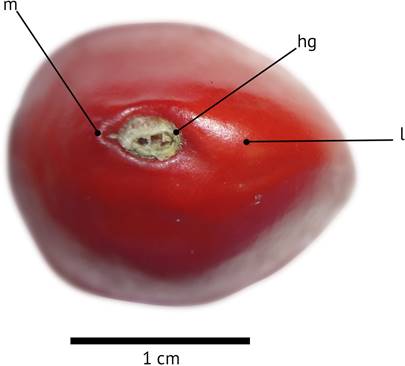
Tropical forests have undergone a high degree of deforestation (Huerta et al. 2014) and many of their plant species are currently in various categories of risk. One such example is Ormosia macrocalyx Ducke (Fabaceae: Papilionoideae), a tree species native to the tropical zones of Latin America (Rudd 1968a), which is classified as endangered in Mexico (SEMARNAT 2010). The species is found in high and medium evergreen forest, as well as in low flooded forest and as secondary vegetation associated with ecosystems featuring different tree species (Ochoa-Gaona et al. 2008b, Pérez-Hernández et al. 2011). Individuals of this species can reach 50 m in height and between 35 and 60 cm in diameter (Ochoa-Gaona et al. 2008b). Timber from this species is used for furniture making, construction, railway ties and canoes (Rudd 1968a, Román et al. 2012). The trees provide shade for cattle and the seeds are used for artisanal jewelry (Ochoa-Gaona et al. 2008a, Baigts 2009). Their fruits are 10 cm long × 3 cm wide, brown in color and are coriaceous dehiscent pods containing 1-6 seeds (normally 2 or 3) (Rudd 1968b). The seeds are 10-13 mm long, 10 mm wide, and 7-8 mm thick and bright red in color (Rudd 1968b, Ochoa-Gaona et al. 2008a). Ormosia macrocalyx plays an important role in CO2 uptake by the forest biomass, and the effect of carbon capture by forests provides an important opportunity in land management to increase the capacity of the soil to respond to disturbances that arise from the intense pressures of both land use and climate change (Banwart et al. 2015). Cernusak et al. (2011) found that O. macrocalyx can reach high nodulation rates (48.9 mg·g-1) on unfertilized soils and provided evidence for a positive relationship between N2 fixation and nodule mass ratio, inferring that this tree has the highest rate of N2 fixation relative to other leguminous species. They estimated the proportion of the plant N acquired by N2 fixation to be about 84 %.
Leguminous seeds present a distinctive physical dormancy due to their hard and impermeable seed coat with sclereid layers (Baskin & Baskin 2004). Breaking of this dormant condition involves rupturing the cover using hot water, chemical or mechanical scarification. For instance, Ormosia arborea (Vell.) Harms requires immersion for 15 min in sulfuric acid in order to obtain 91 % germination (da Silva et al. 2014), while Ormosia nitida Vogel requires 10 min in the acid to achieve 96.25 % or mechanical scarification to achieve 93.75 % germination (Lopes et al. 2006).
Studies of the reproductive physiology and propagation of O. macrocalyx are scarce in Mexico; however, an evaluation of germination in the field and under nursery conditions produced a mean germination of seeds of 75 % after 100 days (Pérez-Hernández et al. 2011). Similar data were recorded by Foster & Delay (1998) for the same species under nursery conditions; the process was improved when the seeds were scarified in the hilar region, achieving 100 % germination by the tenth day. Seeds with no pregerminative treatment presented 41.1 % germination (Foster 2008).
It is notable that seeds remain adhered to the fruit for a long period of time, possibly in a similar manner to Ormosia arborea, the seeds of which can remain attached to the mother plant for up to two years; studies conducted on some Papilionoideae demonstrate the genetic regulation of fruit abscission (Couzigou et al. 2016). Foster & Delay (1998) state that the mimetic seeds of O. macrocalyx deceive birds of the family Psittacidae in order to achieve dispersion, which often takes place at times when succulent or “fleshy” fruits are scarce; These authors also state that, while the most important dispersion in this species may be conducted by birds and rodents, these dispersers appear be insufficient to maintain O. macrocalyx populations alone. The fact that seeds remain attached to the fruit on the tree for a long time represents an obstacle to the successful propagation of the tree, since germinative response could be variable and dependent upon seed age. Given the low seed rain, an adequate seed bank cannot be created; it is known that the most appropriate strategy for the restoration of degraded landscapes is recolonization of the native flora through seed banks (Abiyu et al. 2016), which are defined as the stock of mature and viable seeds deposited in the soil surface. Ideally, this seed bank should be abundant in order to obtain a high production of seedlings and to guarantee the permanence of the species, and it is also a necessary mechanism for secondary succession (Martins & Engel 2007). Another characteristic is that the seeds of O. macrocalyx themselves seem to present physical dormancy (Sautu et al. 2007). The establishment of native species in degraded areas is a valuable option to counteract the problem of biodiversity loss. These species may allow soil recovery and establishment of a native flora and fauna due to the fact that they are well adapted to the environment. Moreover, appropriate management can bring economic and ecological benefits in the short, medium and long term (Moya-Roque & Tenorio-Monge 2013, Casermeiro et al. 2015). Seeds of native trees are readily available to small producers, lowering production costs and avoiding dependence on distributors (Vázquez-Yanez et al. 1999, Casermeiro et al. 2015). However, limitations to their use include a lack of technical information about seed biology and nursery management (Britos-Paniagua et al. 2013). Studies of the feasibility of conserving genetic diversity and seed viability of native species are therefore essential to meet the demand for seedlings for commercial purposes, reforestation or recovery (Dresch et al. 2014).
In general terms, both fruit and seed must be mature: dryness is indicative of maturation and in this condition they respond favourably to germination (Srimathi et al. 2013; Silva et al. 2015); in the dehiscent fruits, this is followed by opening, which is an evolutionary characteristic that allows the dispersion of the seeds and occurs when these has dehydrated completely (McAtee et al. 2013). In legumes, the process is accompanied by lignification of the fruit covers in order to avoid damaging the seeds with excess moisture and to allow them to maintain dormancy until environmental conditions are favorable (Smýkal et al. 2014). Due to the high ecological and economic importance of O. macrocalyx, this study was conducted to further the knowledge relating to its germinative behavior, as well as to generate information that can contribute to reforestation, restoration or conservation programs involving this tree, especially in view of the scarcity of studies of its seed physiology to date.
The study tested the two following hypotheses: 1) The stages of dehiscence of fruits of O. macrocalyx will influence the speed and percentage of germination of its seeds; and 2) pregerminative treatments will improve the germination process of the seeds stored under refrigeration.
Material and methods
Plant material and trial location. This study was conducted through two independent experiments: 1) an evaluation of the relationship between fruit maturity and seed germination rate, and 2) an assessment of the germinative response of stored seeds under refrigeration for 17 months subjected to four treatments, comprising three pregerminative treatments and an untreated control. Both experiments used fruits and seeds that had been harvested in the experimental field of the División Académica de Ciencias Biológicas at the Universidad Juárez Autónoma de Tabasco (17°59’ N; 92°58 W), in Villahermosa, Tabasco, Mexico. The climate at this site is warm wet (Am), according to García (1981), with mean annual precipitation of 1,954 mm.
Experiment 1: Effect of fruit maturity. Seasonal fruits were harvested at three states of maturity: closed, semi-closed (3 mm open) and open. In all cases, the fruit was dry with a dark brown-grey appearance and the seeds were immediately sown in polystyrene germination trays with 112 individual cells, each of 8 mL in volume containing peat moss as a substrate. A seed was considered to have germinated when the storage cotyledons emerged from the surface of the substrate. The test duration was 30 days.
Experiment 2: Effect of pregerminative treatments. Seeds were taken from open fruits and refrigerated (4 °C) for 17 months in brown paper bags placed within a sealed container. Seeds were sown in 15 cm Petri dishes, using wet paper towel as a substrate. Seed treatments consisted of: control; soaking in water for 24 h; mechanical scarification with sandpaper (sanding); mechanical scarification with sandpaper + application of GA3 (1 % gibberellic acid) at sowing. In all cases, scarification was applied in the chalazal region in order to avoid damaging the embryo. The test duration was 60 days
Germination. Germination was classified following Lobo et al. (2014). Both experiments (1 and 2) were established under laboratory conditions (28.4 ± 2.1 to 26.2 ± 1.6 °C max-min temperatures), under artificial fluorescent light (5 μmol mm-2 s-1), 6 hours of light/18 hours of darkness. The variables evaluated were germination percentage (GP); germination velocity (GV): accumulated curve of GP vs. time; germination initiation (GI): number of days required for the germination process to initiate; germination rate (GR): ∑(GP/D), where GP = germination percentage on each day of evaluation, and D = day of evaluation; corrected germination rate (CGR): 100 × GR/FGP, where FGP corresponds to the final GP (Dewir et al. 2011) and T50: number of days at which the seeds attained 50 % of their final GP (Kodde et al. 2012).
Statistical analysis. Experiment 1 was conducted using a completely randomized design consisting of five replicates, each of 20 seeds, for each of the three fruit maturity states (total seed number 300). Experiment 2 comprised four treatments tested in a completely randomized design with 4 replicates, each of 25 seeds (total seed number 400). The results were evaluated through ANOVA and a Tukey test, using the software Statistix v.8 (Analytical Software, Tallahassee, FL, USA). Percentage data were transformed following the arcsine function prior to analysis, in order to normalize data distribution.
Results
Experiment 1. Germination in O. macrocalyx was considered to be epigeal phanerocotylar with reserve cotyledons, in the sense described by De Voguel (1980). Germination initiated with elevation of the reserve cotyledons, protected by the seed coat. The coat was subsequently eliminated and the epicotyl emerged with a pair of bright green, ovate, entire edged, foliate and opposite paracotyledons, with a round base and a largely acuminate apex (Figure 1).
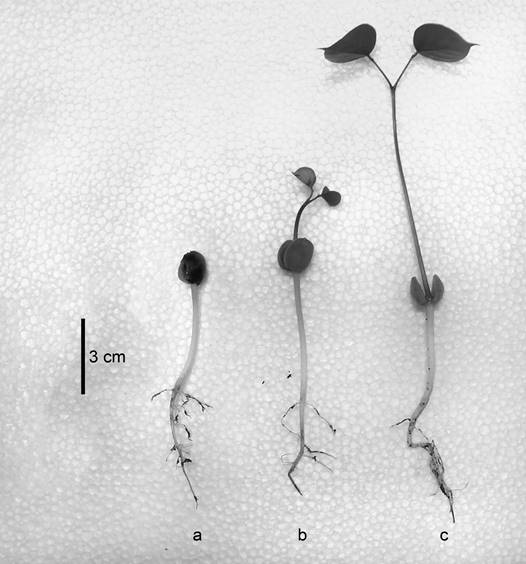
Figure 1 Seedlings of Ormosia macrocalyx, showing the radicle, hypocotyl and reserve cotyledons (a), epicotyl and paracotyledons (b, c)
Germination percentage was ≥ 97 % in all three fruit maturity treatments (mean of 99 %), with no significant differences (Tukey, P > 0.05) among treatments (F2,12 = 2.54) (Table 1). No differences were detected for the number of days required for GI (F2,12 = 2.43), since the seeds initiated germination at practically the same time (10-11 days after sowing). However, GR and CGR, which reflect seed vigor during germination, were lower (Tukey, P ≤ 0.05) in the seeds of the open fruits (F2,12 = 23.1; F2,12 = 27.1). The same occurred with T50, since seeds from the open fruit took almost four days longer to reach T50, and presented the slowest germination (lower GP), reaching the maximum value at day 26, ten days later than the seeds from closed and semi-open fruits (F2,12 = 7.44) (Figure 2).
Table 1 Effect of fruit maturity on germination of Ormosia macrocalyx seeds. GP: germination percentage; GI: germination initiation; GR: germination rate; CGR: corrected germination rate; T50: time to attain 50 % of the final GP.
| Treatments | GP | GI (days) |
GR (% day-1) |
CGR | T50 (days) |
|---|---|---|---|---|---|
| Seeds from closed fruit | 100 ± 0.0 a | 10.2 ± 0.4 a | 8.3 ± 0.1 a | 8.3 ± 0.1 a | 12.0 ± 0.0 a |
| Seeds from semi-open fruit | 97.0 ± 4.4 a | 10.6 ± 0.5 a | 7.8 ± 0.1 a | 8.0 ± 0.1 a | 12.0 ± 0.0 a |
| Seeds from open fruit | 100 ± 0.0 a | 11.4 ± 1.3 a | 6.0 ± 0.8 b | 6.0 ± 0.6 b | 15.8 ± 3.1 b |
Within each column, means followed by different letters differ statistically according to Tukey test (P ≤ 0.05)
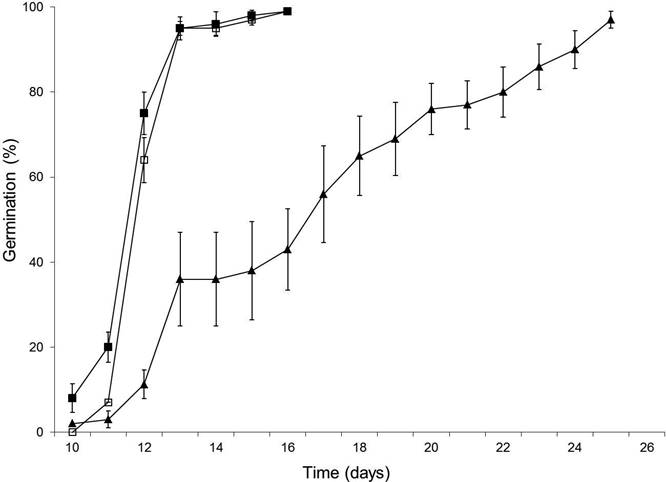
Figure 2 Germination of seeds as a function of time in Ormosia macrocalyx, according to fruit dehiscence: closed fruits (black squares), semi-open fruits (empty squares) and open fruits (triangles). Vertical bars represent one standard error (where these are not displayed it means their length is less than the marker size)
Experiment 2. The highest percentages of germination were obtained by the mechanical scarification treatments or by the combination of this with gibberellic acid, presenting values of 68.0 and 61.3 %, respectively (Table 2). This indicates that, following 17 months of storage, seeds of O. macrocalyx showed a decrease in GP compared to seeds of the same type from fruit that were not stored (GP ≥ 97 %).
Table 2 Effect of pregerminative treatments on germination of Ormosia macrocalyx seeds. GP: germination percentage; GI: germination initiation; GR: germination rate; CGR: corrected germination rate T50: time to attain 50 % of the final GP; GA3 (gibberellic acid).
| Treatments | GP | GI (days) |
GR (% day-1) |
CGR | T50 (days) |
|---|---|---|---|---|---|
| Control | 20.0 ± 4.0 b | 26.0 ± 2.4 a | 0.6 ± 0.1 b | 3.0 ± 0.1b | 28.0 ± 1.7 a |
| Mechanical scarification (sanding) | 68.0 ± 14.4 a | 12.0 ± 0.0 b | 4.5 ± 1.0 a | 6.6 ± 0.2 a | 14.0 ± 3.4 b |
| Soaking in water for 24 h | 12.0 ± 4.0 b | 28.6 ± 3.7 a | 0.3 ± 0.0 b | 2.7 ± 0.3 b | 29.6 ± 6.3 a |
| Mechanical scarification (sanding) + GA3 (at 1 %) | 61.0 ± 12.6 a | 12.0 ± 0.0 b | 4.5 ± 1.0 a | 7.5 ± 0.2 a | 12.0 ± 0.0 b |
Within each column, means followed by different letters differ statistically according to a Tukey test (P ≤ 0.05)
The treatments that undergo mechanical scarification, either alone or combined with gibberellic acid, showed the highest germination rates and the shortest time required for both GI and T50 (F3,9 = 38.1) (Figure 3). Both treatments differed significantly (Tukey, P ≤ 0.05) from the control and from the seeds that had been soaked for 24 hours (F3,9 = 22.7) (Table 2).
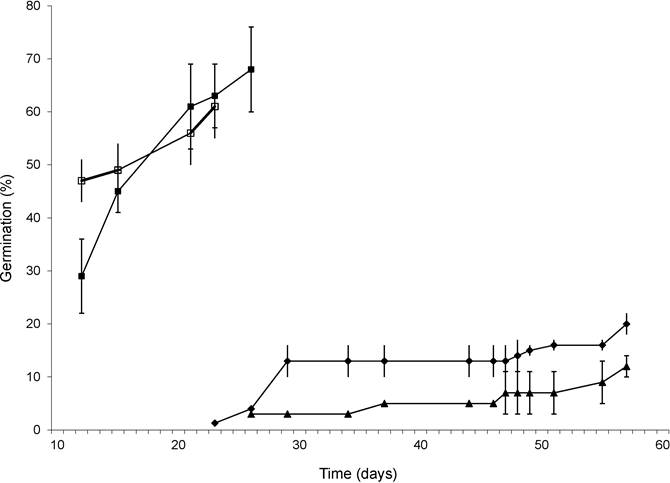
Figure 3 Germination of seeds as a function of time in Ormosia macrocalyx under three pregerminative seed treatments plus an untreated control: water soaking for 24 h (black triangles), mechanical scarification (black squares) and mechanical scarification + GA3 at 1 % (empty squares), control (rhombus). Vertical bars represent one standard error (where these are not displayed it means their length is less than the marker size)
Germination rate (GR) tended to show low values compared to those found in experiment 1. While GR corrected in relation to the percentage of germination (CGR) increased notably with respect to the initial GR (in non-stored seeds), the overall values still remained moderately low. No differences were found between the control and the water soaking treatment in any of the variables (Table 2).
Discussion
Experiment 1. Ormosia macrocalyx shows epigeal phanerocotylar germination, which is similar to other species of the same genus: Ormosia fastigiata Tul. and Ormosia discolor Benth, but differs from that of O. arborea, which presents semi-hypogeal germination (Lobo et al. 2014). It has been demonstrated that epigeal seedlings produce biomass more rapidly than hypogeal seedlings (Ibarra-Manríquez et al. 2001). Furthermore, O. macrocalyx possesses two types of cotyledons, known as reserve and foliar (paracotyledons) cotyledons, with the former being the main source of energy reserves and the latter responsible for photosynthesis. These results show the great capacity of O. macrocalyx seeds for environmental adaptation, compared to those of other tropical species (Soriano et al. 2013). However, other factors such as seed predation and soil humidity (Pérez-Hernández et al. 2011) play an important role in seedling survival in the field.
Recently formed O. macrocalyx seeds of closed fruits (T1) did not require pregerminative treatments and showed high germination values, as described by Bewley et al. (2013) in reference to the seeds contained in recently formed fruits, indicating that they reach physiological maturity followed by an increase in their ability to rapidly germinate, in addition to the fact that the seed coat has still not lignified. Pérez-Hernández et al. (2011) found similar results: field and nursery germination was 75 and 87 %, respectively. However, due to the presence of a hard seed coat, the seeds under physical dormancy have germination rates below 1 % (Foster & Delay 1998) or do not germinate at all without a pregerminative treatment (Baskin & Baskin 2004). Norsworthy & Oliveira (2009) state that leguminous seeds can be permeable when the macrosclereids found in the hilar region become separated as a result of the effect of high temperatures. In the case of O. macrocalyx, this region is characterized by the fractured hilar slit in recently formed seeds, and by the possible presentation of a lens (Figure 4). These structures have been shown to be important zones for water imbibition in legume seeds (De Paula et al. 2012). High temperatures also allow degradation of lipids in the seed coat, facilitating water permeability (Zeng et al. 2005), a process that is further assisted by the thinner tissue present in this seed region (Varela & Albornoz 2013).
Germination rates (GR) were higher or similar to those reported in O. nitida and Bauhinia cheilantha (Bong) Steud (Lopes et al. 2006, Arruda et al. 2015), the values of which were 0.54 and 3.53 % day-1, respectively. It should be stressed that these rates are inversely dependent on the time elapsed from sowing to initiation of germination. In our study, therefore, germination commenced ten days after sowing in all three treatments (GI, Table 1) and the corresponding germination rates were low: 8.3 % day-1, 7.8 % day-1 and 6.0 % day-1 for closed, semi-open and open fruit, respectively, but similar to those obtained with the yellowish-brown fruits of Dalbergia cochinchinensis Pierre (8.17%) (Hung 2003). In contrast, Arruda et al. (2015) obtained high germination rates in Acacia polyphylla DC. (38.43 % day-1) and Anadenanthera colubrina (Vell.) Brenan (31.19 % day-1) (both Fabaceae). Seeds of O. macrocalyx, tested with no pregerminative treatments, germinated after a 10 - 11 day period (Table 1), which represents a relatively short period for this species, although it is similar to that reported by different authors for O. macrocalyx (Sautu et al. 2007, Pérez-Hernández et al. 2011, Román et al. 2012). Different responses have been observed depending on particular species. In our experiment, the seeds were tested without pregerminative treatment, thus the 10-11 day period required for initiation of germination (Table 1) represents a relatively short period for O. macrocalyx.
Statistical differences were found among treatments for germination rates and T50, with the lowest values recorded in seeds from open fruits (Table 1). The difference in germination time between this type of seed and other agricultural seeds should be noted. For example, the shortest T50 of this species (12.0 days) was still much longer than that of seeds of cultivated leguminous plants, which can achieve 50 % germination in less than 3 days with no pregerminative treatment, e.g., Glycine max (L.) Merr. (Sadeghi et al. 2011) and Phaseolus vulgaris L. (Bayuelo-Jimenez et al. 2002). Ormosia macrocalyx seeds obtained from completely open fruits that were exposed to the environment, presented an important delay in germination initiation (almost 4 days) relative to those from closed or semi-open fruits. Taking into consideration the notion proposed by Dam et al. (2009) that fruit and seeds pass through phases of histological differentiation, seed filling and desiccation, our results suggest that dry but still closed O. macrocalyx fruits contain seeds with mature and viable embryos. Once the fruits are open (dehiscence), their seeds become subject to desiccation, which promotes dispersion, with the consequent development of seed dormancy (Silva et al. 2014). Seed dormancy is a natural strategy that maximizes the probability of seedling establishment, limits opportunities for germination in the short term and prevents germination when conditions are not favorable (Moïse et al. 2005, Baskin et al. 2006, van Klinken & Goulier 2013). Anatomically, the cells of the seed cover lignify in order to reduce their permeability (Hudson et al. 2015). The condition of permanence of the seeds in the fruit is considered to represent an aerial seed bank, such as that described in Parkia pendula Benth. ex Walp. (Fabaceae), some of the seeds are dispersed at maturity while the rest remain on the tree, attached to the pendulous pods (Orozco-Segovia & Sánchez-Coronado 2009). This serves to disperse the seeds over a period of time, thus reducing the impact of predation; as well as providing a period of permanence in the soil until the environmental conditions are favorable for germination (De Oliveira et al. 2006).
Experiment 2. When applying mechanical scarification treatments to previously stored seeds, we found a GP of 68 % compared to 20 % in the untreated control. In other studies, seed scarification produced 100 % germination in O. macrocalyx (Foster & Delay 1998), 95 % in O. nitida (Lopes et al. 2006) and more than 90 % in O. arborea (Marques et al. 2004, Gonçalves et al. 2011). However, other authors obtained germination of just 22.75 % for O. nitida (Basqueira et al. 2011); this low response was attributed to the presence of phenolic compounds in the seed. Similarly, Mews et al. (2012) found that mechanically scarified seeds of Ormosia paraensis Ducke, presented only 28.4 % germination and suggested that the abrasion may have affected the viability of the embryo.
Even though the corrected germination rate (CGR), which is adjusted in relation to the percentage of germination, increased markedly with respect to the original germination rate (GR), the overall values still remained moderately low: 4.53 and 4.59 % day-1, which corresponds to the best treatments in this experiment (Table 2). Beikmohammadi et al. (2012) report high germination rates (13.2 % day-1) in the leguminous plant Colutea buhsei (Boiss.) Shap.
With regard to GI, Mews (2012) found that O. paraensis only required 28 days for initiation of germination in scarified seeds, compared to 35 days in the control. In addition, shorter germination times were recorded in scarified O. nitida seeds (7.8 days) (Lopes et al. 2006) and O. arborea (11 days) (Marques et al. 2004). Those results indicate that scarification is required to fracture the sclereid cells of the seed coats of Ormosia species in order to initiate germination, a common treatment for leguminous seeds that present deep dormancy due to the presence of a hard coating (Mantoan et al. 2012, Freitas et al. 2013, Silva et al., 2015). In the case of the control and the water soaking treatment, GI was delayed and initiation time was much longer than in the seeds with scarification treatments (Table 2). One possible explanation for this delay is that water imbibition, gaseous exchange and radicle emergence will all be hampered, as described by Baskin & Baskin (2004), Finch-Savage & Leubner-Metzger (2006). This response suggests the notion that dry storage promotes seed dormancy (Baskin et al. 2006), which represents a beneficial strategy for avoiding the initiation of germination under unsuitably stressful dry weather conditions (Gremer & Sala 2013).
The combination of mechanical scarification and gibberellic acid application as a pregerminative treatment produces a response in O. macrocalyx seeds that is similar to that produced by the use of scarification alone. Linkies & Leubner-Metzger (2012) and Zhang et al. (2012) described scarification as rupturing the seed coat and allowing chemical exchanges to occur, triggering the germinative process, while the gibberellic acid breaks the physiological dormancy by lengthening the embryonic axis. A low germination rate (< 23 % day-1) was observed in O. arborea seeds subjected to a concentration of GA3 ≤ 20 mg·L-1 (Curiel & Moraes 2011), while a germination similar to that found in this study (66.2 %) was obtained in C. buhsei, when treatments of concentrated sulfuric acid and gibberellic acid (100 mg·L-1) were combined (Beikmohammadi et al. 2012).
In our experiment, the highest germination percentage reached by the stored and scarified seeds (68 % GP) did not reach the highest values obtained in the first experiment (nearly 100 % GP), suggesting a loss of germinative vigor associated with the long-term seed storage. Hong & Ellis (1996) and Ferreira & de Oliveira-Gentil (2003) have stated that a loss in seed viability will occur with storage under refrigeration, possibly caused by the low temperatures and embryo desiccation, as is the case in intermediate seeds. Similar results were found in Coccoloba uvifera L. (Polygonaceae) after 15 months (Vargas-Simón & Pire 2010) and in Gliricidia sepium (Jacq.) Walp. after 12 months of storage under the same conditions (Reis et al. 2012).
Sautu et al. (2006) report a germination rate of 49 % in O. macrocalyx seeds stored for three years at 20°C and with a moisture content of 13.5 %. In a previous experiment (Georgina Vargas-Simón, unpublished data), O. macrocalyx seeds stored under refrigeration (4 °C) for 36 months showed only 5 % germination after soaking for 24 h, but germination reached 58 % following scarification with sandpaper. Román et al. (2012) also showed that O. macrocalyx seeds can be stored at 20 °C and remain viable for more than 36 months; however, these authors do not specify the germination percentage achieved after this storage period.
Our germination values confirm the effectiveness of scarification to break physical dormancy and suggest a loss of viability with increased storage time under refrigeration, factors to be taken into account when planning the seed nursery management of this species. Studies and findings about successful seed storage methods should be promoted, because field survival following direct sowing of this endangered species is very low (less than 4 %) (Pérez-Hernández et al. 2011). For this reason, the alternatives for conservation of germplasm must include: assisted natural regeneration (passive restoration), protecting the remnants of tropical forest in which it is found (Abiyu et al. 2016); or harvesting of the seeds and producing plants in greenhouses for subsequent introduction into the ecosystem in cuestion. Considering that this is a late species with requirements for shade in its first stage of life (Krause et al. 2012), it can be transplanted at four months of age in order to maximize its possibility of survival (Villacís et al. 2016).
Conclusions
Fresh seeds of O. macrocalyx, taken from closed or semi-open fruits, present high germination success. Seeds from open fruits present a lower germination rate, although the final germination percentage seems to be unaffected. When seeds are stored for 17 months under refrigeration (4 °C), their viability decreases markedly, as reflected in low values of germination percentages and rates, in addition to a delay in germination initiation and time taken for the seeds to attain 50 % of their final percentage of germination. The highest germination percentages are achieved with scarified seeds, either alone or in conjunction with application of gibberellic acid. Moreover, seeds collected from non-dispersed fruits for undefined periods of time require pregerminative treatments to ensure the seed coat rupture necessary to break the physical dormancy. These seed handling details may contribute to the recovery and conservation of O. macrocalyx, which is of particular value considering the scarcity of studies on the reproductive physiology and propagation of this important tree species.











 nueva página del texto (beta)
nueva página del texto (beta)