecotypes of Typha domingensis were acclimatized in the Faisalabad conditions at Botanic Garden Research Area, University of Agriculture, Faisalabad for a period of six months. The vegetative buds of almost equal size were randomly detached from mother plants and grown in waterlogged conditions. The experiment was organized in complete randomized design with four levels of salinity viz. 0, 100, 200 and 300 mM NaCl and five replicates. After 8 weeks of growth in the salt treatment, plants were carefully collected from the medium and washed thoroughly with distilled water.
Anatomical attributes. For the anatomical studies, five plants of similar age were selected and a piece of leaf 2 cm long was taken from the longest tiller were used. Following the procedure as described by Ahmad et al. (2016), material was fixed in formalin acetic alcohol (v/v formaline 10 %, acetic acid 5 %, ethyl alcohol 50 % and distilled water 35 %) fixative for 48 h and subsequently transferred to acetic alcohol (v/v acetic acid 25 %, and ethanol 75 %) solution for long-term storage. Permanent free-hand sectioning slides were prepared by serial dehydrations in ethanol using standard double-stained technique of safranine and fast green stains. Measurements were obtained using an ocular micrometer, which was calibrated with a stage micrometer. Photographs were taken with the help of a camera equipped light microscope (Nikon 104, Japan).
Biochemical attributes. To determine the biochemical parameters, supernatant was separated from fresh leaves (1.0 g), sliced in citrate buffer and incubated for 1 h and spectrophotometer (Hitachi 220, Japan) was used for biochemical analysis. Proline was estimated by the method described by (Bates et al. 1973). The proline concentration was determined from a standard curve and calculated on fresh weight basis as follows:
µmole proline g-1 fresh weight = (µg proline ml-1 × ml of toluene/115.5) / (g of sample)
For glycinebetaine, dried plant material (0.5 g) was mechanically shaked with 20 ml of deionized water for 24 h at 25 ºC and then filtered following a method of Grattan & Grieve (1998). For total amino acids, fresh leaves (1.0 g) were chopped in 10 mL of citrate buffer (pH 5.0) and incubated for 1h at room temperature and centrifuged at 15,000 rpm at 15 ºC for 10 min. The supernatant was separated and used to measure the total free amino acids following the method of Moor & Stein (1948).
For total soluble proteins (Lowry et al. 1951), fresh leaf material (0.2 g) was chopped in 5 mL of phosphate buffer (0.2 M) of pH 7.0 and was ground. The ground leaf material was centrifuged at 5,000 × g for five min. The supernatant was used for protein determination. Total soluble sugars were determined by following (Yemm & Willis 1954). Plant material (0.1 g) was extracted in 80 % ethanol solution. Dried plant material was ground well in a micromill and the material was sieved to 1 mm sieve of micromill. Plant material (0.1 g) was extracted in 80 % ethanol solution. The extract was incubated for 6 h at 60 ºC. This extract was used for the estimation of total soluble sugars.
Redundancy analysis (RDA). Data was subjected to multivariate RDA to correlate anatomical and physiological attributes along salinity gradient using software CONACO (v. 5 for Windows, http://www.canoco5.com). The data for all anatomical and physiological parameters recorded in this study were standardized before RDA analysis. Then a multivariate direct gradient model was fitted and all variables were plotted on RDA Axis 1 and 2 (Figures 3 and 4)
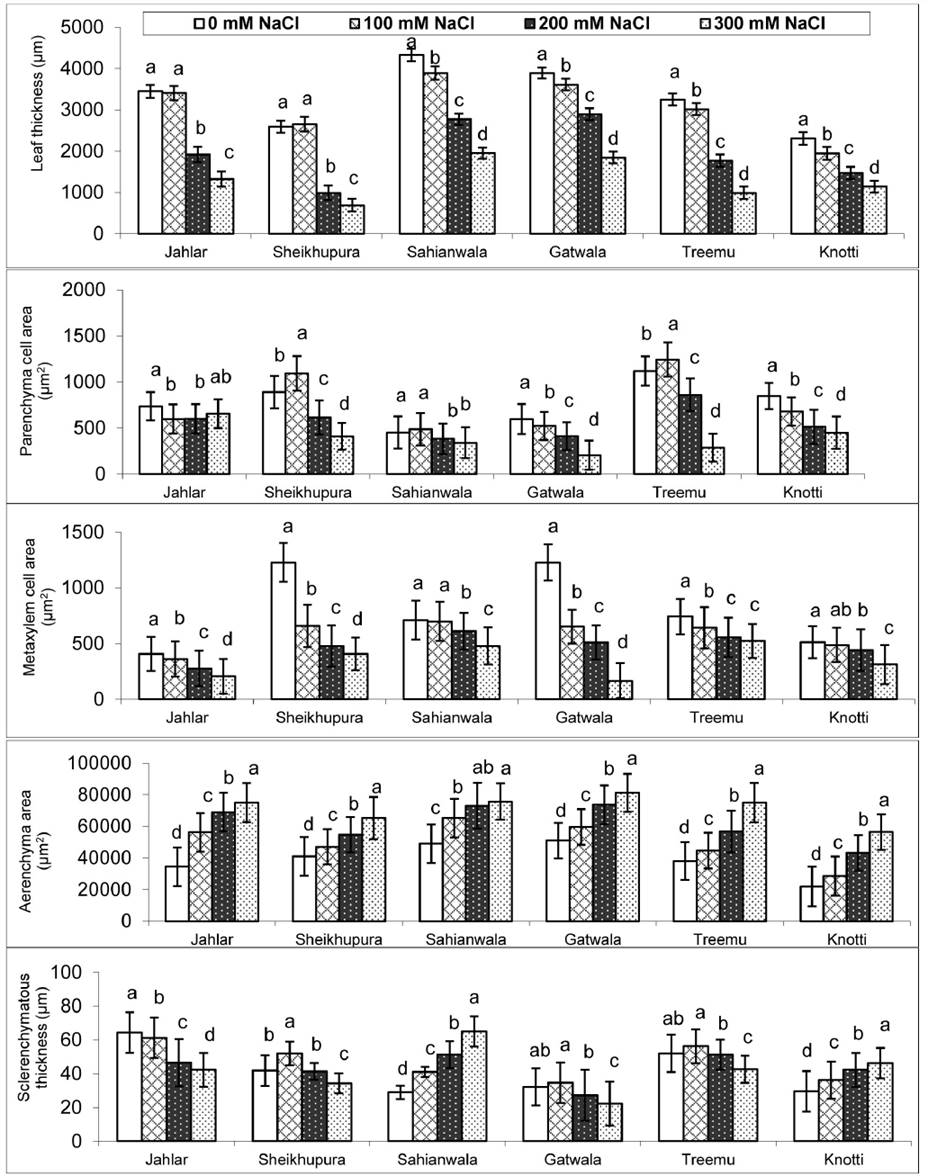
Figure 1 Anatomical leaf characteristics of six ecotypes of Typha domingensis under different levels of salt stress.

Figure 2 Osmotic organic compounds of six ecotypes of Typha domingensis under different levels of salt stress.
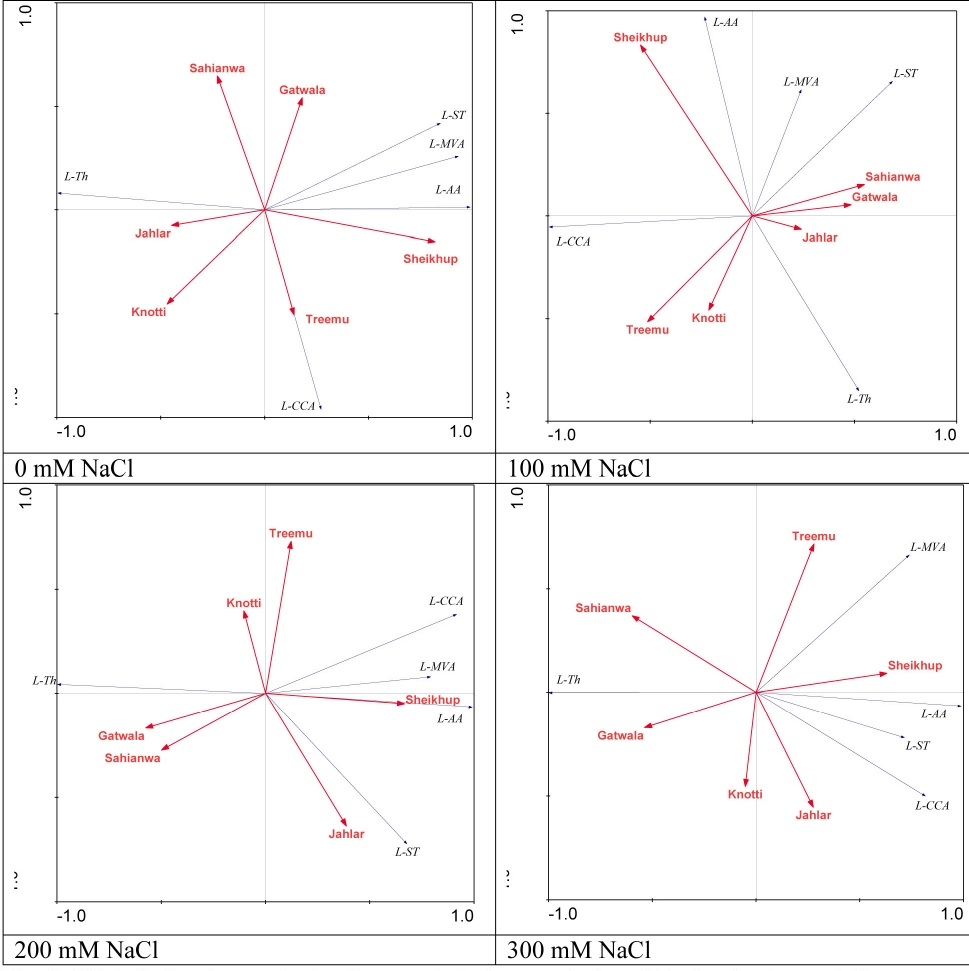
Figure 3 RDA Ordination analysis of anatomical characteristics of Typha domingensis ecotypes under different levels of salt stress. Legends: L-Th = Leaf thickness; L-CCA = Leaf cortical/parenchyma cell area; L-St = Leaf sclerenchyma thickness; L-AA = Leaf arenchyma area; L-MVA = Leaf Metavessel area.
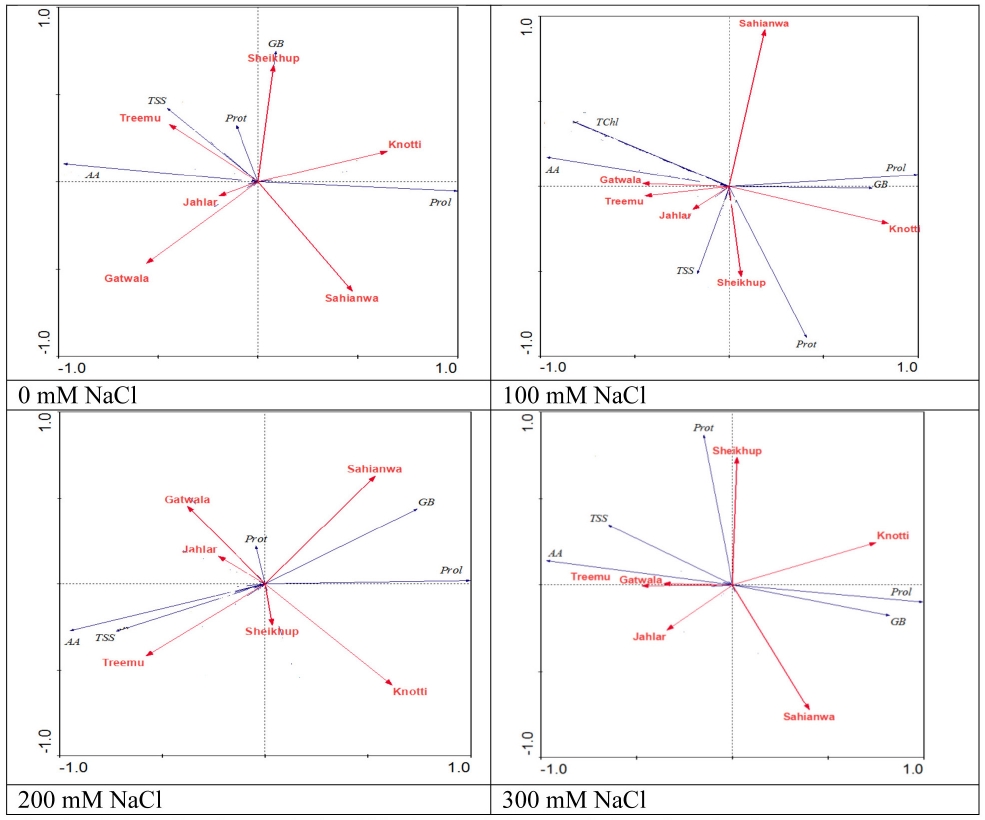
Figure 4 RDA Ordination analysis of biochemical characteristics of Typha domingensis ecotypes under different levels of salt stress. Legends: Prol = Proline; GB = Glycinebetaine; Prot = Total proteins; AA = Total amino acids; TSS = Total soluble sugar.
Statistical analysis. Four salinity levels were maintained during the experiment. The experiment was planned in completely randomized design (CRD) with five replicates. The data was also subjected to statistical analysis using Microsoft Excel software and Minitab statistical software for ANOVA and LSD for comparison of mean values.
Results
Leaf anatomy. Under salt stress, leaf thickness was progressively reduced in Typha ecotypes from Sahianwala, Gatwala, Treemu and Knotti in response to saline medium. Jahlar and Sheikhupura ecotypes remained stable at low level of salinity (100 mM NaCl) but, as the salt levels increase the value related to leaf thickness was significantly decline in these ecotypes. At low level of salt stress, leaf parenchyma cell area in Sheikhupura and Treemu ecotype was increased but in Gatwala, there was significant decline in leaf parenchyma at 300 Mm Nacl. The ecotypes from Jahlar and Sahianwala remained more stable at low level of salt stress. Ecotypes from Sheikhupura and Gatwala revealed a distinct and significant decrease in metaxylem cell area under saline conditions. Up to 100 mM NaCl stress, metaxylem cell area remained constant but at 300 mM NaCl a reasonable decrease in metaxylem cell area was noticed in Sahianwala and Knotti. In Treemu ecotype this parameter decreased up to 200 mM NaCl but thereafter, at higher salt level this value remains stable and did not show any increase. Aerenchyma cell area was consistently increased in all Typha ecotypes in response to external salt levels of medium. The most prominent increased was recorded in Treemu, Knotti and Sheikhupura ecotype. A variable response of sclerenchymatous thickness was recorded in ecotypes of Typha along the salinity gradient. Sahianwala and Knotti ecotype showed consistent increase in sclerenchyma thickness with increase in salt stress at 200 Mm NaCl. A slight increase in sclerenchyma thickness was recorded in Sheikhupura, Gatwala and Treemu was observed at 200 Mm NaCl but thereafter a progressive decline was noticed (Figure 1 and Plate 3 and 4).
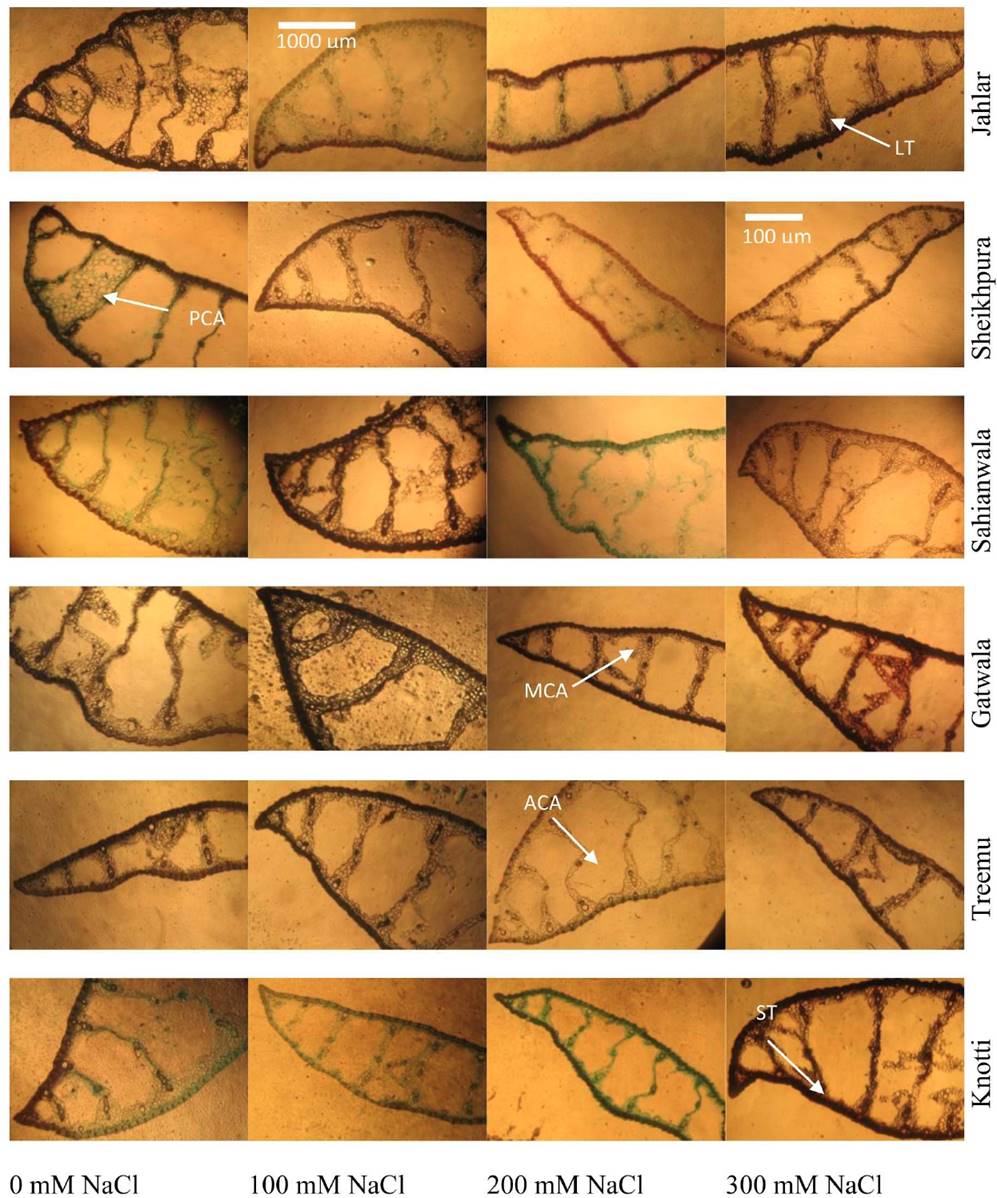
Plate 3 Leaf structural adaptations (tip portion) of six ecotypes of Typha domingensis Pers. under different levels of salt stress. (LT: Leaf thickness, PCA: Parenchyma cell area, MCA: Metaxylem cell area, ACA: Aerenchyma cell area, ST: Sclerenchyma thickness).
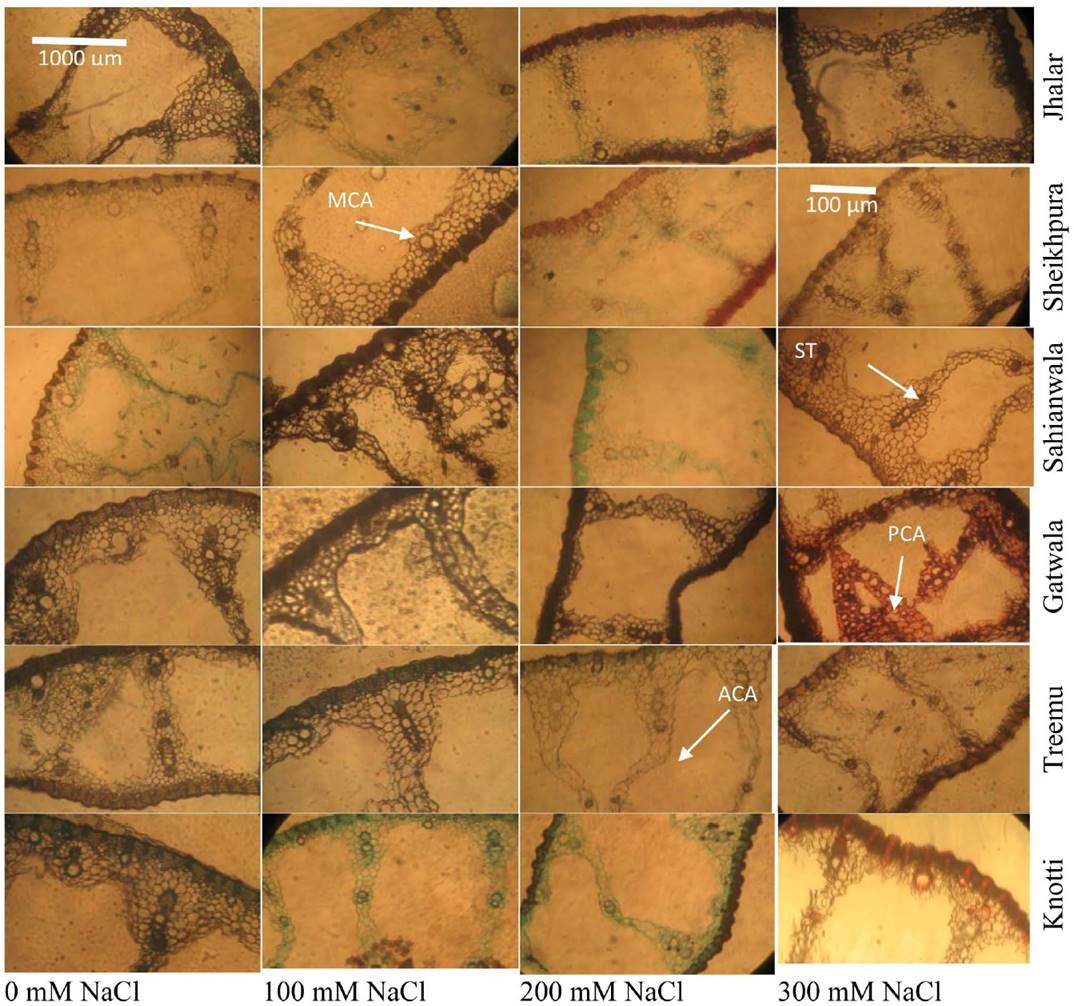
Plate 4 Leaf structural adaptations (mid portion) of six ecotypes of Typha domingensis Pers. under different levels of salt stress. (PCA: Parenchyma cell area, MCA: Metaxylem cell area, ACA: Aerenchyma cell area, ST: Sclerenchyma thickness).
Organic Osmolytes. A prominent enhancement in proline content was observed in all ecotypes of Typha plants in response to saline levels of growth medium. A significant increase in proline content was recorded in Knotti and Gatwala however, a non-significant increase was recorded in the rest of the ecotypes under saline toxicity. The maximum proline accumulation was recorded in Knotti ecotype. The glycinebetaine content was significantly high in all ecotypes of Typha under increasing levels of salt. This accumulation was comparatively higher in Sahianwala followed by Knotti ecotype. Gatwala and Knotti ecotype showed prominent increase in total soluble protein content with increasing level of salinity. The ecotype from Sheikhupura, Treemu and Sahianwala showed remarkable increase in protein content only at higher 300 mM NaCl level of stress but the pattern of variation remains unchanged at low levels 100 and 200 mM NaCl of salinity. All six ecotypes of Typha showed a progressive and significant increase in total soluble sugars under different levels of salt stress. The Knotti ecotype revealed significantly higher accumulation of sugars as compared to Sheikhupura ecotype where accumulation of sugars was decreased under saline environment. Jahlar, Gatwala and Sheikhupura ecotype revealed a consistent and significant increase in total free amino acids under different levels of salt. At low level of salinity 100 mM NaCl, Sahianwala ecotype showed increasing trend as compared to controlled conditions. The ecotype from Treemu and Knotti showed significant increase in total free amino only at higher levels 200 and 300 mM NaCl of salt stress (Figure 2).
Redundancy analysis (RDA). Among leaf anatomical characteristics, only parenchyma cell area was strongly associated with Treemu, and no other characteristics showed any association with leaf anatomical characteristics at 0 mM NaCl level. At 100 mM NaCl salinity level, no association was recorded among habitats and anatomical characteristics. At 200 mM NaCl, Sheikhupura was strongly associated with aerenchyma area and metaxylem area, and weakly associated with parenchyma cell area. Jahlar was strongly associated with sclerenchyma thickness. At the highest salt level, there was again no association among anatomical characteristics and different habitats (Figure 3).
Most of the biochemical characteristics were associated with Treemu at control (0 mM NaCl). However, glycinebetaine was related to Sheikhupura and proline to Knotti. At 100 mM NaCl level, Gatwala showed a strong correlation with total soluble sugars in all treatments and proteins were associated with Sheikhupura, and glycinebetaine and proline with Knotti. Association of these osmolytes was further changed at 200 mM salt level where again Treemu showed strong association with total soluble sugar and total amino acids. Glycinebetaine and proline showed association with Sahianwala. At the highest salt level (300 mM NaCl), Gatwala and Treemu was associated with total free amino acids and Sheikhupura with proteins (Figure 4).
Discussion
Differentially adaptive ecotypes of Typha domingensis responded differently to salt stress in relation to leaf anatomical and biochemical parameters. Each ecotype responded individually to different kind level of salt stress. Jahlar, hyper-saline Salt Lake in district Khushaab, Pakistan, where soil EC is more than 16 dSm-1, and the area is surrounded by mountains predominately of sand stone. Jahlar lake along with some other lakes like Uchaali and Kabeki inhabits some rare and endangered waterfall species like white headed duck. Another wetland in salt range is Knotti with soil EC over 35 dSm-1. The high quantity of salt in both these wetlands is due to brine water springs. Sahianwala (Faisalabad) is another salt affected wetland with EC nearly 19 dSm-1. Soil around Sahianwala is highly saline and canal irrigation system is resulted a huge hyper saline wetland along both sides of the canal. Sheikhupura is an industrial city and the effluents from variety of industries is heavily metal toxic, where Ni metal is up to 40 mg kg-1 in the soil. Gatwala and Treemu are the fresh water habitats. The Gatwala ecotype was collected along irrigation canal whereas, that from Treemu along the river Chenab. Both have fertile non saline soil with low Ni content.
Leaf anatomical characteristics like leaf thickness, cortical cell area and metaxylem cell area significantly increased along with increasing salt level, as was also reported in many plant species like in Cenchrus ciliaris and Cynodon dactylon (Mukhtar et al. 2013), as in wheat (Aldesuquy 2014). However, ecotype from Sahianwala has relatively thick leaves mainly due to storage parenchyma and therefore more space for dumping off toxic ions like Na+ and Cl- as well as to conserve water under limited moisture availability.
Leaf architectural design is an essential tool to study the environmental changes as it is the most responsive plant organ to different environmental stresses (Cavusoglu et al. 2007, Ahmad et al. 2016). Leaf anatomical characteristics such as leaf thickness, parenchyma cell area and metaxylem area was decreased in all ecotypes under increasing stress levels. Leaf thickness is an extremely useful source to examine the stress response. In our study, leaf thickness was more pronounced and showed a sharp decrease under increasing salt stress levels in Sheikhupura, Gatwala and Treemu ecotypes. Whereas, decrease in leaf thickness in Jahlar, Sahianwala and Knotti ecotypes revealed no significant variation under stressed condition. Decline in leaf thickness under stress condition has been observed by many researchers (Delorge et al. 2014). Witkowski & Lamont (1991) observed a reduction of the leaf thickness as well as a decrease of the mesophyll portion in number and size. This indicates a limitation of cell growth since division and cell expansion would be more affected as a result of osmotic and water stress in the mesophyll cells (Carcamo et al. 2012). Reduction of leaf thickness may reduce the ability of plants to take up water under salt stress (Munns 2002, Atabayeva et al. 2013).
A consistent reduction in leaf perenchyma cell area was noticed in all ecotypes under stressed condition except Treemu ecotype in which this parameter was increased. However, parenchyma cell area was less affected in Jahlar, Sahianwala and Knotti ecotypes under increasing levels of salts, this may be due to increased cell vacuolar volume for storing the ions and osmolytes in plant body to tolerate the osmotic stress resulted from harsh environmental conditions (Flowers & Colmer 2008, Ali et al. 2009).
Larger vessels in plants are efficient in nutrient and water conduction (Dolatabadian et al. 2011) and metaxylem vessel seems to be directly related to efficient transport of water and nutrients from the soil, and these might be of greater importance under stressed conditions (Grattan & Grieve 1998). A consistent decrease in metaxylem area was recorded in all ecotypes along with increased stress levels except in Jahlar ecotype in which it is almost stable. However, ecotypes of Sheikhupura and Gatwala had larger metaxylem vessels as compared to others. Larger vascular bundle size under saline stress has been reported (Ali et al. 2009), which support the fact of hydraulic safety as the larger vessels are more prone to embolism/cavitation under water stress condition (physiological drought) created by salinity (Dolatabadian et al. 2011).
Aerenchyma is a characteristic feature of Typha domingensis, as of many other hydrophytes of aquatic saline wetlands (Zhang et al. 2015b). One of the most common effects of increasing stress tolerance was the formation of aerenchyma. All ecotypes showed consistent increase in aerenchyma formation with increase in salt stress level. The maximum increase was observed in Gatwala ecotype that could be an important feature of this ecotype to survive and excrete toxic ions and overcome gaseous exchange problems during physiological drought (Hameed et al. 2010). Aerenchyma is responsible for gaseous exchange under anaerobic conditions for normal metabolic processes in plants inhabiting water logged areas (Nawaz et al. 2013, Al-Maskri et al. 2014, Di Bella et al. 2014).
Sclerification is one of the most widely reported mechanism for the protection of plants under harsh droughty environment. Increased sclerification has also been reported by several researchers in many salt tolerant/ halophytic plant species (e.g., Nawaz et al. 2013, Hameed et al. 2010). Sclerification responded differently in different ecotypes as the stress level of the growth medium increased. High amount of sclerification in Sahianwala and Knotti ecotypes was reported which can be related to their ability of water conservation under salinity and this might be one of reason for their better degree of tolerance to high salinity. Enhanced formation of sclerenchymatous tissues in leaf and other plant organs is the immediate response to physiological drought created in plants due to other environmental stresses (Al-Maskri et al. 2014). The major function of sclerification is to decrease water loss by evapo-transpiration from the plant body and reduce tissue collapse (Hameed et al., 2014). Increased sclerefication has also been reported by several researchers in many salt tolerant/ halophytic plant species (e.gNawaz et al. 2013, Hameed et al. 2010).
One of the prominent feature of Typha domingensis is the accumulation of organic osmolytes like total free amino acids, proline, glycinebetaine, total proteins and total soluble sugars. This phenomenon has already been recorded in number of plant species and populations as the result of salt stress (Jogaiah et al. 2014, Saum et al. 2013). The Sahianwala ecotype depended more on the accumulation of the glycinebetaine whereas, Knotti ecotype on proline accumulation. Glycinebetaine and proline accumulation is a protective mechanism of many plant species to avoid turgor loss under limited moisture conditions and has previously been recorded in relatively more tolerant species such as Vicia faba L. (Ashraf & Foolad 2007, Gill et al. 2014.) The Sahianwala ecotype can be rated as more tolerant as accumulated both proline and glycinebetaine in high amount. In relation to other osmolytes, all ecotypes responded almost similarly as salt level of the medium increased.
Environmental stresses, in particular salinity can cause severe loss in turgor in plants and as compensation accumulation of organic osmolytes is a defense mechanism of many plant species (Zhang et al. 2015a, Gupta & Huang 2012).
It can be safely concluded from this study that differently adaptive ecotypes of Typha domingensis responded differently to salt stress in relation to anatomical and biochemical parameters. Overall performance of ecotypes under salt stress was reasonably good. The main factor involved in the confrontation of these ecotypes under salt affected habitat was high accumulation of osmolytes in particular proline and glycinebetaine and structural modifications like leaf thickness, leaf parenchyma and aerenchyma area and high amount of sclerification. All these features may contribute towards water conservation which is vital for the survival of the species under salt and physiological drought condition.











 nueva página del texto (beta)
nueva página del texto (beta)


