Transition metals Cu, Fe, Mo and Zn are naturally present in ground and are essential micronutrients for all plant metabolism, fulfilling critical structural and catalytic roles throughout the cell in various subcellular compartments. They are involved in a wide variety of critical processes such as transcription, translation, ATP production in the mitochondria and scavenging of toxic free radicals (Tamayo et al. 2014). However, high level of these metals could be toxic, leading to disruption of vital physiological or biochemical functions and ultimately to cell death (Gangwar et al. 2014, Shahid et al. 2014).
One of the most commonly metal present at low concentrations in soil is copper (Cu). Owing to copper mining, waste deposition, smelting and agricultural practices, e.g. use of pesticides, it tends to accumulate in high and toxic concentrations in the ecosystems (Singh et al. 2010). Nevertheless, copper is an essential component of several proteins and enzymes, and it is required in trace amounts for various metabolic activities of the plants (Elleuch et al. 2013). It has been demonstrated that an increase in copper concentration (from 0.1 µM to 10µM) adversely affects plant metabolism by inhibiting nutrient uptake, enzyme activities, disturbing photosynthesis, respiration, cellular transport and many other metabolic processes (Peng et al. 2012). Indeed, it is able to generate directly oxidative stress via potent redox activity through Fenton and Haber-Weiss reaction (Liu et al. 2014). The adaptive response to oxidative stress consists in a complex antioxidant system including several ROS-scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), guaicol peroxidase (GPX), ascorbate peroxidase (APX), gluthatione reductase (GR), as well as non enzymatic compounds such as ascorbate, gluthatione, carotenoids, tocopherols and metallothioneins (Thounaojam et al. 2012). It has already been established that some plant species including rice (Thounaojam et al. 2012), Populus deltoides W. Bartram ex Marshall (Guerra et al. 2009), Indian mustard (Brassica juncea L. Czern), rapeseed (Brassica napus L.) (Feigl et al. 2013) and some hyperaccumulators like Haumaniastrum katangense (S. Moore) P.A.Duvign. & Plancke (Peng et al. 20012) and Crassula helmsii (Kirk) Cockayne (Küpper et al. 2009) developed different defense systems in response to Cu-induced stress. Nevertheless, the effects of Cu on both enzymatic and non-enzymatic defence systems in medicinal species have not received much attention.
Taking into account these observations, we focused our attention on the North African medicinal plant Marrubium vulgare L. (Horehound) with the aim to study the influence of soil copper concentration on its pharmacological properties. Indeed, Marrubium vulgare possess various pharmacological activities including anti-inflammatory, vasorelexant, anti-hypertensive, analgesic, antioedematogenic, antimicrobial, anti-diabetic, hepatoprotective and antioxidant activities (Sahpaz et al. 2002, El Bardai et al. 2003, El Bardai et al. 2004, Meyre-Silva et al. 2005, Stulzer et al. 2006, Masoodi et al. 2008, Boudjelal et al. 2012, Akther et al. 2013, Boulila et al. 2015). The leaves and young flowering stems have been proven to be responsible for tonic, aromatic, stimulant, expectorant, diaphoretic and diuretic activities (Amessis-Ouchemoukh et al. 2014). Previous studies already demonstrated that M. vulgare is able to tolerate water deficit (Habibi & Ajory 2015). In such studies, general responses of plants to stress (growth, mineral nutrition and photosynthesis) have been considered and little attention has been paid to the antioxidant response of M. vulgare to metal stress. In line with this consideration, the aim of the present contribution is to explore the impact of copper concentrations on nutrient uptake and on the enzymatic and non enzymatic antioxidant system of M. vulgare. Assessment of such effects is crucial to achieve a more comprehensive picture of the response of this medicinal plant to Cu toxicity.
Material and methods
Chemical and reagents. All chemicals and reagent were of the highest purity available:
2,2-diphenyl-1-picrylhydrazyl radical (DPPH), polyvinylpyrrolidone (PVP) and charcoal were obtained from Sigma Aldrich (Milan, Italy). Copper sulfate (CuSO4) was purchased from Fluka, (Germany). Potassium chlorides(KCl), Magnesium chloride (MgCl2), Sodium carbonate, Sodium nitrite and Ethylenediaminetetra acetic acid (EDTA) were purchased from Merck (Darmstadt, Germany). Folin-Ciocalteau reagent (FCR), aluminum chloride (AlCl3), gallic acid, Sodium hydroxide (NaOH), nitro-blue tetrazolium chloride (NBT), riboflavin, ascorbic acid, hydrogen peroxide (H2O2), methionine and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma Aldrich (St. Louis, MO, USA).
Plant growth and copper treatment. Seeds of Marrubium vulgare were issued from a non-contaminated wild population in the region of Béja (Northwestern Tunisia; latitude 36° 43’ 30”(N), longitude 9° 10’ 51” (E), altitude 255 m). The experiments were conducted on August 2014 and lasted 4 months. Initially, 20 seeds × 3 replications for each treatment were sown at the depth of 2 cm in plastic pots (26 × 22 cm) fitted with commercial peat and sand (1:2, v/v) and maintained under greenhouse conditions (naturally lit with sunlight, with a temperature range of 20-25 °C, relative humidity range of 50-80 %). Seedlings were pre-cultivated for one month and then treated with 0 (control), 80, 200 and 300 mg/L CuSO4. After 4 weeks of treatment, the seedling percentage was determined for each treatment by the following formula:
Seedlings have been separately transplanted into individual pots (18.2 × 14.2 cm). The treatment with 0 (control), 80, 200 and 300 mg/L CuSO4 was continued for 8 weeks. Nutrient solutions were renewed every 4 days. Irrigation was done up to field capacity in each pot. At the end of the experiment, 4 plants for each treatment were harvested for various analyses. Cu concentration was selected based on its ability to inhibit the germination rate of Marrubium vulgare seeds (Table 1). Statistical analysis (ANOVA) showed that seedling percentage (%) varied significantly as a function of Cu concentration. Based on the statistical analysis, 80, 200 and 300 mg/L Cu have been selected and the effect of metal stress on exposed samples evaluated.
Table 1 Seedling percentage (%) of Marrubium vulgare grown under 80, 200 and 300 mg/L CuSO4. Data are the mean ± SEM of three independent experiments. Different letters indicate that differences from control values were statistically significant (p < 0.05).
| Treatments CuSO4 (mg/L) |
Number of sown seeds |
Number of germinated seeds |
Total number of germinated seeds |
Seedling percentage (%) |
|---|---|---|---|---|
| 0 | 20 | 9 | 30 | 50 ± 6.67 a |
| 20 | 9 | |||
| 20 | 12 | |||
| 80 | 20 | 7 | 21 | 35 ± 3.33 b |
| 20 | 8 | |||
| 20 | 6 | |||
| 200 | 20 | 5 | 17 | 28 ± 2.22 b |
| 20 | 6 | |||
| 20 | 6 | |||
| 300 | 20 | 4 | 15 | 25 ± 3.33 b |
| 20 | 6 | |||
| 20 | 5 |
Percentage values based on three replications.
a) p < 0.01; b) p < 0.05
Determination of metal content in leaves. Dried leaves (100 mg) were digested with a nitric and perchloric acid mixture (3:1 v/v). Copper, Fe, K, Mg and Ca were determined by atomic absorption spectrometry (Perkin Elmer-model 2380).
Determination of enzymes activity. Fresh leaves (200 mg) were homogenized with 50 mM potassium-phosphate buffer (pH 7) containing 0.1 mM EDTA and 1 % (w/v) polyvinylpirrolidone (PVP). The homogenate was centrifuged at 15,000g for 20 min, and then the supernatants were collected and subsequently assayed for catalase (EC 1.11.1.6), superoxide dismutase (EC1.15.1.1) and ascorbate peroxidase(EC1.11.1.11) activities.
Catalase (CAT) assay.-Total CAT activity was assayed by measuring the initial rate of disappearance of H2O2 according to the method of Aebi (1984). The reaction mixture (1 mL) contained 50 mM K-phosphate buffer (pH 7), 15 mM H2O2 and 50 µL of enzyme extracts. The decrease in absorbance at 240 nm was monitored for 2 min and CAT activity was expressed as units (µmol of H2O2 decomposed per minute) per g FW.
Superoxide dismutase (SOD) assay.-The SOD activity was measured according to the method of Beyer & Fridovich (1987) and expressed as unit of SOD per g FW. The reaction mixture (3 mL) contained 50 mM K-phosphate buffer (pH 7.8), 0.1 mM EDTA, 14.3 mM methionine, 82.5 mM nitroblue tetrazolium (NBT) and 2.2mM riboflavin. The reaction was started by adding enzyme extracts under illumination (15 min, 5000 Lux). The color intensity of the chromogen in the reaction mixture was measured at 590 nm. An enzyme free system was used as a negative control.
Ascorbate peroxidase (APX) assay.-The activity of APX was determined according to Chen & Asada (1989) method by monitoring the decrease in absorbance at 290 nm. The reaction mixture (2mL) consisted of 50 mM Na-phosphate buffer (pH 7.0) containing 0.1 mM EDTA, 0.25 mM ascorbate, 1mM H2O2 and 100 µL of enzyme extract. The APX activity was expressed as nmol of ascorbate per g FW.
Determination of total phenolic and flavonoid contents
Extract preparation.-Basing on our experience [Martino et al. (2008), Gaggeri et al. (2012), Rossi et al. (2017)], extracts have been prepared applying a Miscrowave Assisetd extraction procedure. Powdered leaves (1 g) were homogenized with 80 % methanol (20 mL) and extracted in a multimode microwave apparatus using a closed-vessel system (MARSX press, CEM Corporation, Matthews, NC, USA) under the following conditions: temperature 60 °C, power 100 w, pressure 120 psi, run time 10 min. Charcoal and PVP were added to the extracts in order to remove chlorophyll and tannins. After 10 minutes the suspension was filtered through Whatman No. 4 filter paper, the solvent removed under reduced pressure and the dry extract obtained stored at 4 °C until analysis.
Determination of total phenolic content (TPC).-Total phenolic content (TPC) was determined based on the method described by Mau et al. (2001). Briefly, an aliquot of 125 µLof extract was added to 500 µL deionized water and 125 µL of the Folin-Ciocalteu reagent. After shaking, the mixture was incubated for 3 min at room temperature. Then, 1250 µL of 7 % Na2CO3 solution was added, the volume adjusted to 3 mL using distilled water, then the extract was mixed vigorously and held for 90 min at room temperature before measuring the optical density at 765 nm. The sample was analyzed in triplicate against a blank Gallic acid was used as a standard,and the TPC was expressed as mg gallic acid equivalents (GAE) per g dry weight (DW).
Determination of total flavonoid content (TFC).- Total flavonoids were determined as described by Zhishem et al. (1999) and Dewanto et al. (2002). A 250 µL aliquot of the diluted extract or standard solution of (+)-catechin was added to a 75 µL of a 5 % NaNO2 solution. After 6 min, 150 µL of a 10 % AlCl3 was added and allowed to stand for 5 min before 0.5 mL of 1M NaOH was added. The mixture was brought to 2.5 mL with distilled water and thoroughly mixed. The absorbance was measured at 510 nm against the blank. Total flavonoid content was expressed as mg catechin/g dry weight (mg CE/g DW).
Free radical scavenging activity (DPPH assay). The DPPH (1,1-diphenyl-2-picrylhydrazyl) scavenging effect of Marrubium vulgare extracts was determined according to Gaggeri et al. (2015). Briefly, 100 µL aliquot of sample solution with different concentration was thoroughly mixed with 3.9 mL of freshly prepared DPPH and allowed to stand for 20 min in the dark. The absorbance was then measured at 515 nm against a blank. Tests were carried out in triplicate. The radical scavenging activity was calculated as follows:
Where: Abscontrol and Abssample are the absorbance of the control (blank) and the sample, respectively.
Statistical analysis. Seedling percentage was determined using 20 seeds of a biological replicate. The results were the average of three independent experiments. The data set representing three biological replicates of each treatment represented a complete randomized design. Statistical analysis of this dataset was conducted using SPSS v19.0 software (Chicago, IL, USA) for windows. ANOVA was used to estimate the significance in differences between the means of different treatments, and the Duncan’s multiple range test at 5 % level (p ≤ 0.05) was used to separate significant means. Normality test of seedling percentage showed that the majority of the observed cumulative values were very near compared to the expected cumulative points.
All data are expressed as the means ± standard deviation of three replicates. The one-way analysis of variance (ANOVA) were used to detect differences among treatments, p-values < 0.05 were considered statically significant.
Results
Effect of copper on seedling percentage and mineral elements uptake. As shown in Table1, the treatment of Marrubium vulgare with increasing amount of Cu (80, 200 and 300 mg/L) gave rise to a decrease in seedling percentage (30, 44 and 50 % reduction, respectively).
The amount of Cu, Fe, K, Mg and Ca was determined in dried leaves (Table 2). As expected, the copper content present in M. vulgare leaves increased significantly in the plants grown under 200 and 300 mg/L of Cu (38 and 55 %, respectively) compared to the control plants (Table 2). On the contrary the results clearly showed that the amount of Fe, K and Ca decreased with increasing Cu concentration (Table 2). No effect of Copper-induced-stress uptake and translocation of Mg has been observed.
Table 2 Mineral concentrations in Marrubium vulgare leaves grown under 80, 200 and 300 mg/L CuSO4. Data are the mean ± SEM of at least three different experiments. Different letters indicate that differences from control values were statistically significant (p < 0.05).
| Treatments CuSO4 (mg/L) |
Cu (µg/ g DW) |
Fe (µg/ g DW) |
K (µg/ g DW) |
Ca (µg/ g DW) |
Mg (µg/ g DW) |
|---|---|---|---|---|---|
| 0 | 29.6 ± 3.4 a | 268.6 ± 14.7 a | 33466 ± 261 c | 11220 ± 1715 b | 2010 ± 599 a |
| 80 | 33.6 ± 2.7 a | 188.0 ± 43.6 b | 33200 ± 226 c | 10146 ± 1335 b | 1953 ± 183 a |
| 200 | 40.8 ± 3.5 b | 219.3 ± 54.9 ab | 23900 ± 196 b | 6076 ± 1703 a | 1970 ± 103 a |
| 300 | 46.0 ± 3.4 b | 169.6 ± 38.9 b | 22800 ± 1037 a | 4963 ± 1199 a | 1900 ± 608 a |
Effects of copper on antioxidant enzymes. The effect of Cu treatment on the enzymatic activity of the leaves extract has been evaluated. The results of Figure 1 showed that SOD and CAT activities increased together with Cu concentration. On the contrary, the activity of the APX enzyme was unaffected by the increase in Cu concentrations.
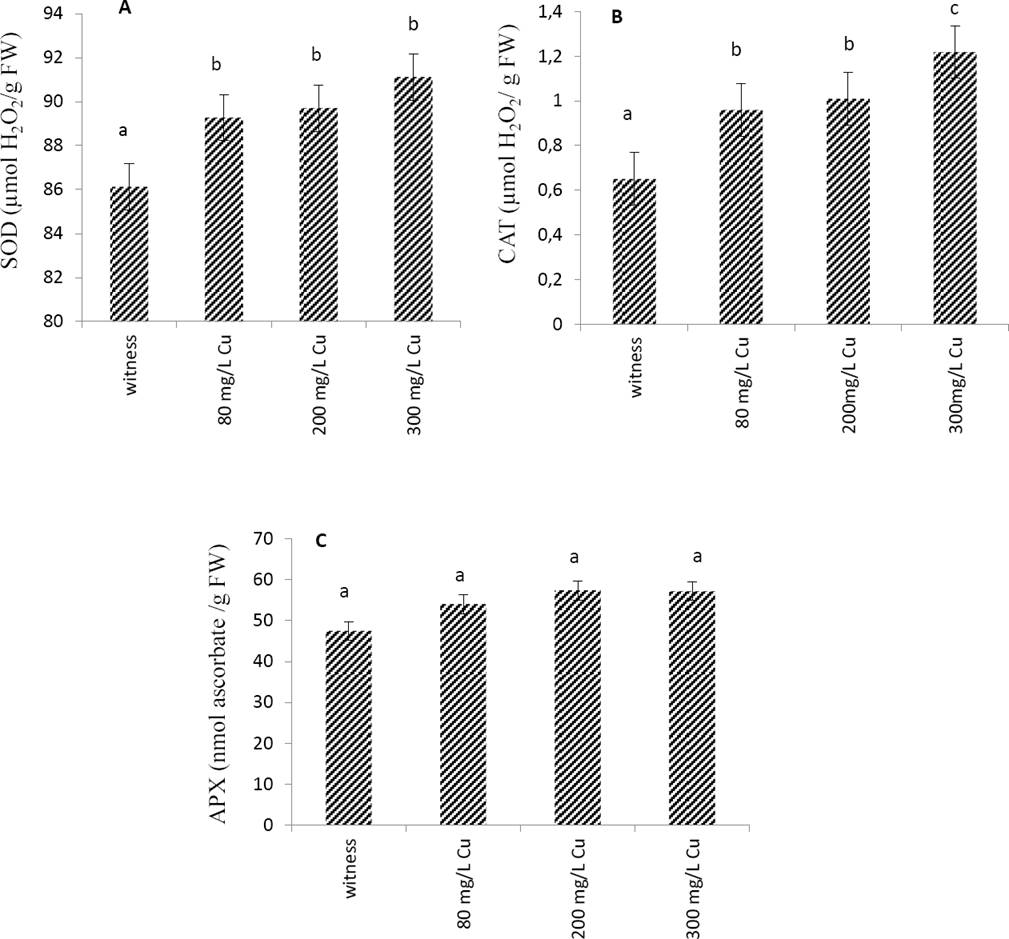
Figure 1 Effect of Cu applied at different doses (0 mg/L: witness, 80 mg/L, 200 mg/L and 300mg/L) on antioxidant enzymes activities, SOD (A), CAT (B) and APX (C) from leaves of Marrubium vulgare. Data are the mean ± SEM of at least three different experiments. Different letters indicate that differences from control values were statistically significant (p < 0.05).
Effects of copper on total phenol and flavonoid contents and the anti-radical activity Marrubium vulgare extract. As shownin Figure 2, total phenol and flavonoid contents increased in a concentration-dependent manner; however, this increase was significant (p < 0.05) only in the extracts obtained in plant treated with 300 mg/L CuSO4.
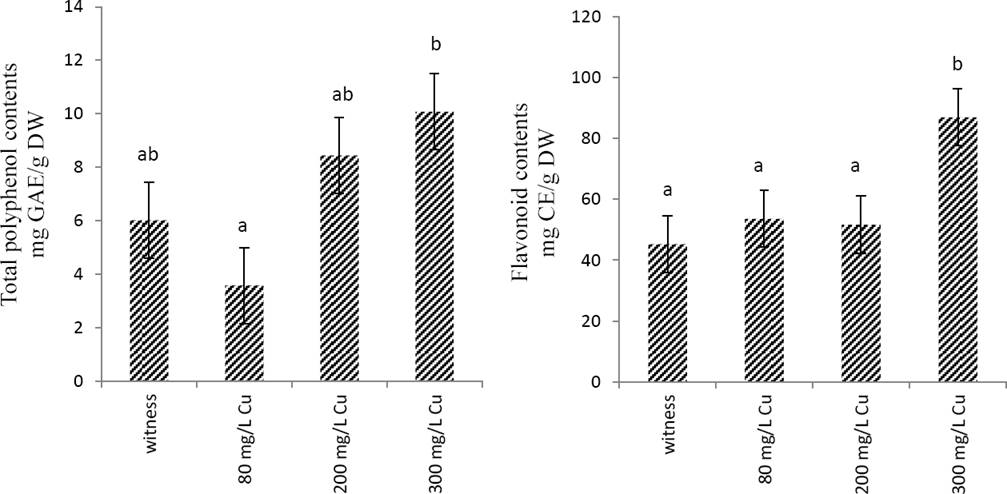
Figure 2 Total polyphenol and flavonoids contents of Marrubium vulgare leaves extracts grown in regular condition (witness) or under copper stress at doses of 80 mg/L, 200 mg/L and 300 mg/L CuSO4. Data are the mean ± SEM of at least three different experiments. Different letters indicate that differences from control values were statistically significant (p < 0.05).
DPPH radical scavenging activities of the extracts (obtained by using 80 % methanol) obtained from not-treated and Cu-treated plant material was evaluated. All the extracts showed a similar IC50 values, ranging from 50 to 50.6 µg/mL (Figure 3).
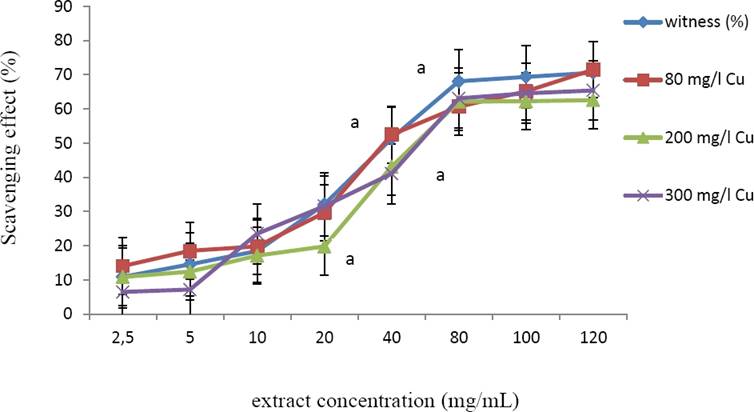
Figure 3 DPPH scavenging activity (%) of methanolic extracts from Marrubium vulgare leaves, grown in normal condition (witness) or under copper stress at doses of 80 mg/L, 200 mg/L and 300 mg/L CuSO4. Data are the mean ± SEM of at least three different experiments. Different letters indicate that differences from control values were statistically significant (p < 0.05).
Discussion
Copper (Cu) is an essential micronutrient for plants, but it is also an environmental pollutant that severely affects plant growth (Adrees et al. 2015). To clarify the effect of such metal, we evaluated the effect of Cu treatment on Marrubium vulgare. Results obtained clearly showed that seedling percentage gradually decreased as copper concentration increased. Cu treatment significantly decreased (p < 0.05) seed germination at concentration of 80, 200 and 300 mg/L as compared to the control. Similar observations in maize have been reported by Gupta & Abdullah (2011) at concentration of 200 mg/L Cu and in tomato when >100ppm concentration of Cu inhibits seedling growth (Ashagre et al. 2013). According to Houshmandfar & Moraghebi (2011), a decrease in seed germination can be attributed to the poor break down of starch and also to alterations inpermeability selection properties of cell membrane. Interestingly, in our conditions, the inhibition of seed germination due to copper treatments can be correlated to the amylase activity which plays an important role during seed germination, as previously reported (Singh et al. 2007, Upadhyay & Pandey 2013). Indeed, it has been demonstrated that amylase activity decreased significantly in Wheat, Maize and Sweet pea plants under the influence of different levels of copper solution (5, 25, 50, 75 and100 ppm) in comparison to controls. Upadhyay & Pandey (2013) hypothesized that the observed decreasing in seed germination percentage is a result of low level of amylase activity.
Our results are also in accordance with studies performed on Brassica juncea and Brassica napus (Feigl et al. 2013) evidencing that Cu excess in the nutrient solution inhibited Fe, K and Ca uptake by competing with their absorption and/or translocation.
Moreover, we proved that Cu was accumulated in the aerial part of Marrubium vulgare, as already observed in cucumber (Alaoui-Sossé et al. 2003) and rice (Thounaojam et al. 2012). In contrast, in Cu-tolerant species like Populus deltoides, Haumaniastrum katangense, fenugreek and mustard rapeseed (Guerra et al. 2009, Peng et al. 2012, Elleuch et al. 2013, Feigl et al. 2013) Cu was preferentially accumulated in roots.
It is well known that an excess of Cu induced oxidative stress in plant enhancing the ROS production. Cu catalyzes the formation of hydroxyl radicals (OH•) from the non-enzymatic chemical reaction between superoxide (O2•-) and H2O2 via Haber-Weiss reaction (Islek & Unal, 2015). ROS can damage cells (Dey et al. 2015) and plants have established both enzymatic and non-enzymatic protective mechanisms. Enzymatic scavengers responsible for the elimination of H2O2, include ascorbate peroxidase, catalase, superoxide dismutase and enzymes of the ascorbate-glutathione cycle and non-enzymatic radicals-scavengers such as ascorbate and glutathione (Wang et al. 2004). Accordingly, we studied the effect of Cu-induced stress on SOD, CAT and APX enzymatic activity Marrubium vulgare leaves extracts. Results clearly evidenced that metal stress is able to induce activation of antioxidant enzymes such as SOD, which converts superoxide (O2•-) into H2O2 (Dey et al. 2015), and CAT, responsible for transformation of H2O2 in water (H2O) and oxygen (O2) (Karuppanapandian et al. 2011), indicating that these enzymes are involved in the detoxification mechanism in M. vulgare, as already observed in other species (Haribabu & Sudha 2011, Thounaojam et al. 2012, Elleuch et al. 2013).These results led to hypothesize that such increment is an adaptive response of plants to cope with Cu-induced oxidative stress. To sum up, our observations are in line with literature data (Elleuch et al. 2013) and suggest that the generation of reactive oxygen species (ROS) under Cu stress and the SOD and CAT activated are an adaptive mechanism to M. vulgare that serves to maintain the ROS at a steady-state level. On the contrary, the activity of APX was not influenced by Cu concentrations, as compared to the control. It is not surprisingly, because catalases (CAT) and peroxidases (APX) have different functions and may have a different behavior in response to Cu stress. Our results are in accordance with that found in other species like Lemna minor L. (Paczkowska et al. 2007) and Triticum aestivum L. (Sairam et al. 2000). Particularly, Sairam et al. (2000) hypothesized that the differences in antioxidant enzymatic activities may be attributed to different genotypes.
Lastly, taking into account that also non-enzymatic antioxidant systems (ascorbate, glutathione, phenols, flavonoids, carotenoids) are involved in the defense system against oxidative stress (Thounaojam et al. 2012), we evaluated the total phenol content and total flavonoid contents as well as the anti-radical scavenging activities of Cu-treated and not-treated plant extracts. Our findings suggest that phenols are involved in Cu detoxification process, having an increase of phenol and flavonoid contents, as already observed in fenugreek (Elleuch et al. 2013). From a physiological point of view, an increase in total phenol and flavonoid content is expected due to their ROS scavenging activity, Cu chelating and detoxifying properties (Gordon & Roedig-Penman 1998). Phenolic components have been also shown to be upregulated in response to Cd (Mishra et al. 2014), Zn (Marichali et al. 2014), Al (Kováčik et al. 2012), Pb (Wang et al. 2011) and Ni (Kováčik et al. 2009).
To verify a possible correlation between phenolic content and free radical scavenging activity, Marrubium vulgare extracts were analysed (DPPH assay). Unexpectedly, no relevant differences in the free radical activities of the extracts were evidenced indicating that the Cu-stress applied to M. vulgare did not affect the non-enzymatic antioxidant activity. These results are not surprisingly. Indeed, contrasting results in the literature, regarding the correlation between free radical scavenging activity (DPPH method) and the amount of total polyphenolic compounds, have been reported. Some authors have evidenced positive correlations (Huda-Faujan et al. 2009, Rebaya et al. 2014), while others have pointed out negative relationships (Hesam et al. 2012, Aksoy et al. 2013). In the current study, we evidenced an absence of significant variation in the antioxidant activity between samples although the significant (p < 0.05) changes in the total phenol and flavonoid contents. Therefore, the anti-radical scavenging activity seems to be not correlated to the phenolic content, but presumably linked to other non-phenolic constituents such as tocopherols and ascorbic acid. Another possible explanation is that the amount of Cu used in our study is below the limit that the plants can tolerate. Studies in this direction are currently in progress.
Collectively, results from the present contribution indicated that Cu-induced stress (80-300 mg/L) negatively affect seedling percentage of Marrubium vulgrae and reduced the uptake and translocation of cationic elements namely Fe, K and Ca. Meanwhile, the activity of the antioxidant enzymes (SOD and CAT) and the content of total phenol and flavonoid were found to increase. To sum up, the increased antioxidant response (enzymatic and non-enzymatic system) might enhance the endurable ability to cope with Cu stress.











 nueva página del texto (beta)
nueva página del texto (beta)


