Angiosperms may have the largest diversity of reproductive systems in nature (Barrett 2010). Even though the great majority of plants are hermaphrodites, dioecious species (plants with only staminate or pistillate flowers) are well represented along angiosperm families (Helibuth 2000). An obvious limitation of populations of unisexual plants is the dependence for reproduction on a pollen vector either biotic or abiotic, making these species particularly sensitive to global changes such as habitat fragmentation. Mexico is a mega diverse country with more than 22,000 species of flowering plants (González-Medrano 2003). Ironically, and tantamount to its biodiversity, Mexico has one of the highest deforestation rates worldwide (Challenger & Dirzo 2009). In particular, the State of Michoacán, where the main part of this study was done, lost more than 800,000 ha. of temperate and tropical forest during the 70´s and 90´s (Bocco et al. 2001) increasing habitat fragmentation and possibly isolation among populations. Fragmentation may reduce population sizes and consequently may affect pollen flow (Rosas et al. 2011), reproductive success and finally the viability of populations (Murren 2002, Leimu et al. 2006). Fragmentation may especially affect biotic pollinated, endemic dioecious species, and it is thus essential to assess their population sizes and sex ratios (Leo et al. 1997, Yu & Lu 2011). A recent review on dioecious species, found that sex-ratio deviates from equality in half of the studied species being male-biased almost twice as common as female-biased (Field et al. 2013). Hypotheses that try to explain male-biased sex ratios on dioecious species, emphasize the divergent allocation to vegetative and reproductive process in males and female plants (Lloyd & Webb 1977, Johnson et al. 2015). Accordingly, a higher reproductive allocation by females (because they need to allocate nutrients to flowers, fruits and seeds, whereas males invest only in flowers) may reduce their vegetative growth, flowering frequency and increase mortality among female plants, increasing male-biased sex ratios (Lloyd & Webb 1977, Espírito-Santo et al. 2003). However, few empirical studies have actually measured total investment in reproduction of males and females (see Avila-Sakar & Romanow 2012, Johnson et al. 2015). In addition, the existence of gender dimorphism in floral traits related to pollinator attraction such as flower size and number of flowers are predicted to evolve through selection on male function rather than on female function in accordance to Bateman´s principle. Therefore, on dioecious species with biotic pollination we expect more and larger flowers on male plants than on female plants (Delph 1996, Vaughton & Ramsey 1998). In extreme cases of floral dimorphism, pollinators may show strong preferences for one sex (usually males), which could even place showy dioecious species at a higher risk of extinction (Vamosi & Otto 2002). In order to estimate the viability of natural populations of the Mexican endemic dioecious animal-pollinated shrub Fuchsia parviflora we estimated their population sizes and sex ratios and evaluated the dimorphism in primary and secondary sexual characters. Specifically, we have the following predictions: 1) Stochasticity in sex ratio will be higher in small populations where a male or female-biased may occur. 2) A positive relationship between population size and number of flowers and fruit production is expected 3) Male individuals will have both, larger floral traits and number of flowers than female plants, in accordance to Batema´s principle.
Materials and methods
Study species and study site. Fuchsia parviflora Lindl. (Onagraceae), is an endemic dioecious, short live, scattered shrub (1.5 - 4 m height) from west-central Mexico (Figure 1) that inhabits the steep canyons and barrancas of the broad-leaved evergreen forest understory occurring between 1,500-2,500 m (Breedlove 1969). The flowers are red with four sepals and four petals forming a cylindrical hypanthium. Staminate flowers have eight stamens and are larger than pistillate flowers (see results), which have four stigmatic lobes and a developed ovary. Bumblebees and hummingbirds have been reported as pollinators (Breedlove 1969). The fruits are small berries, black when mature, dispersed by birds, and each fruit contains 10-20 seeds (Breedlove 1969). Some individuals obtained from seeds growing at the greenhouse, began to flower after one year of being planted (E. Cuevas unpublished data) and no clonal reproduction has been observed.
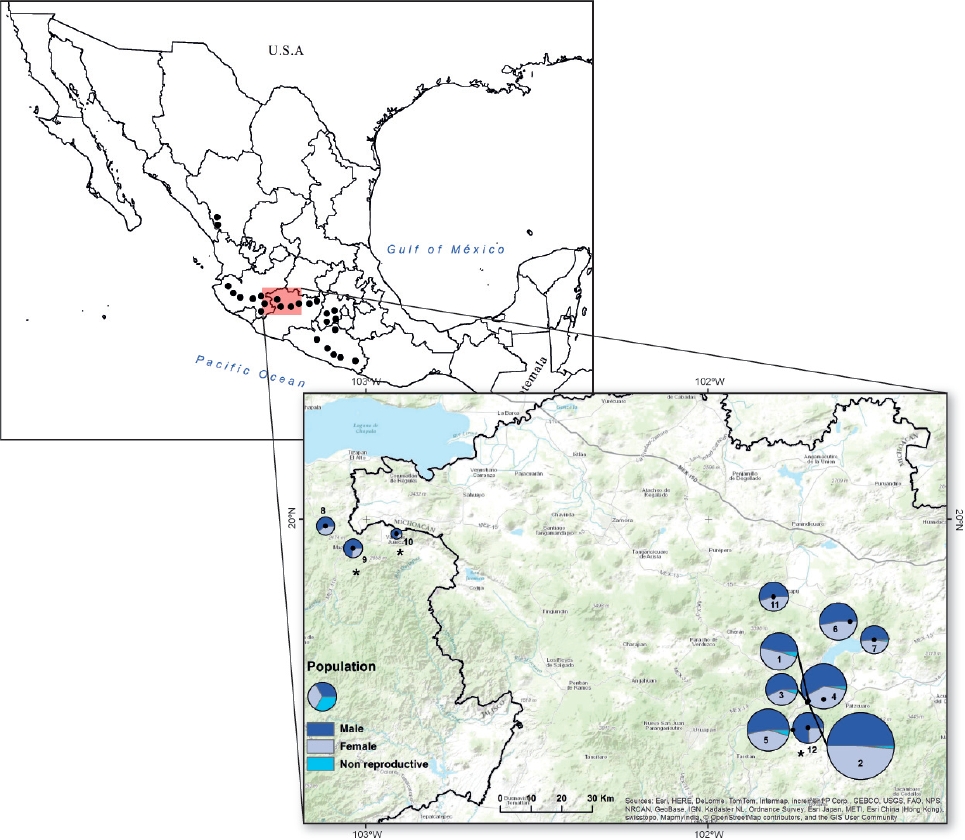
Figure 1 The distribution of Fuchsia parviflora (black dots) in accordance to Breedlove (1969). The location of the 12 studied populations, the number inside or aside each pie chart indicates the population number. The size of each pie chart is proportional to the population size (i.e. population 2 = 544 individuals and population 10 =14 individuals). Non-reproductive individuals are only shown in the first seven populations. An overall male-biased was found and the asterisk indicates populations where the number of male plants was significantly higher (Probability of success: P.S) = 0.72, P = 0.01, for population 9; P.S = 0.78, P = 0.05 for population 10; and P.S = 0.75, P < 0.001, for population 12.
Sex ratio. This study was carried out during 2012 and 2013 in natural populations of F. parviflora in the states of Michoacán and Jalisco, Mexico (Figure 1). We used herbarium specimens from the three most important herbaria of Mexico (MEXU, IEB and ENCB) as a source of candidate locations for natural populations. We visited the candidate locations during the flowering peak (August-September), trying to cover the distributional range of the species; however, in some cases it was not possible to find the plants after exhaustive searches (i.e., Sinaloa and Estado de Mexico) and in other cases the sites were not visited for lack of security (i.e., Guerrero). Distances among populations range between 3 and 185 km. Once we located a population, we estimated population size and sex ratio. In populations along the rivers (1, 2, 3, 8 and 11), we checked both sides of the river for 1-2 km depending on the particular conditions in order to locate all identified individuals. We stop plant searching after walking 200 m without finding a plant. Populations in some cases were delimited by lakes (population 5) or by small villages (6 and 12). In other cases, we established two quadrats (100 × 100 m) and registered all individuals inside (population 3). Operational sex ratio was estimated from reproductive individuals, by examining the morphology of two or three flowers per plant, and was estimated as: males/(males + females), so that low values of 0.5 indicate female bias and higher values of 0.5 indicate male bias. In total, we estimated sex ratio in twelve populations. Five populations were measured only once (populations 8-12) in 2012, and the rest of the populations were measured three times in 2013 (populations 1-7). We performed three censuses during the same flowering season in 2013 (July, August and September), to know whether flowering sex ratio changed along the year within each population. No differences were found among censuses in any population (chi-square test for homogeneity of proportions; in all cases df = 2 and P > 0.05) and thus data from the third census was chosen for analysis, given that sample size was highest. During all censuses for populations 1-7 we also counted the number of immature individuals to have an estimate of the number of non-reproductive individuals in each population.
Gender differences in plant and floral traits. During the flowering peak (August-September) in each of the 12 populations, plant height and number of flowers was estimated in 20 individuals / gender / population except for populations 9, 10 and 12 where a lower number of individuals was found. In addition, floral tube and corolla diameter (three flowers per plant / gender) were estimated in 20 individuals per gender in the first seven populations. Floral longevity was estimated only in one population using 12 plants per gender and three flowers per plant. Each flower was tagged the day it opened and checked every day until flowers wilted. Finally, the number of fruits per plant (20 female plants / population) was estimated in November 2013 in the first seven populations.
Data analyses. Sex ratio was first analyzed for all twelve populations pooled, and then for each population independently. We performed binomial tests for all populations (pooled data) and then for each population, to determine whether sex ratio (proportion of male: female plants) was significantly different from 0.5. We tested for differences in sex ratio among populations using a generalized linear model, assuming a binomial distribution. The relationship between population size, (log-transformed) and: a) relative male frequency, b) number of flowers per plant and c) fruit production was explored by means of linear correlations. To compare the number of flowers between genders and populations, a generalized linear mixed model was employed using gender as fixed factor and population as a random effect, assuming a Poisson distribution, and correcting for over-dispersion. Comparisons of plant height, floral tube and corolla diameter (log-transformed) between morphs were performed using linear mixed models, using plant gender as a fixed effect and population as a random effect. For the number of flowers, data from the second census (flowering peak) was used. The mean values of floral tube and corolla diameter per plant were used for the analyses. All statistical analyses were performed in R 2.9.2 (R Core Development Team).
Results
Sex ratio. The number of flowering individuals per population varied from 14 to 544, although most populations showed between 100 and 200 individuals (Figure 1) and the number of immature individuals ranged between 1 and 10 % (Figure 1). Overall 1,864 flowering individuals were found in the twelve populations, and the number of male plants (1,038) was significantly greater than that of females (826; overall sex ratio = 0.6, Probability of success = 0.52; P < 0.03; Figure 1). In three populations the number of male plants was significantly higher than that of females according to exact binomial tests, after Bonferroni correction (Figure 1). According to the GLM, sex ratio was significantly different among populations (Deviance = 63.7, df = 11, P < 0.001). A negative significant correlation was found between population size and relative male frequency (r2 = -0.45, P = 0.02, Figure 2), however, no relationship was found between population size and fruit production (P = 0.35, average fruit production per plant 58 ± 8.8) or population size and male (P = 0.13) or female (P = 0.39) flower display.
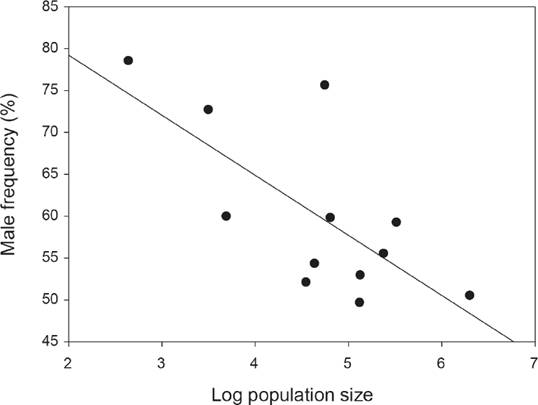
Figure 2 A significant negative relationship between population size (log-transformed) and male frequency for the 12 studied populations of Fuchsia parviflora.
Gender differences in plant and floral traits. The height of mature plants (133.14 cm ± 2.22) was different among populations (F11,1833 = 23.8, P < 0.0001) but did not differ between genders within populations (F12,1833 = 0.24, P = 0.99; Figure 3A). The average number of flowers per plant (20.28 ± 0.9) differed among populations (Deviance = 15,304, df = 11, P < 0.0001) and also the interaction between genders within population (Deviance = 1,530, df = 1, P < 0.0001). In most populations, male plants produced more flowers than females (Figure 3A); however, this difference was significant only in some populations (Figure 3B). Average staminate flower longevity (3.8 days ± 0.16) did not differ from that of pistillate flowers (3.6 ± 0.18; F1, 48 = 0.94, P = 0.33). In the seven studied populations, staminate flowers had wider corolla diameters (5 to 7.6 mm) than pistillate flowers (corolla diameter 4.7 to 6.4; F6,823 = 44.87, P < 0.001; Figure 3C). Staminate flowers also had consistently longer floral tubes (range 7.7 to 11.5 mm), than pistillate flowers (range 3.9 to 6.1; F7,823 = 118.48, P < 0.001; Figure 3D).
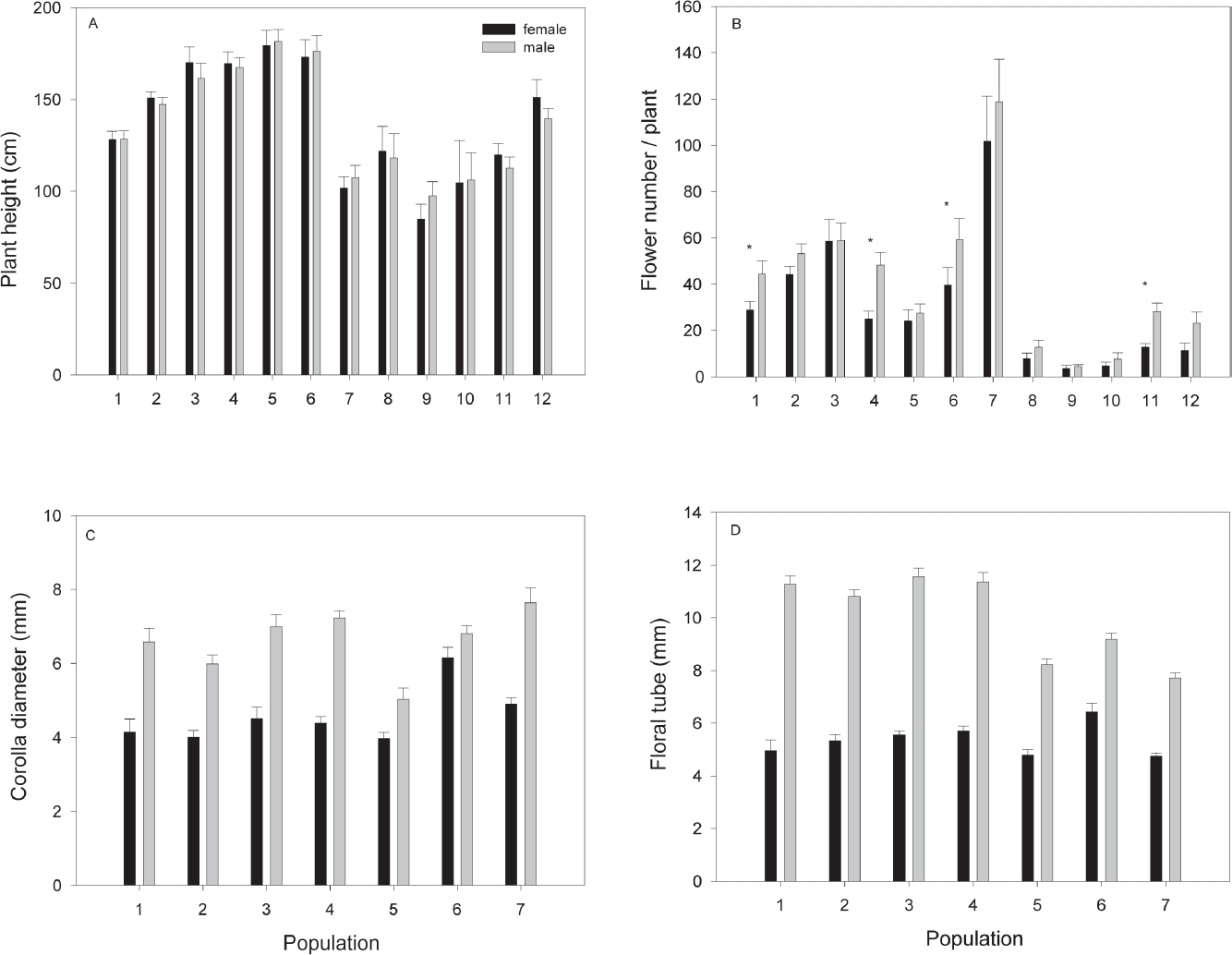
Figure 3 Means (± 1 SE) for plant characters and floral traits by population and gender in Fuchsia parviflora. A) Plant height. B) Flower display, the asterisk shows populations where males had significantly more flowers than female plants, (P < 0.05). In population two, the difference was near significance (P = 0.06). C) Corolla diameter. D) Average floral tubes length. In all populations staminate flowers had wider corollas diameter and longer floral tubes than pistillate flowers (P < 0.001, for all populations).
Discussion
Sex ratio. Recent reviews of sex ratio in dioecious species report that male-biased sex ratios are almost twice as frequent as female-biased sex ratios (Sinclair et al. 2012, Field et al. 2013). In particular, male-biased sex ratio was associated to fleshy fruits, biotic pollination and seed dispersal (Sinclair et al. 2012). Fuchsia parviflora, shows those characteristics and a significant overall male-biased sex ratio was found (P < 0.03). Nevertheless, only three populations had significantly more males and two of those populations had also the lowest number of flowering individuals (Figure 1). Therefore, in those cases male-bias sex ratio is probably related to population size, as the negative association between male frequency and population size suggests (Figure 2). However, if biased sex ratio was only due to stochastic events, we expect to find male and female-biased sex ratios. The fact that we only found male-biased sex ratio suggests that other factors may favor males versus female plants.
The relatively low number of immature individuals found in the studied populations (Figure 1) suggests a low recruitment of individuals or may also be the consequence of a short juvenile stage, as suggested by some individuals that began flowering during their first year of growth. The fact that sex ratio did not vary along the flowering season gives us more confidence on our sex ratio estimations. Few studies have reported the effect of population size on sex ratios (Soldaat et al. 1997, Somanathan & Borges 2000, Yu & Lu 2011). If most individuals in a small population are males this may reduce the chances of fruit production and therefore the viability of populations. On the contrary, male-biase could also result in abundance of pollen competition and thus greater genetic quality of offspring, lower probability of fruit and seed abortion and therefore greater fruit production in the available females, as suggested by the relatively high fruit production found on the evaluated populations (1-7). Unfortunately, we could not estimate fruit production in some of the smaller populations. Yu & Lu (2011) found that small populations of the dioecious Pistacia chinensis had a male-biased sex ratio, compared with larger, non-fragmented populations. They found that male-biased populations were established in soils with low nitrogen availability and they concluded that fragmentation affects soil nitrogen conditions at the microhabitat scale. We did not measure micro-environmental conditions in our populations, but further investigation on aspects such as soil nitrogen and water content could clarify whether they are relevant in determining sex ratio in F. parviflora. Data of sex ratios from seeds planted in a common garden from five natural populations of F. parviflora, showed no evidence of biased sex ratio at flowering, suggesting that abiotic factors, such as soil nutrients or biotic interactors as herbivores (Avila-Sakar & Romanow 2012) may affect male and female plants differently.
Flower number may have important consequences for pollinator attraction (Barrett & Hough 2013). In Fuchsia parviflora, flower number in males was barely higher than that of female plants in most populations evaluated, even though floral longevity did not differ between genders. This result is in accordance with other studies in animal-pollinated dioecious plants (Delph et al. 2005); however, the higher number of flowers in males was only significant in three populations (Figure 3B). In other species, male plants produced many times more flowers than female plants (Bond & Maze 1999, Delph et al. 2002). Consistently with the study by Delph et al. (1996), staminate flowers were significantly longer and wider in all evaluated populations. The same pattern has been found in other dimorphic species of Fuchsia where pistillate flowers are smaller (Godley 1955 in F. excorticata and F. perscande, Arroyo & Raven 1975 in F. microphylla and F. thymifolia, Atsatt & Rundel 1982 in F. lycioides, González et al. 2016. in F. obconica). The floral differences found between sexes may contribute to pollinators preferentially visiting staminate flowers and may potentially promote pollen limitation. However, so far no evidence of pollen limitation has been found in natural populations (González et al. 2016). Furthermore, no asexual seed production was found in flowers excluded from pollinators (González et al. 2016), which highlights the dependence on pollinators for seed production in this species. In addition, pistillate flowers of F. parviflora were preferentially visited by insects (bees and wasp) and staminate flowers by hummingbirds. However, despite these differences in preferences visits between genders, fruit production was apparently not affected (after comparing natural vs. pollen supplemented flowers). This visitation pattern may be a consequence of the differences in flower size between genders, given that nectar production and concentration did not differ between male and pistillate flowers (González et al. 2016).
Dioecious species with biotic pollination are particularly sensitive to stochastic events which may increase biases in sex ratios resulting in a low fruit production. Furthermore, gender differences in flower size and number may also decrease fruit production. Although some populations in this study showed a slight male biases sex ratio, male flowers were larger and in some populations male floral display was higher, fruit production seem not to be affected on the evaluated populations.











 nueva página del texto (beta)
nueva página del texto (beta)


