Galls are atypical growths of plants that offer food, shelter and protection for the gall inducer or its progeny (Cornell 1983, Schönrogge et al. 2000, Hernández-Soto et al. 2015). Close to 13,000 species of gall-inducing insects have been recorded in plants (Shorthouse et al. 2005), although Espírito-Santo & Fernandes (2007) estimated a larger value of gall-inducing species ranging from 21,000 to 211,000 species. The family Cynipidae (Hymenoptera) contributes with approximately 1,400 species, constituting the second-largest source of gall-inducing insects, followed by the family Cecidomyiidae: Diptera (Csóka et al. 2005, Liljeblad & Ronquist 1998, Nieves-Aldrey & Fontal-Cazalla 1999, Ronquist & Liljeblad 2001). Each species produces its own gall type, which is anatomically and physiologically distinct from that of other related species (Shorthouse et al. 2005).
Nearly a thousand species of gall-inducing wasps have been reported (Hymenoptera: Cynipidae: Cynipini) in oak species (Fagaceae: Quercus) (Kinsey 1936, Weld 1960, Stone et al. 2002), and they are among the most structurally complex and diverse galls (Liljeblad et al. 2008). Gall structures reflect the primary characteristics of the insect inducer, representing an extension of their phenotype (Stone & Cook 1998). The host species of the Cynipini are almost exclusively limited to the Quercus genus (Liljeblad et al. 2008), although some use other hosts within the Fagaceae family, such as Castanea, Castanopsis, Lithocarpus and Chrysolepis (Nieves-Aldrey 2001, Stone et al. 2002, Liu & Ronquist 2006).
Each species of wasp from the Cynipini tribe is generally associated with inducing galls in practically all the organs (e.g., the leaves, roots, stems, and catkins) of their host species (Weld 1960, Ronquist 1994, 1995, Liljeblad & Ronquist 1998, Nieves-Aldrey 2001, Stone et al. 2002, Csóka et al. 2005). This association between wasps and oaks determines the global distribution pattern of this family of insects as well as the richness of the cynipid community in a specific locality (Nieves-Aldrey 2001, Stone et al. 2002).
In Europe, approximately 280 cynipid species have been recorded in 25 species of European oaks, which represents all the tribes of the Cynipidae (Nieves-Aldrey 2001, Stone et al. 2002, Rokas et al. 2003). Most of these endophagous insects have a life cycle with a spring-summer sexual generation and an autumn asexual generation, i.e., they exhibit an alternation of generations (Stone et al. 2002). During each generation, cynipid species have the ability to induce specific and complex galls on oaks, and in the majority of cases, they form a different gall morphotype each generation (e.g., sexual and asexual). Another characteristic in some species of cynipids is host alternation (heteroecy), which occurs every generation (Askew 1984, Cook et al. 1998, 2002).
Due to the diversity and structural complexity of the galls induced by cynipids, they are considered to be a microcosm of intense ecological activity since they support at least three trophic levels (gall tissue, gall-inducing wasps and occupants, and parasites and predators), which makes them a complex and interesting community for ecological-evolutionary studies (Nieves-Aldrey 2001, Ronquist & Liljeblad 2001, Hayward & Stone 2005).
It is estimated that there are approximately 500 oak species in the world and that the greatest species richness occurs in Mexico (Manos et al. 1999). Mexico is considered one of the centers of diversification for the Quercus genus with 161 species, of which 76 are located in the Lobatae section (red oaks), and 61 species are endemic to Mexico. In addition, the genus has 81 species in the Quercus section (white oaks) with 47 endemic species and four species in the Protobalanus section (intermediate oaks), with one endemic species (Valencia-Ávalos 2004).
One issue that is unclear is the specificity of cynipids to their host plants. In some European species, the alternation of hosts from different series of oaks has been reported at each reproductive stage, which indicates that the specificity may not be exclusively limited to a particular host species (Askew 1984, Stone et al. 2001, 2002). The specificity of gall-forming wasps has been poorly reviewed in the literature (Stone et al. 2002), and in Mexico, this group of insects and their ecological interactions have been poorly studied. Although the collection effort has not been as intense as in other countries, the species richness of cynipids has been estimated to range from 250 to 700 species based on the high richness of Mexican oak species (Nieves-Aldrey 2001). It is thus important to develop a strategy for the collection, breeding and identification of this group of insects in Mexico based on the distribution of oak species.
Based on the above priorities, it is necessary to study the species richness of cynipid species in Mexico, the diversity of gall morphology and the degree of gall specificity to their hosts. Therefore, the specific objectives of this study were to determine 1) the variations in wasp species richness among different oak sections in Mexico; 2) the morphological variation of galls induced by wasps on different oak species; and 3) the relationship between the cynipid species richness and the amplitude in the geographic distribution of the host oak species.
Materials and methods
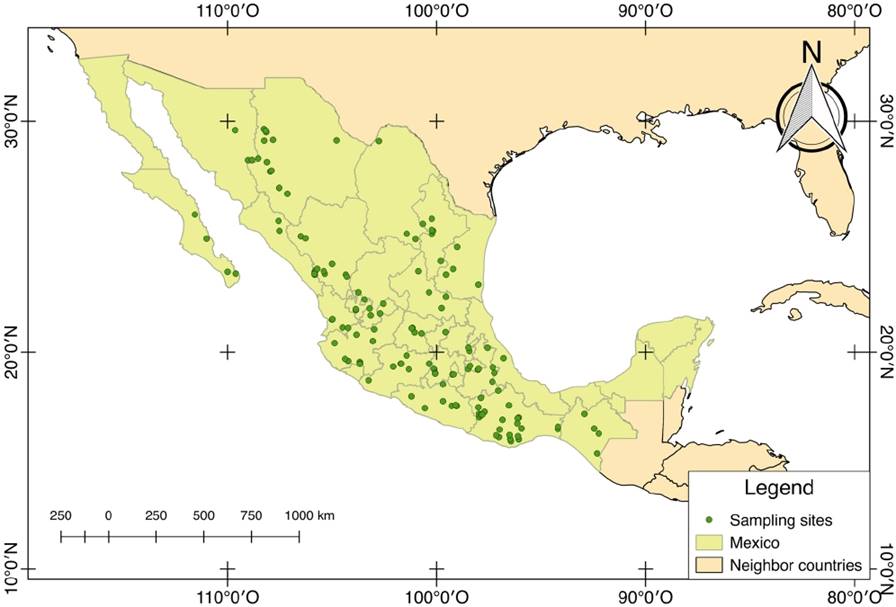
A literature review of cynipid species associated with oaks was conducted, leading to the construction of a database to locate the species and establish them as starting points for the collection. Sampling was performed from March to August for four years (2008-2012) in 120 localities in total. For each oak species, at least five localities were visited. Geographic coordinates were taken at each location to define the distribution pattern of the cynipids associated with the different Quercus species (Figure 1).
Once the different oak species were located, an exhaustive search for galls was conducted at each site by checking each structure (i.e., apical buds, leaves, petioles, branches, stems, roots, catkins and acorns) of at least ten trees from each available Quercus species. In addition, herbarium specimens were collected to identify the oak species.
The collection of cynipids was based on gall morphology and was taken from across the country. A total of 80 oak species that were associated with this guild of herbivores was analyzed. For this study, each distinct gall morph is considered a potentially distinct species. We classified the galls according to the number of larval chambers as unilocular (a single chamber) or multilocular (with several chambers) and with or without ornaments (spines, hairs).
The collected galls were brought to the laboratory for growth and subsequent taxonomic determination. The taxonomy of the species is being determined in collaboration with Dr. José Luis Nieves-Aldrey from the National Museum of Natural Sciences in Madrid, Spain.
Results
A total of 224 distinct gall morphotypes induced by cynipids was found in 73 of the 80 oak species under analysis, of which 40 correspond to the Lobatae section (red oaks) and 33 to the Quercus section (white oaks). Galls were not found in the following seven oak species: Q. canbyi, Q. gentryi, Q. crispifolia and Q. hypoleucoides from the Lobatae section and Q. glabrescens, Q. glaucoides and Q. lancifolia from the Quercus section. The largest number of morphotypes was found in leaves (125), followed by branches (37), buds (31), petioles (20), catkins (5), acorns (3) and roots (3) (Figure 2). A total of 126 gall morphotypes were found in the Quercus section, and 113 were found in the Lobatae section.
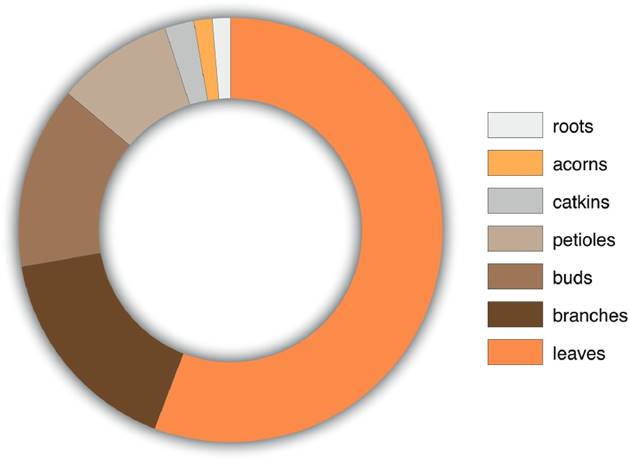
Figure 2 Number of gall morphotypes in each plant organ of oaks. Total number of galls = 224. 1 = leaves (125 galls; 55.8%); 2 = branches (37; 16.5%); 3 = buds (31; 13.8%); 4 = petioles (20; 8.9%); 5 = catkins (5; 2.2%); 6 = acorns (3; 1.3%); and 7 = roots (3; 1.3%).
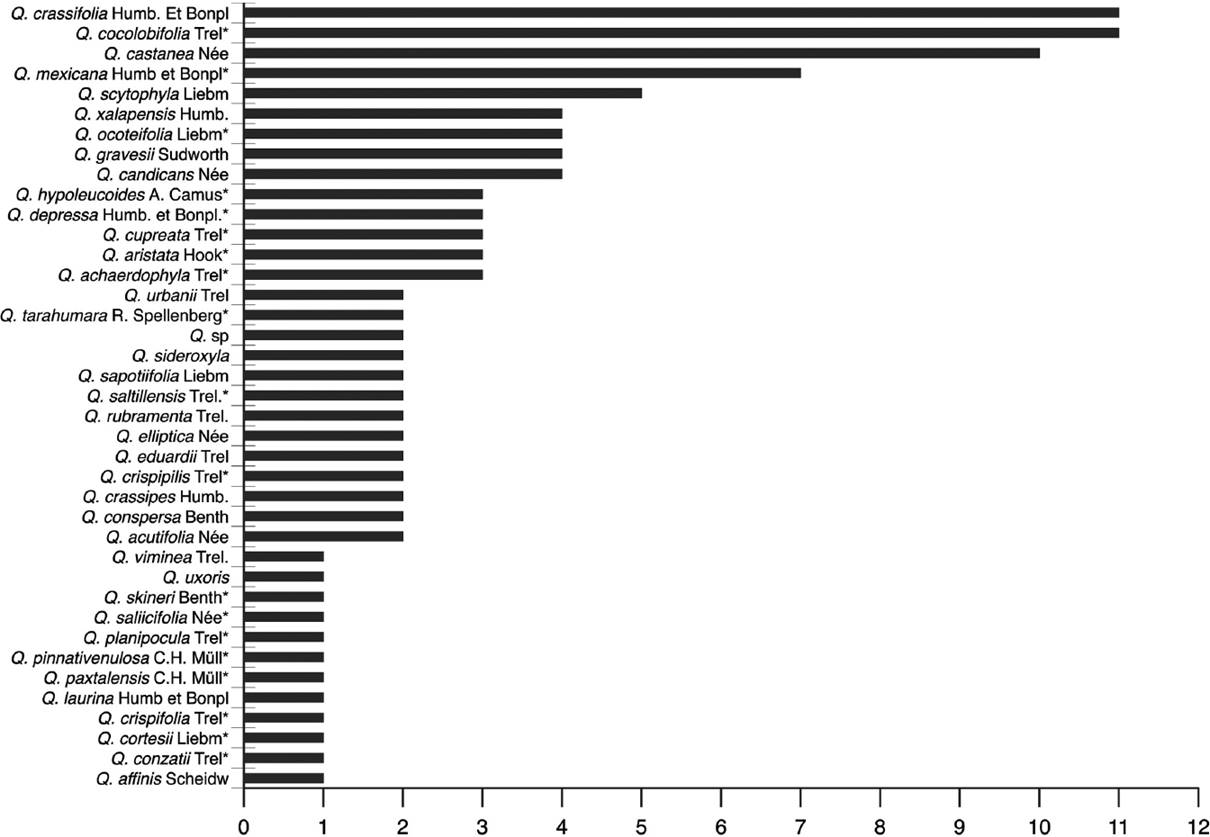
The number of gall morphotypes associated with each oak species, their taxonomic section, and the amplitude of geographical distribution and whether they are endemic to Mexico is summarized in Table 1. In the Lobatae section (red oaks), the species with the greatest number of morphotypes are Quercus crassifolia (11), Q. castanea (10) and Q. mexicana (7). The remaining species have one to five distinct morphotypes (Figure 3). In the Quercus section (white oaks), the oak species with the greatest number of gall morphotypes were Q. microphylla (20 morphotypes), followed by Q. resinosa (17) and Q. magnoliifolia (14). The remaining species have one to five distinct associated morphotypes (Figure 4). As in the case of section Lobatae, the species with the greatest number of morphotypes are those with a wide geographical distribution.
Table 1 Number of gall morphotype associated to each oak species in the section Lobatae (red oaks) and section Quercus (white oaks). Oak endemic species to Mexico is indicated by an asterisk. The type of gall morphotype: U = unilocular, M = multilocular, s/o = without ornaments and c/o = with ornaments.
| Section | Species | Collected states | Number of gall morphotypes |
Unique gall in a population |
Type of gall morphotype |
|---|---|---|---|---|---|
| Lobatae | Q. acutifolia Née | Mich., Oax., Jal. | 2 | Ms/o(2) | |
| Q. acherdophylla Trel. * | Hgo., Pue. | 3 | Us/o(1), Ms/o(2) | ||
| Q. affinis M.Martens & Galeotti. | Gto., Pue., Hgo., Ver., S.L.P. | 1 | Ms/o | ||
| Q. aristata Hook & Arn* | Jal., Nay. | 3 | Us/o(1), Ms/o(2) | ||
| Q. candicans Née | Chih., Chis., Dgo., Gro., Jal., Mich., Nay., Oax | 4 | Us/o(1), Ms/o(3) | ||
| Q. castanea Née | Chis., Col., Dgo., Gto., Gro., Hgo., Jal., Mich.,Oax. | 10 | Us/o (2) | Us/o(3), Ms/o(3), Uc/o(2), Mc/o(2) | |
| Q. coccolobifolia Trel.* | Chih., Jal., Nay. | 11 | Us/o(4), Ms/o(4), Uc/o(2), Mc/o(1) | ||
| Q. conspersa Benth. | Chih., Gro., Jal., Mich., N.L. | 2 | Ms/o(2) | ||
| Q. conzattii Trel.* | Dgo., Jal., Oax., Zac. | 1 | Ms/o | ||
| Q. cortesii Liebm.* | Chis., Oax., Ver. | 1 | Ms/o | ||
| Q. crassifolia Bonpl. | Chih., Chis., Dgo., Mich., Oax. | 11 | Us/o(4), Ms/o(4), Uc/o(2), Mc/o(1) | ||
| Q. crassipes Bonpl. | Col., Gto., Hgo., Mich. | 2 | Ms/o(2) | ||
| Q. crispifolia Trel.* | Chis., Oax. | 1 | Ms/o | ||
| Q. crispipilis Trel.* | Chis. | 2 | Uc/o(1), Ms/o(1) | ||
| Q. cupreata Trel. & C.H.Mull.* | N.L. | 3 | Us/o(1) | Us/o(1), Ms/o(2) | |
| Q. depressa Bonpl.* | Hgo.,Oax., Ver. | 3 | Us/o(1), Ms/o(2) | ||
| Q. eduardi Trel. | Chih., Dgo., Mich., Ver. | 2 | Us/o(1), Ms/o(1) | ||
| Q. elliptica Née | Chis., Jal., Mich., Sin. | 2 | Us/o(1), Ms/o(1) | ||
| Q. gravesii (Sarg.) Sudw. | Coah. | 4 | Us/o(1), Uc/o(1), Ms/o(1) | ||
| Q. hypoleucoides A.Camus* | Chih., Coah., Dgo. | 3 | Us/o(1), Ms/o(2) | ||
| Q. laurina Bonpl. | Gro., Gto., Hgo., Mich., Pue., Tlax., Ver. | 1 | Ms/o | ||
| Q. mexicana Bonpl.* | Coah., Hgo., N.L., Tamps., Tlax., Ver. | 7 | Uc/o(1) | Us/o(2), Ms/o(3), Uc/o(1), Mc/o(1) | |
| Q. ocoteifolia Liebm.* | Chis. , Oax. | 4 | Uc/o(1) | Us/o(1), Uc/o(1), Ms/o(2) | |
| Q. paxtalensis C.H.Müll.* | Chis. | 1 | Us/o | ||
| Q. pinnativenulosa C.H.Müll.* | N.L. ,Ver. | 1 | Us/o | ||
| Q. planipocula Trel.* | Mich, Gro., Sin. | 1 | Us/o | ||
| Q. rubramenta Trel. | Gro., Oax. | 2 | Us/o(1), Ms/o(1) | ||
| Q. salicifolia Née* | Gro., Jal., Mich. | 1 | Ms/o | ||
| Q. saltillensis Trel.* | Coah., N.L. | 2 | Us/o(1), Ms/o(1) | ||
| Q. sapotifolia Liebm. | Chis., Hgo., Oax., Ver. | 2 | Uc/o(1), Ms/o(1) | ||
| Q. scytophylla Liebm. | Gro., Jal., Mich., Pue. | 5 | Us/o(2), Ms/o(2), Mc/o(1) | ||
| Q. sideroxyla Bonpl | Ags., Chih. Coah., Dgo., Jal., Zac. | 2 | Us/o(1), Ms/o(1) | ||
| Q. sp. | Tamps., Ver. | 2 | Us/o(1), Ms/o(1) | ||
| Q. skinneri Benth.* | Chis., Oax. | 1 | Us/o | ||
| Q. tarahumara R. Spellenb., J.D. Bacon & Breedlove* | Chih, Sin, Dgo. | 2 | Us/o(1), Mc/o(1) | ||
| Q. urbanii Trel. | Dgo., Gro., Sin. | 2 | Us/o(1), Ms/o(1) | ||
| Q. uxoris McVaugh | Col., Jal., Oax. | 1 | 1 Uc/o | Uc/o | |
| Q. viminea Trel. | Ags., Dgo., Gto., Nay. | 1 | Ms/o | ||
| Q. xalapensis Bonpl. | Hgo., Ver. | 4 | Us/o(1), Uc/o(1), Ms/o(2) | ||
| Quercus | Q. alpescens Trel.* | Hgo., N.L. | 2 | Ms/o (2) | |
| Q. arizonica Sarg. | Chih., Coah., Dgo. | 3 | Us/o (1), Mc/o (2) | ||
| Q. corrugata Hook* | Chis, Gro., Ver. | 2 | Uc/o(1), Ms/o(1) | ||
| Q. depressipes Trel. | Chih., Zac. | 1 | Ms/o(1) | ||
| Q. deserticola Trel. | Gto., Jal., Mex., Mich., Oax., Qro., Sin. | 3 | Uc/o(1), Ms/o(2) | ||
| Q. frutex Trel.* | Hgo., Mex., Oax., Pue. | 1 | Uc/o(1) | ||
| Q. fusiformis Small | Coah., N.L. | 2 | Ms/o (2) | ||
| Q. germana Schltdl. & Cham. * | Oax., Pue., Ver. | 5 | Us/o(1), Uc/o(1), Ms/o(2), Mc/o(1) | ||
| Q. glaucescens Bonpl. | Gro., Jal., Mich., Sin. | 4 | Us/o(1), Ms/o(3) | ||
| Q. greggii (A.D.C) Trel. | Coah., Dgo., N.L. | 6 | Us/o(1), Uc/o(1), Ms/o(2), Mc/o(2) | ||
| Q. grisea Liebm. | Ags., Chih.,Coah., Gto., Jal. | 5 | Us/o(1), Uc/o(1), Ms/o(2), Mc/o(1) | ||
| Q. laeta Liebm. | Ags., Coah., Dgo.,Gto., Hgo., Jal., Mex., Mich., Zac | 7 | Us/o(1), Uc/o(2), Ms/o(2), Mc/o(2) | ||
| Q. leiophylla A.D.C. * | Oax., Pue. | 6 | Us/o(1), Uc/o(1), Ms/o(2), Mc/o(2) | ||
| Q. liebmannii Oerst ex Trel* | Gro. | 5 | Us/o(1), Uc/o(1), Ms/o(2), Mc/o(1) | ||
| Q. magnoliifolia Née | Col.,Gro., Hgo., Jal., Méx., Mich., Oax., Pue., Sin. | 14 | Us/o(1), Uc/o(1), Ms/o(1) | Us/o(4), Uc/o(4), Ms/o(4), Mc/o(2) | |
| Q. martinezii C.H.Mull* | Gro., Jal., Nay., Mich. | 1 | Mc/o (1) | ||
| Q. microphylla Née | Ags.,Gto., Nay. | 20 | Us/o(6), Uc/o(4), Ms/o(6), Mc/o(4) | ||
| Q. monterreyensis Trel. & C.H.Mull.* | N.L. | 3 | Us/o (1), Mc/o (2) | ||
| Q. oblongifolia Torr. | Chih., Coah., Son. | 1 | Ms/o(1) | ||
| Q. obtusata Bonpl. | Dgo., Gro., Gto., Jal., Mich. | 4 | Us/o(1), Ms/o(3) | ||
| Q. oleoides Schltdl. & Cham. | Chis., S.L.P., Oax. | 1 | Mc/o (1) | ||
| Q. oocarpa Liebm.* | Jal., Nay. | 3 | Us/o (1), Mc/o (2) | ||
| Q. peduncularis Neé | Chis., Col., Nay. | 2 | Ms/o (2) | ||
| Q. polymorpha Schltdl. & Cham. | Chis., Hgo., N.L. | 2 | Us/o(1), Ms/o(1) | Us/o(1), Ms/o(1) | |
| Q. potosina Trel.* | Ags., Dgo., S.L.P. | 1 | Ms/o(1) | ||
| Q. pungens Liebm. | Chih. | 1 | Ms/o(1) | ||
| Q. repanda Michx..* | Hgo., Tlax. | 1 | Ms/o(1) | ||
| Q. resinosa Liebm. | Ags., Dgo., Gto., Jal., Mich. | 10 | Uc/o(1) | Us/o(2), Uc/o(2), Ms/o(4), Mc/o(2) | |
| Q. rugosa Née | Ags., Chis., Col., Gro., Gto., Mich., Mor., N.L., Zac. | 3 | Us/o(1), Uc/o (1), Ms/o(1) | ||
| Q. segoviensis Liebm. | Chis. | 3 | Ms/o (1) | Us/o (1), Ms/o (2) | |
| Q. splendens Née* | Nay., Jal., Mich. | 1 | Ms/o(1) | ||
| Q. tuberculata Liebm. | B.C., Nay., Son. | 2 | Us/o(1), Ms/o(1) | ||
| Q. turbinella Greene* | B.C., Son. | 1 | Ms/o(1) |
States abbreviations. Ags.: Aguascalientes; BC: Baja California; Coah.: Coahuila; Col.: Colima; Chih.: Chihuahua; Chis.: Chiapas; Dgo.: Durango; Gro.: Guerrero; Gto.: Guanajuato; Hgo.: Hidalgo; Jal: Jalisco; Méx.: Estado de México; Mich.: Michoacán; Mor.: Morelos; Nay: Nayarit; N.L.: Nuevo León; Oax.: Oaxaca; Pue.: Puebla; S.L.P.: San Luis Potosí; Sin.: Sinaloa; Son: Sonora; Tamps.: Tamaulipas; Tlax.: Tlaxcala; Ver.: Veracruz; Zac.: Zacatecas.

Figure 3 Number of gall morphotypes according to the morphology in the two sections of oaks (Lobatae and Quercus). A) Unilocular without ornaments. B) Unilocular with ornaments. C) Multilocular without ornaments. D) Multilocular with ornamentes.
Only 31.5 % of the 73 oak species (13 red oaks and 10 white oaks) had only a single associated gall morphotype; 24.7 % (12 red and 6 white oaks) had two gall morphotypes, 16.4 % (6 red and 6 white oaks) had three gall morphotypes, and the remainder (27.4 %) had more than 4 morphs. Of the 13 species of red oaks with a single gall morphotype, 9 are endemic to Mexico, and of the 10 white oaks, 6 are endemic.
We found 63 galls with a single larval chamber (unilocular) with smooth surface (without ornaments) (36 in Lobatae and 27 in Quercus), 35 unilocular with ornaments (14 Lobatae and 21 Quercus), 109 multilocular without ornaments (56 Lobatae and 53 Quercus) and 32 multilocular with ornaments (7 Lobatae and 25 Quercus) (Figure 5).
Discussion
In this study, we found a great diversity of galls induced by wasps on oaks as host species (Figure 6). The assumption that gall-inducing wasps from the Cynipidae family exhibit a high degree of specialization to their host plants in the Quercus genus (Weld 1960, Ronquist 1995, Liljeblad & Ronquist 1998, Nieves-Aldrey 2001, Stone et al. 2002, Csóka et al. 2005) should be reviewed based on the results of this study. Most of the oak species studied here had more than one gall morphotype, and in some cases, they had an unexpectedly high number of gall morphotypes throughout their geographical distribution. In particular, three species of white oaks [Q. microphylla (20 morphotypes), Q. resinosa (17) and Q. magnoliifolia (14)] and three red oaks [Q. crassifolia (11), Q. coccolobifolia (11) and Q. castanea (10)] had a considerable number of galls collected from throughout their broad distribution in Mexico. These oak species have a wide range of geographic distribution occurring in different environmental conditions that could represent diverse ecological niches occupied by different gall wasps. It is also necessary to study the degree of host specialization of these galls through their entire geographic range in future studies.

Figure 6 Photos of gall morphotypes in different oak species in Mexico. 1. Quercus conzati (petiole) Jal. 2. Q. sideroxyla (branch) Chih. 3 y 4. Q.magnoliifolia (leaves) Gro. 5. Q. resinosa (catkin) Mich. 6. Q. crassifolia (bud) Chis. 7. Q. ocoteifolia (yema) Oax. 8. Q. uxoris (leaf) Oax. 9. Q. sp. (branch) Ver. 10. Q. castanea (branch) Mich. 11. Q. segoviensis (catkin) Chis. 12. Q. polymorpha (leaf) NL. 13. Q. arizonica (leaf) Coah. 14. Q. segoviensis (leaf) Chis. 15. Q. cupreata (root) NL. 16. Q. obtusata (leaf) Dgo. 17. Q. microphylla (acorn) Gto. 18. Q. laurina (branch) Hgo. 19. Q. gregii (leaf) Dgo. 20. Q. laeta (bud) Mich. 21. Q. rugosa (bud) Jal. 22. Q. conspersa (branch) NL. 23. Q. frutex (leaf) Pue. 24. Q. viminea (bud) Dgo. 25. Q. deserticola (leaf) Sin. Abbreviations of each locality (state) as indicated in Table 1.
In these cases, in particular, these oak species could be considered super hosts, since they have galls throughout their entire distribution and because galls occur over the entire structure of the tree. They also have morphotypes that are specific to certain localities, which may indicate endemism.
In the case of the endemic oak species, many have only a single morphotype, and even similar morphotypes had variations in bark texture or the inside the gall itself. It can therefore be assumed that they are distinct species and probably endemic and a detailed ecological and taxonomic study would need to be conducted for each of them.
By contrast, very unique morphs were discovered, which were only found on the leaves of oaks in the Quercus section, for example, Q. magnoliifolia, Q. resinosa, Q. laeta, Q. deserticola, Q. rugosa and Q. obtusata. According to Pujade-Villar et al. (2010), there is a new genus called Kinseyella, although confirming it would require a thorough review of both the gall and the gall inducers to know whether it is the same genus and a distinct species, or whether it is the same species of gall inducer in all the hosts listed here.
In the case of Q. magnoliifolia and Q. resinosa, recent studies showed that these oak species form hybrids in regions where their geographic distributions overlap (Albarrán-Lara et al. 2010), generating new adaptive zones for the formation of new species of gall insects (Pérez-López et al. 2016). These zones are also suggested by the Q. crassifolia × Q. crassipes complex (Tovar-Sánchez & Oyama 2004) in which three gall morphotypes were exclusively associated with the hybrid individuals found there (Tovar-Sánchez & Oyama 2006). However, this pattern cannot be generalized, since in another hybrid complex formed by Q. affinis × Q. laurina (González-Rodríguez et al. 2004, González-Rodríguez & Oyama 2005, González-Rodríguez et al. 2005), only one gall morphotype was found in each of the species in this study (see Table 1).
The galls induced by cynipids can be classified according to the number of larval chambers (e.g., unilocular and multilocular) and their ornamentation (e.g., without ornamentation and a smooth surface, or with hair and spines). The highest numbers of galls for both sections are multilocular galls without ornamentation, and they are usually associated with the branch of the host. According to Ronquist (1995, 1999) and Rokas et al. (2003), this gall morphotype is the most primitive, suggesting the colonization of the host by cynipids. Confirming this colonization would require a phylogenetic and comparative biological study to locate these species relative to European species and assess their degree of relationship.
There is, however, a peculiar morph in the Lobatae section that is unilocular, globular, with or without ornamentation, and similar to galls from the Amphibolips genus (Nieves et al. 2012). This finding may indicate that there is a particular association between this endemic group of oaks with a genus of cynipid inducers, an interaction so specific that it could be considered a process of speciation that often occurs in the hosts of this section of oaks (Nieves et al. 2012).
The collection effort is a key factor in this type of study. Maldonado-López et al. (2015a, b, 2016) recorded up to 40 morphologically distinct galls that were induced by wasps on Q. castanea throughout its geographic distribution. This finding suggests that studies with both greater temporal and spatial collection intensity, focused on a single species of oak throughout its entire distribution, can produce this type of result.
Conclusions and perspectives
Gall-forming wasps (Hymenoptera: Cynipidae: Cynipini) represent a very diverse group with ecologically interesting characteristics (Nieves-Aldrey 1998, 2001, Ronquist & Liljeblad 2001, Hayward & Stone 2005). In this study, an important effort to add new records to this guild of insects in Mexico was undertaken. However, it is necessary to continue this type of study to determine the total species richness of wasps in Mexico as well as their phylogenetic relationships, and finally, to establish their patterns of diversification. Mexico represents one of the two most important centers of oak diversification in the world, especially for red oaks (section Lobatae). These collection efforts must be accompanied by taxonomic studies that elucidate the discovery of new species (Nieves-Aldrey et al. 2012) and even genera as observed in some of the collections made in this study.
One important and characteristic aspect of the cynipids associated with oaks is that they are cyclically parthenogenetic, which has been a source of confusion during their taxonomic classification. Thus, studies should focus on finding both morphological (Hernández-Soto et al. 2015) and molecular tools to resolve this problem, in addition to elucidating which processes cause this phenomenon.
At the population level, it is important to determine the dispersal distances of individuals and their patterns of colonization in both themselves and their new hosts through either ecological studies (Schönrogge et al. 1994, 1999) or genetic studies using molecular markers (Castillejos-Lemus 2016).











 nueva página del texto (beta)
nueva página del texto (beta)