Several species of seeds undergo physical dormancy caused by a testa that prevents water from permeating to the embryo (Baskin 2003) as the result of one or two layers of palisade cells (Baskin & Baskin 2004). Seeds of different species within the Fabaceae family such as Lupinus spp. exhibit this type of dormancy (Ali et al. 2011). Several methods exist for breaking the physical dormancy of hard-testa seeds. These methods are generally classified as physical (mechanical scarification, temperature alternation and shock, among others), chemical (acid scarification and soaking in alkaline solutions) and biological (use of enzymes or fungi) (Baskin & Baskin 2014). These methods accelerate germination because they permit water permeability and gas exchange by wearing down or fracturing the testa (Qihe et al. 2006). Different methods have been tested to break the dormancy of seeds from the Lupinus genus; the effects vary depending on the species and the environment where the seed developed. In Lupinus exaltatus, which comes from areas prone to fires within the state of Jalisco, treatment with a temperature of 150 °C, simulating forest fire, breaks physical dormancy and increases the germination percentages up to 92 % (Zuloaga-Aguilar et al. 2011). High temperatures inhibit the germination of L. polyphyllus, L. lepidus and L. albicaulis seeds, but alternating cold temperatures, and pre-germination treatments that included moist heat increased germination by > 50 % (Elliott et al. 2011). Scarification with sulfuric acid for 15 min has increased the germination of L. montanus seeds, but possible intraspecific variation exists. A germination percentage of 62.5 % was achieved for seeds from La Joya, Mexico (Hernández-Ferretiz et al. 2008), whereas 100 % of seeds from the Cumbres del Ajusco National Park germinated (Acosta-Percástegui & Rodríguez-Trejo 2005).
When dormancy is broken and water and gases can reach the embryo, the metabolism of reserve compounds is reactivated, hydrolyzing or synthesizing new compounds (Bewley et al. 2013). This mobilization of seed reserves during the first phase of germination is an essential process for embryo development and the later formation of seedlings (Satyanarayana et al. 2011). Changes in the reserve compounds have been observed when Cyclocarya paliurus seeds are released from dormancy by scarification. In this species, starch decreased by 45 %, crude protein by 45 % and lipid by 11 %, but soluble sugars increased by 101.5 % (Shen-zou & Jia-yuan 2007). With Oryza sativa seeds, a hydrothermal treatment at 50 °C for 15 min increased not only the percent germination but also the respiration rate and α-amylase activity (Tung & Serrano 2011).
Although it is documented that the process of releasing physical dormancy involves changes in the seed reserves when germination is promoted and the application of scarification treatments can modify these changes, there are few studies that document the changes in Lupinus seeds. For this reason, the objectives of this study were as follows: a) to evaluate the effect of different scarification treatments on the rate and percent germination of Lupinus spp. seeds and b) to determine changes in the contents of protein, soluble sugars, reducing sugars, amino acids, polyphenols and abscisic acid during germination after applying pre-germination treatments.
Materials and methods
Biological material. Mature seeds of Lupinus campestris Schltdl. & Cham. (Lc) and Lupinus exaltatus Zucc (Le) were collected on September 20, 2014, at 2,866 and 3,066 m asl, respectively, in the region of the Serdán and Libres Valleys, Puebla, Mexico. Seeds of Lupinus montanus Kunth (Lm) were collected on November 8, 2[R1]014, at 3,442 m asl in the same region. Healthy seeds were selected and conserved at 4 °C for approximately two months before analysis. A topographic test using tetrazolium chloride was performed on the seeds prior to analysis. The percent viability was 93.3 % for L. exaltatus, 90.6 % for L. campestris and 96.6 % for L. montanus.
Pre-germination treatments. Before applying the pre-germination treatments, triplicate lots of 50 seeds of each species were disinfected with a 3 % sodium hypochlorite for 2 min (except treatment PG1) and washed with sterile distilled water. The pre-germination treatments were as follows: PG1, immersion in 98 % H2SO4 for 15 min; PG2, immersion in sterile wet sand at 80 °C for 5 min; PG3, alternating temperatures of 35 °C for 8 h followed by 16 h at 25 °C in sterile wet sand; PG4, dry heat at 80 °C for 7 min in sterile sand; PG5, dry heat at 150 °C for 1 min in sterile sand; and PG6, immersion in H2O at 80 °C for 1 min. A control was included with untreated seeds.
After each pretreatment, the seeds were placed in plastic Petri dishes between layers of sterile absorbent paper in a growing chamber (Thermo Scientific, model 818, USA) at 20 °C with white LED light for 12 h followed by a 12 h dark period at 15 °C. For each species, 50 seeds were placed in the Petri dishes in triplicate. The percent germination (GP) and germination rate (GR) were assessed at 3, 5, 10 and 15 days after seeding (das). For GP, a seed was considered germinated when the radicle was ≥ 2 mm long. GR was determined using the formula GR = ∑ (NGSi)/t, where NGSi is the number of germinated seeds on day i and t is the time from sowing to germination of the last seed. During germination, the seeds were watered daily with room temperature sterile distilled water. Following each germination period, the seeds were dried in a forced air oven at 50 °C for 48 h (Tonguc et al. 2012). They were then ground in a mortar and de-fatted for 2 h with 95 % hexane in a 1:10 flour/hexane mixture (w/v) with constant shaking at 280 rpm at 4 °C. The mixture was centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was decanted and the remaining fat-free precipitate was left to dry at room temperature until the solvent had completely evaporated. The dried precipitate was stored until use at 4 °C.
Biochemical analyses. Biochemical analyses were conducted 0, 3, 5 10 and 15 das in the two pre-germination treatments that significantly increased germination (PG1 and PG6) and the control, using all of the seeds per treatment and replication. All of the analyses were performed in triplicate and the results were expressed in mg g-1.
Soluble protein. The total protein content was estimated using the method of Lowry et al. (1951). To 100 mg of previously de-fatted flour, 2.5 mL of a solution of 0.3 mol L-1 tris-HCL, pH 8.0, and 0.01 mol L-1 β-mercaptoethanol was added. The mixture was vortexed and left to incubate for 1 h with shaking every 10 min. It was then centrifuged at room temperature for 20 min at 1,100 × g, and the supernatant was collected to determine the protein content (Sussulini et al. 2007). Of the collected and diluted (1:4) supernatant, 0.02 mL was taken and 0.7 mL Lowry solution was added; the mixture was left to incubate in darkness for 20 min. After incubation, 0.1 mL 2N Folin Ciocalteu reagent was added and the mixture was left to incubate for 30 min in darkness. The protein concentration was determined based on a 1 mg mL-1 standard solution of bovine serum albumin (BSA). The absorbance was measured at 750 nm.
Total soluble sugars, reducing sugars and amino acids. For the extraction, 200 mg of previously de-fatted dry sample of each species and treatment was weighed in triplicate. Three milliliters of absolute ethanol was added to each sample, which was then placed in a water bath at 70 °C for 5 min. After incubating in the water bath, the supernatant (extract) was recovered in a 50 mL Erlenmeyer flask. This process was repeated four times. The extract was dried in a forced-air oven at 70 °C until completely dry. It was then re-suspended in 1 mL distilled water and stored at -20 °C until analysis.
Total soluble sugars. The total soluble sugars were determined with the Somogy (1952) method using a 100 µL sample with 300 µL distilled water and 3 mL Anthrone. This mixture was vortexed and left to cool on ice for 5 min. It was then placed in a water bath at 100 °C for 10 min. The tubes were then left to cool at room temperature. The total soluble sugars concentration was determined based on a 2.5 mg mL-1 standard solution of glucose. The absorbance was read at 625 nm.
Reducing sugars. To 0.5 mL of the sample, 1.5 mL distilled water and 1 mL copper tartrate alkaline solution was added. The samples were placed in a water bath at 100 °C for 10 min and then left to cool at room temperature. One mL arsenomolybdate reagent was added, and then 6 mL distilled water was added. For the calibration curve, concentrations from 0 to 0.25 mg mL-1 were prepared from a standard solution of 2.5 mg mL-1 glucose. The absorbance was read at 620 nm.
Total amino acids. The amino acid content was determined using the method of Yemm & Cocking (1995). We used 0.1 mL of the sample, added distilled water to a 1 mL volume and added 1 mL of the reagent ninhydrin. The resulting solution was vortexed and then placed in a water bath at 100 °C for 15 min, after which the solution [R2]was placed in cold water and 5 mL 80 % ethanol was added and left to incubate at room temperature for 10 min. For the calibration curve, concentrations of 0 to 100 µg mL-1 were prepared from a standard solution of 100 µg mL-1 leucine. The absorbance was read at 570 nm.
Total polyphenols. Of the dry de-fatted sample, 250 mg was weighed in triplicate. The polyphenols were extracted by shaking (vortexed for 1 min) with 1 mL 80 % methanol. The samples were then placed in a water bath at 50 °C for 15 min and centrifuged at 10,956 × g for 15 min. The supernatant was recovered and the residue was again washed and mixed with 0.5 mL 100 % methanol. Both supernatants were recovered and the volume was increased to 1.5 mL with distilled water and stored at -20 °C protected from light. For quantification, 1.5 mL distilled water, 0.1 mL 50 % Folin-Denis reagent and 0.2 mL 15 % anhydrous sodium carbonate were added to 0.2 mL of the extract. These were vortexed and left to incubate for 30 min in darkness. For the calibration curve, concentrations of 0 to 100 µg mL-1 were prepared from a stock solution of 100 µg mL-1 gallic acid, which was used to make standard solutions. The absorbance was read at 765 nm.
Abscisic acid (ABA). The concentration of abscisic acid was determined following the methods of Khadri et al. (2006) and Kishwar et al. (2011). The analysis was conducted with untreated seeds, with treated seeds immediately after the application of 80 °C water for 1 min, and with treated seeds of more than 30 das. From these samples, 0.5 g of fresh material was macerated with 5 mL of an extraction solvent (80 % methanol and 10 mg L-1 butylatehydroxitoluene) and incubated at 4 °C for 1 h. After incubation, the mixture was centrifuged at 10,956 × g for 15 min. The supernatant was collected and two additional extractions were made of the precipitate with 1 mL extraction solvent. The three supernatants were aggregated and 30 mg polyvinyl-polypyrrolidone (PVPP) was added to eliminate plant pigments and other non-polar compounds that might interfere with the immunoassay.
The supernatant obtained was concentrated to dryness [R3]and re-suspended in 0.5 mL 25 mM Tris-HCl buffer, pH 7.5, which contained 100 mM NaCl and 1 mM magnesium chloride. ABA was quantified by immunoassay (ELISA) using the PGR-1 detection kit specific for this hormone (Sigma PGR1-1KT). Aliquots of 0.1 mL sample and 0.1 mL tracer solution were added to each well and incubated at 4 °C for 3 h. During incubation, the ABA of the sample competes with the tracer molecule bound to the enzyme alkaline phosphatase because it binds to the ABA-specific monoclonal antibodies fixed on the ELISA plate. After incubation, the plates were washed twice with 0.1 mL PBS Tween washing solution, 0.2 mL substrate solution (paranitrophenyl phosphate) was added, and the plates were incubated at 37 °C for 60 min to produce the enzyme reaction. The absorbance was read at 405 nm with an ELISA plate reader. Quantification of ABA was based on an ABA standard curve (0-1,000 pmol mL-1).
Statistical analysis. GP data were arcsine square root transformed (Elliott et al. 2011) to obtain an approximate normal distribution of the percentages. These data, together with GR and biochemical analyses, were subjected, by species, to an analysis of variance in a completely randomized design in a factorial array. The first factor consisted of the eight pre-germination treatments and the second factor consisted of the four time intervals (0, 3, 5, 10, 15 das) and their interactions (Treatment*Time). A Tukey test was used to distinguish differences among treatments, and Pearson correlations were performed using SAS statistical software (SAS, 2010).
Results
Percent germination and germination rate (GP and GR). The GP of the three Lupinus species was significantly affected (P < 0.05) by the application of the pre-germination treatments (Table 1). PG2 and PG6 surpassed the control (7 % GP) for L. exaltatus. The treatment with H2O at 80 °C for 1 min (PG6) was the most effective treatment (41 % GP) (P < 0.05), followed by the treatment with 98 % H2SO4 for 15 min (34 % GP), surpassing the control by 33 % and 27 %, respectively. For treatment PG6, the greatest increase in germination was observed on days 10 and 15, whereas with PG1, germination began rapidly in the first days after seeding. Treatment PG5 (150 °C for 1 min) inhibited germination in this species. A similar effect was observed on the GP of L. campestris seeds, for which the PG1, PG2, PG5 and PG6 treatments surpassed the control. For this species, PG6 was the best treatment, increasing the GP to 69 %. The greatest increase in germination for this treatment occurred at 10 das. For L. montanus, the PG1 and PG6 treatments surpassed the control, but there was no significant difference between them (P < 0.05). The effect of the pre-germination treatments on the average GR can be seen in Figure 1. In the three species, the treatment with concentrated sulfuric acid (PG1) showed the greatest increase in the average GR relative to the control, followed by PG6.
Table 1 Effect of pre-germination treatments on percent germination of L. exaltatus, L. campestris and L. montanus seeds at different times during the germination process (3, 5, 10 and 15 days).
| Species, das | Germination (%) | ||||||
|---|---|---|---|---|---|---|---|
| Control | PG1 | PG2 | PG3 | PG4 | PG5 | PG6 | |
| L. exaltatus | |||||||
| 3 | 4.82d | 23.4bc | 2.82d | 2.94d | 0d | 0d | 5.33d |
| 5 | 7.36d | 28.4b | 4.56d | 3.34d | 0d | 0d | 5.33d |
| 10 | 7.76d | 31.4ab | 7.36d | 3.34d | 0.4d | 0d | 22.67bc |
| 15 | 7.76d | 34.4ab | 12.3cd | 4.28d | 5.79d | 0d | 41.33a |
| L. campestris | |||||||
| 3 | 3.2f | 25.51cd | 2.58f | 3.91f | 3.85f | 1.6f | 4f |
| 5 | 4.22f | 27.29cd | 3.38f | 4.31f | 6.36f | 2f | 4f |
| 10 | 4.62f | 31.27cd | 8.14f | 5.43f | 8.21f | 3.2f | 62.67a |
| 15 | 4.62f | 34.05c | 22.39cde | 6.5f | 11.26ef | 21.5de | 69.33a |
| L. montanus | |||||||
| 3 | 6c | 25.06ab | 2.33c | 1.5c | 4.59c | 1c | 5.33c |
| 5 | 7.33c | 27.56a | 2.33c | 1.5c | 4.59c | 1.5c | 5.33c |
| 10 | 7.33c | 32.98a | 3.67c | 1.5c | 5.59c | 3c | 30.67a |
| 15 | 7.33c | 37.33a | 12.83bc | 2c | 9.67c | 10.5c | 36a |
Control: untreated seeds; PG1: H2SO4 for 15 min; PG2: H20 at 80 °C for 5 min; PG3: wet sand at 35 °C for 8 h followed by 16 h at 25 °C; PG4: dry sand at 80 °C for 7 min; PG5: sand at 150 °C for 1 min; PG6: water at 80 °C for 1 min. das = days after seeding. Different letters within species indicate significant differences (P<0.05).
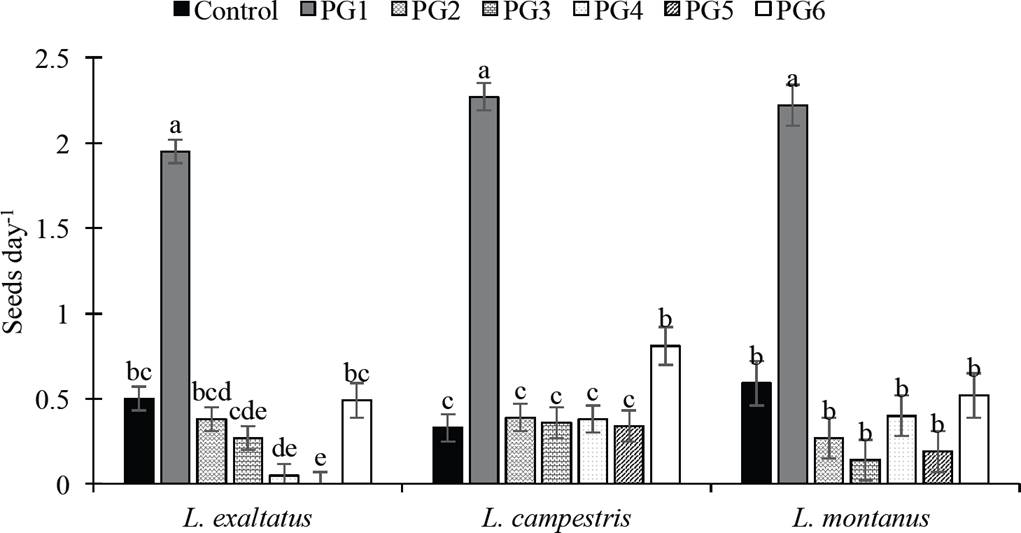
Figure 1 Effect of pre-germination treatments on germination rate (GR) of Lupinus seeds. Control: untreated seeds; PG1: H2SO4 for 15 min; PG2: H20 at 80 °C for 5 min; PG3: wet sand at 35 °C for 8 h followed by 16 h at 25 °C; PG4: dry sand at 80 °C for 7 min; PG5: sand at 150 °C for 1 min; PG6: water at 80 °C for 1 min. Different letters within treatments indicate significant differences (P < 0.05).
Soluble protein. During germination, both increases and decreases in the concentration of protein were observed among species. The treatment with water at 80 °C for 1 min (PG6) increased the protein concentration by 13 % relative to the control in L. exaltatus (Figure 2a), and the sulfuric acid treatment (PG1) decreased protein on days 3 and 5 by 239 and 47 %, respectively, relative to the control, but the protein concentration increased gradually thereafter. In L. campestris (Figure 2c), the treatment with 98 % H2SO4 for 15 min (PG1) had the opposite effect on protein mobilization, beginning with an increase on day 3 (21 and 24 %) relative to the control and decreasing on day 15 (-26 and -20 %). In L. montanus seeds (Figure 2e), the protein concentration increased significantly with both PG1 and PG6, similar to L. exaltatus. However, the Pearson’s coefficients did not show a significant correlation between soluble protein concentration and PG or GR in the species under study, with the exception of GR in L. exaltatus (Table 2).
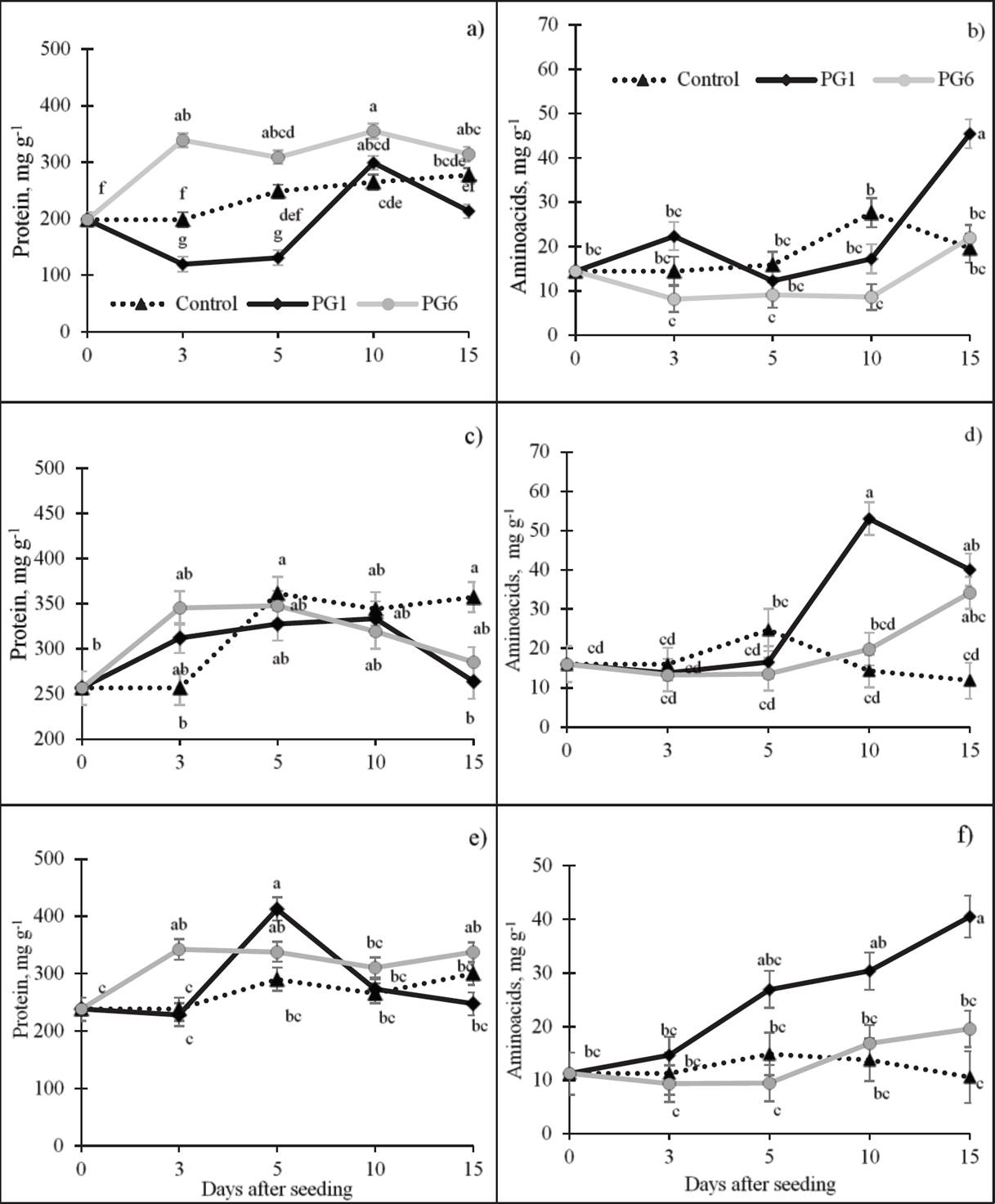
Figure 2 Effect of pre-germination treatments on the concentrations of soluble proteins and amino acids in seeds of L. exaltatus (a, b), L. campestris (c, d) and L. montanus (e, f) during germination. Control: untreated seeds; PG1: H2SO4 for 15 min; PG6: wet sand at 80 °C for 1 min. Different letters within treatments indicate significant differences (P < 0.05).
Table 2 Pearson’s correlations between percent germination (GP) and germination rate (GR, seeds day-1), and biochemical traits in L. exaltatus, L. campestris and L. montanus seeds, by effect of germination pretreatments.
| Protein | Amino acids | Total soluble sugars |
Reducing sugars |
Total polyphenols |
|
|---|---|---|---|---|---|
| L. exaltatus, mg g-1 | |||||
| GP | -0.14 | 0.38* | -0.45*** | -0.002 | 0.10 |
| GR | -0.44*** | 0.08 | -0.29*** | -0.06 | -0.05 |
| L. campestris, mg g-1 | |||||
| GP | -0.25 | 0.60*** | -0.40** | -0.53*** | 0.29* |
| GR | -0.06 | 0.06 | -0.42** | -0.63*** | -0.05 |
| L. montanus, mg g-1 | |||||
| GP | 0.15 | 0.72*** | -0.31* | -0.02 | 0.72*** |
| GR | 0.01 | 0.34* | -0.31* | -0.40* | 0.43 |
*, **, ***Significant differences at P < 0.05, P < 0.01, P < 0.001, respectively.
Amino acids (AA). A highly significant, positive relation was observed between the AA concentration and the GP for the three species under study (Table 2). During germination, the concentration of AA increased in L. exaltatus (Figure 2b) under PG1 (22.3 mg g-1) relative to the control and then decreased between 5 and 10 das. On day 15, the AA concentration increased and surpassed the control by 130 %. The AA concentration in treatment PG6 was the lowest of all the treatments throughout the period of germination. In L. campestris seeds (Figure 2d), the AA concentration decreased slowly from 3 to 5 das (43 %) relative to day 0 in PG1 and PG6. The highest level of synthesis was observed at 10 and 15 das, surpassing the control by 239 and 189 %, respectively. In PG1 with L. montanus seeds (Figure 2f), the average amino acid concentration increased with germination time, surpassing the control by 283 % at 15 das.
Total soluble sugars (TSS). A highly significant, negative relation was observed between TSS concentrations and both GP and GR in the species under study (Table 2). The highest average concentration of TSS was observed in the control (381.47 mg g-1). In contrast, the two pre-germination treatments exhibited a decrease in the TSS concentration relative to the control. On average, the greatest decrease in these sugars occurred at 3 das (47 %, relative to day 0), possibly related to initial germination. The TSS concentration in L. campestris seeds (Figure 3c) exhibited the same pattern as the L. exaltatus seeds, decreasing in PG1 and PG6 throughout germination. The highest TSS concentration occurred in the control (397.28 mg g-1). Similarly, the TSS decreased in pre-treated L. montanus seeds (Figure 3e) relative to the L. montanus control. The highest concentration of TSS was in the control treatment (398.02 mg g-1).
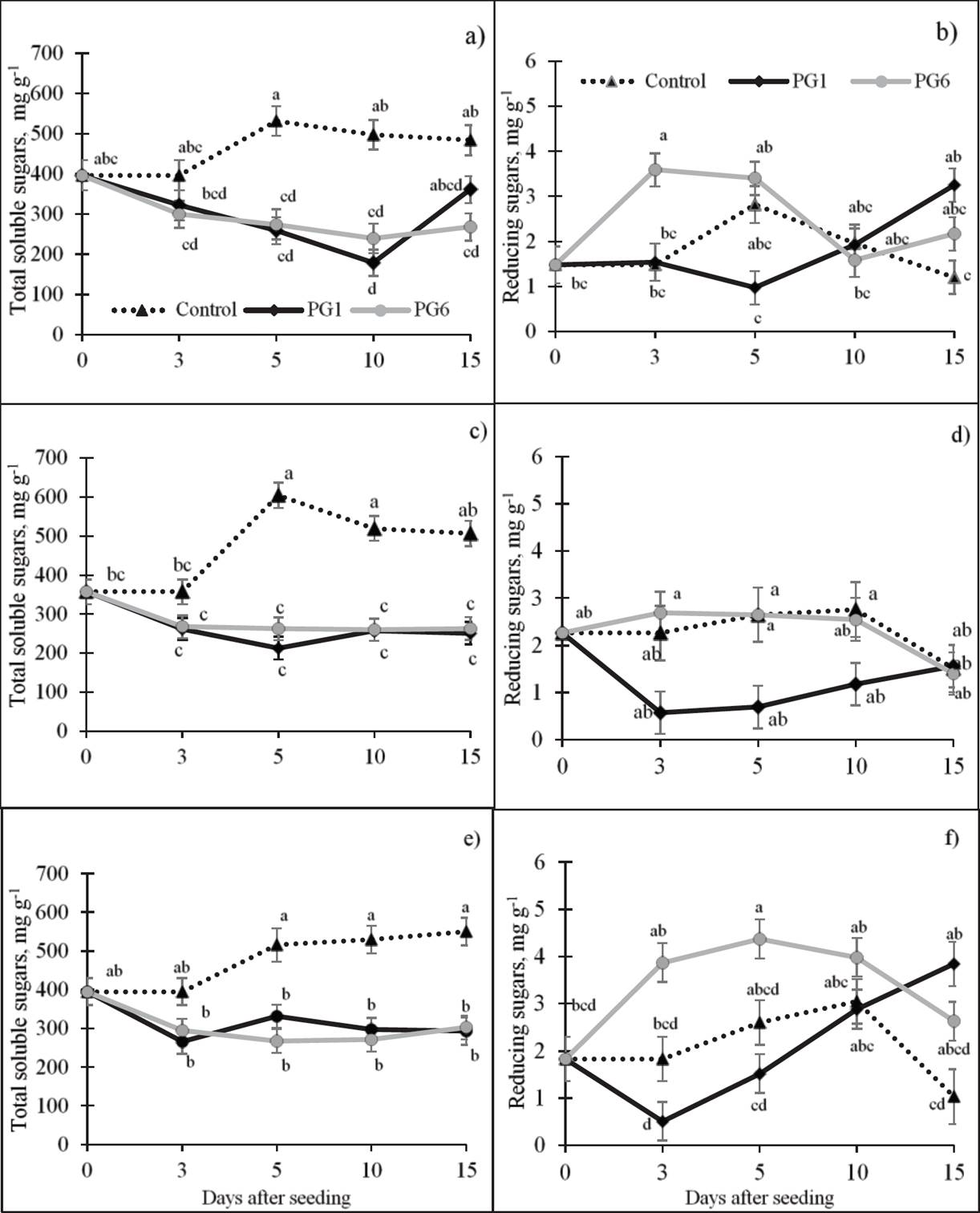
Figure 3 Effect of pre-germination treatments on the concentrations of soluble and reducing sugars (dry basis) of L. exaltatus (a, b), L. campestris (c, d) and L. montanus (e, f) seeds during germination Control: untreated seeds; PG1: H2SO4 for 15 min; PG6: wet sand at 80 °C for 1 min. Different letters within treatments indicate significant differences (P < 0.05).
Reducing sugars (RS). The Pearson’s coefficients did not show a significant correlation between the RS concentration and PG or GR, with the exception of L. campestris (Table 2). In the three species, RS increased with PG6 and decreased with PG1 relative to the control (Figure 3). In the case of L. campestris (Figure 3d), the greatest decrease in the average RS concentration, relative to the control, was in PG1 (27 %). In contrast, under PG6, the RS concentration increased with germination time as did the control.
Total polyphenols (TPP). The concentration of phenolic compounds (Figure 4) was significantly affected by PG1 and PG6 in L. montanus, where a highly significant, positive correlation with GP was observed (Table 2). In this species (Figure 4c), a TPP increase of 114 % at 10 das was observed relative to day 0.
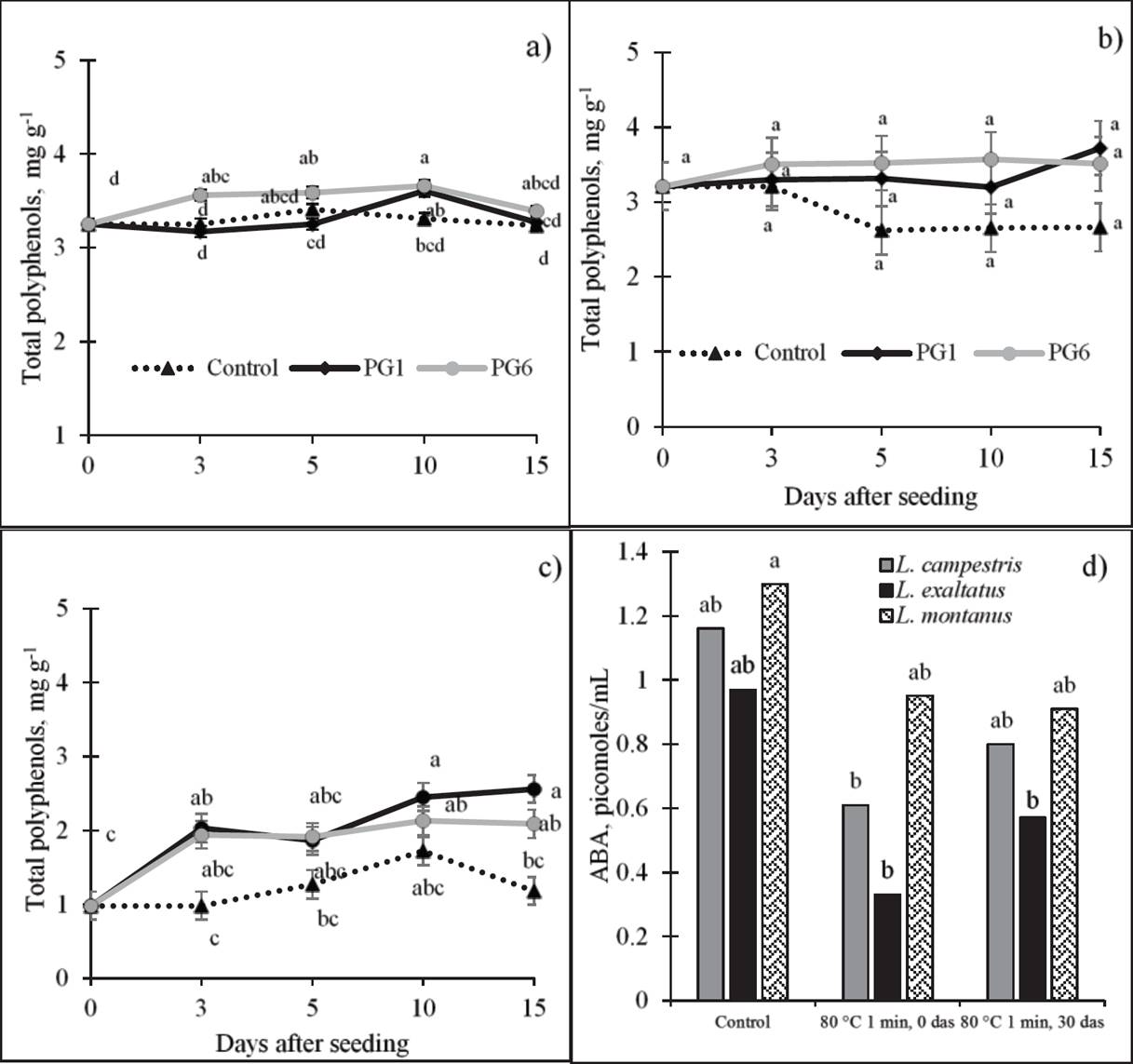
Figure 4 Effect of pre-germination treatments on total polyphenol concentration (dry basis) of L. exaltatus (a), L. campestris (b) and L. montanus (c) seeds, and on abscisic acid (ABA) concentration (d) during germination. Control: untreated seeds; PG1: H2SO4 for 15 min; PG6: wet sand at 80 °C for 1 min. das = days after seeding. Different letters within treatments indicate significant differences (P < 0.05).
Abscisic acid (ABA). To observe the behavior of ABA before and after the application of a treatment that increased germination, we evaluated the pre-germination treatment with water at 80 °C for 1 min (PG6). The concentration of ABA decreased with the application of PG6 in the three species, particularly in L. campestris and L. exaltatus (Figure 4d). L. montanus showed the highest ABA concentration (1.30 pmoles mL-1) in its seeds, followed by L. exaltatus (0.97 pmoles mL-1) and L. campestris (0.77 pmoles mL-1). After the application of the treatment, at 30 das, the ABA concentration increased again in the three species, but it did not reach the levels found in untreated seeds (1.3 pmoles mL-1).
Discussion
Percent germination and germination rate. The increase in the GP of the three species after the application of the pre-germination treatments, relative to the control, confirms the presence of physical dormancy in these seeds. In general, the three species responded favorably to the treatment with H2O at 80 °C for 1 min (PG6), which surpassed the treatment with sulfuric acid (PG1) in L. campestris and L. exaltatus. The GP response to PG6 differed among the species. On day 15, the GP was 69 % for L. campestris, 41 % for L. exaltatus and 36 % for L. montanus. The efficiency of this treatment has also been observed in Lupinus lepidus seeds (Elliott et al. 2011) and in the seeds of other legumes such as L. leucaena cv. Cunningham, in which a GP of 55 % was achieved for seeds exposed to water at 80 °C for 2 min (Sánchez et al. 2005). According to Tiryaki & Topu (2014), thermal treatments such as abrupt changes in temperature cause the seed testa to crack because the cells expand in the hot water and then contract when exposed to ambient temperature.
The treatment with sulfuric acid was also able to break the seed dormancy of the species studied, although the germination percentages were lower than those reported for other Lupinus species, and in our study were GP values were even different for the same species from different regions of Mexico. With 12 h of exposure, 80 % germination of L. varius seeds was obtained by Karaguzel et al. (2004) and with 90 min of exposure, a GP of 50 % germination of L. campestris was achieved by Gutiérrez-Nava et al. (2010), whereas in L. montanus exposed for 15 min, a GP of 75 % or 100 % was obtained, depending on the origin of the seed (Acosta-Percástegui & Rodríguez-Trejo 2005, Hernández-Ferretiz et al. 2008). In general, the application of H2SO4 increased GR in the three species studied as has been observed in other species. Although it has been mentioned that the application of strong acids increases germination by weakening the testa walls and permitting radicle protrusion, the acids can damage the embryo and produce abnormal seedlings if the concentration and exposure time are not appropriate (Kimura & Islam, 2012).
The treatment with dry sand at 150 °C (PG5) inhibited the germination of L. exaltatus seeds, but did not inhibit L. campestris or L. montanus seeds. This suggests that after a fire event, L. exaltatus from region of the Serdán and Libres Valleys in Puebla would not germinate with subsequent moist conditions. Negative germination results were also reported by Robles-Díaz et al. (2014) for L. elegans and L. rotundiflorus seeds from the state of Jalisco, Mexico, when dry heat treatments were applied (120 and 150 °C). Variation in the species’ response to these pretreatments among seeds from different regions was also manifest in the results of Zuloaga-Aguilar et al. (2011), who applied treatment PG5 on L. exaltatus collected in the state of Jalisco from an area prone to forest fires and achieved 93 % germination. The negative effect observed in this study may have been due to rapid imbibition through the cracks produced by the treatment, causing the solid-state cell walls to pass through a liquid crystalline phase and become temporarily permeable (Schelin et al. 2003), affecting germination.
The variable responses observed among Lupinus seeds under study and those of the same species collected in different regions of Mexico, to the application of the same pre-germination treatment, could be explained by differences in the moisture content of the seeds at the moment of seed maturation. Baskin & Baskin (2014) mentioned that when the moisture content of physically dormant seeds is >14 %, they become permeable, but when the moisture content is < 10 %, physical dormancy persists even when the seeds are placed in a moist environment. Another explanation could be differences in the age of the seed, which could lead to harder seeds due to moisture loss during maturation or storage (Baskin & Baskin, 2014).
Mobilization of reserves. According to the literature, it is in phase I of the germination process that the greatest mobilization of reserves occurs; among these reserves are proteins and sugars (Nonogaki et al. 2010, Bewley et al. 2013). During this phase, proteins are catabolized, which provides amino acids for the synthesis of de novo proteins to support seedling growth (Bewley et al. 2013). After the application of the treatments that increased the GP, both increases and decreases in protein concentration were observed during germination relative to the control in the species under study, but these changes were not related to the GP. In other species, however, increases in protein were observed after the application of a H2SO4 treatment (Benítez-Rodríguez et al. 2014). However, the application of treatments that included high temperatures (50 °C) to break dormancy in Cucumis sativus L. seeds did not significantly affect the soluble protein concentration (Amritphale et al. 2000).
The increase in the AA concentration in response to pre-germination treatments appears to be associated with GP. For the treatments in which the GP was high, there was an increase in the concentration of AA. Several authors have indicated that the increase in AA concentration during germination is due to protein degradation, which results in the release of AA. The reduction in the AA concentration, typically at 3 and 5 das in these three species, has also been observed in Arabidopsis thaliana seeds after the application of a scarification treatment at 4 °C and in Lens culinaris and Pisum sativum seeds (Joosen et al. 2013, Rosental et al. 2014). In other species, an increase in the AA concentration during germination has also been observed (Kuo et al. 2004).
It was evident that the application of the pre-germination treatments reduced the soluble sugar content in the three species and that this decrease was associated with a high GP and GR. This decline in soluble sugars has been observed during the germination of Erythrina velutina Willd. (Ribeiro et al. 2012) and Lupinus luteus L. at 20 °C (Zalewski et al. 2010) and during the release from dormancy of Malus domestica embryos treated with HCN (Bogatek et al. 1999). This reduction in soluble sugars in response to pre-germination treatments that promote germination, particularly PG1 and PG6, may be related to the initial activation of seed metabolism, which provides the energy for germination prior to the mobilization of reserves. However, the use of sugars varies depending on the species; they may be used during germination phase I and II or during radicle growth in phase III (Pritchard et al. 2002). The greatest decrease in RS in L. campestris, which occurred with treatment PG1, was related to the GP and GR, probably due to the high demand for energy by embryo growth. Moreover, the RS concentration under PG6 increased during the first 10 das, because the SS decreased in L. exaltatus and L. montanus, reflecting the mobilization and hydrolysis of the polysaccharides present in the seeds. These polysaccharides can be strongly hydrolyzed by hydrolases, which may be responsible for the incremental change in reducing sugars during germination (Rosental et al. 2014). It has also been observed that the application of pre-germination treatments, including hot water, to break dormancy in Oriza sativa seeds increase α-amylase activity (Tung & Serrano 2011). This enzyme hydrolyzes polysaccharides during germination to form simpler sugars, such that enzyme activities may be related to the increase in RS observed in the treatment with water at 80 °C for 1 min (PG6).
Total polyphenols. During radicle protrusion, several biochemical processes are activated. These processes can change the composition of primary and secondary metabolites, which in turn can affect the activity of phenolic compounds (Xu et al. 2009). This increase in the average concentration of TPP with the 98 % H2SO4 for 15 min (PG1) and H2O at 80 °C for 1 min (PG6) treatments appears to be related to the GP in L. montanus seeds. Different researchers have described an incremental change in TPP concentration during the seed germination process of Vigna radiata, Avena nuda, Cicer arietinum, L. campestris, and Chenopodium quinoa (Troszynska et al. 2006, Xu et al. 2009, Tarzi et al. 2012, Jiménez-Martínez et al. 2012, Carciochi et al. 2014). Cevallos-Casals & Cisneros-Zevallos (2010) also found an increase in the phenolic compound content in seeds of 13 species including mungbean, alfalfa, fava, fenugreek, mustard, wheat, broccoli, sunflower, soybean, radish, kale, lentil and onion, during the passage from dormancy to non-dormancy. According to López-Amorós et al. (2006), the increase in TPP is related to the activation of hydrolase and polyphenoloxidase enzymes, which increase their activity during germination.
Abscisic acid. The decrease in ABA observed after the application of the heat treatment (PG6) is related to the GP and the hormone balance ratio between ABA and gibberellins (Bewley et al. 2013). ABA acts as a regulator of germination, inhibiting it at high concentrations (Toh et al. 2008). This decrease in ABA has been observed in Arabidopsis seeds in response to a heat treatment at 34 °C (Toh et al. 2008). A similar decrease in ABA was observed in Hordeum distichon cv Triumph seeds after applying a treatment with H2SO4 to break dormancy (Wang et al. 1998).
In general, the results showed that the seeds of the three species under study responded to treatment with moist heat at 80 °C for 1 min by increasing germination, the concentration of amino acids and reducing sugars, and decreasing soluble sugars.











 text new page (beta)
text new page (beta)


