Growing human pressure on terrestrial ecosystems represents one of the most important threats to biodiversity, especially in the tropics (Godfray et al. 2010, Melo et al. 2013, FAO 2014). Therefore, the planet is suffering rapid and dramatic changes across the majority of biomes (Foley et al. 2005). Considering the current high rates of deforestation in most of the tropical countries (Lindenmayer et al. 2006), it is projected that areas with old-growth forests will become increasingly scarce and fragmented (Köster et al. 2009, FAO 2014). Human population growth and the intensification of agricultural practice are the major factors that threaten old-growth forests and their associated biodiversity in the tropics (Wright 2005), due to their conversion into cropland, grassland for cattle and human settlements (Foley et al. 2005). Consequently, complete floristic inventories documenting which species are affected by human interference are urgently needed (DeClerck et al. 2010).
The Mesoamerican region including Mexico is considered as a hotspot of plant diversity, meaning that it is very rich in endemic species, but also highly threatened (Myers et al. 2000). The main reason is the loss of primary vegetation due to high deforestation and urbanization rates (Wright & Muller-Landau 2006). Within Mexico, the state of Veracruz is considered a priority site for national and global conservation of biodiversity due to its outstanding geographical characteristics, such as the complex topography and the transition between tropical and temperate zones (Olguín 2011). Veracruz covers an area of 72,420 km2 of which less than 20 % consists of natural vegetation, with a high degree of habitat fragmentation (Gómez-Pompa et al. 2010, CONABIO 2011). Nevertheless, Veracruz hosts a large number of angiosperms (6,876 species) that represents about 31 % of the flora of Mexico (Villaseñor & Ortíz 2012), and thus it is considered as the country´s third richest state in angiosperms after Oaxaca and Chiapas (Rzedowski 1993, Villaseñor & Ortíz 2014). The state is also known for having all vegetation types registered in Mexico (Gómez-Pompa & Castillo-Campos 2010), according to the classification of Rzedowski (2006). Despite being a region with high species richness, there are still many parts of the state which lack a reliable floristic inventory (Gómez-Pompa et al. 2010), especially in remote montane areas, such as our study area. Until now, no floristic research along this elevational gradient has been conducted, taking into account terrestrial herbaceous angiosperms, human land use intensity and geoecological conditions.
In the last two decades studies about diversity patterns of tropical vegetation along elevational gradients have received substantial consideration (Vázquez & Givnish 1998, Colwell et al. 2008, Willinghöfer et al. 2012), but the focus considering different taxonomical plant groups is unevenly distributed because most of the research is concentrated in the most species-rich herbaceous family (e.g., Poaceae, Asteraceae, Araceae) of every study area (Willinghöfer et al. 2012). However, many other herbaceous angiosperm families, such as Orchidaceae, Zingiberaceae and Begoniaceae, are significant elements in the composition of tropical vegetation (Willinghöfer et al. 2012, Cicuzza et al. 2013).
Nevertheless, terrestrial forest herbs have been little studied from a floristic and biogeographic point of view. As a result, there is little knowledge about how herbaceous angiosperm associations change along elevational gradients, and if they exhibit similar patterns like other plant groups (Willinghöfer et al. 2012). Only a few relevant studies have been realized in pastures (Lira-Noriega et al. 2007) and coffee plantations (Ramos et al. 1983) or focusing on single families, such as Poaceae (Hernández et al. 1990, Mejía-Saulés et al. 2002), Orchidaceae (Sosa & Platas 1998, Salazar 1999) and Asteraceae (Villaseñor et al. 2006).
Further studies on the geographical distribution of the floristic elements of central Veracruz are necessary in order to better understand its complex mix of plant species (Villaseñor 2010). Inventories of specific groups of plants or particular geographic areas contribute to the completion of the national flora of Mexico and form the basis for the appropriate management of the natural resources (Martinez-Camilo et al. 2012). This kind of data can also provide information about the degree of endemism and endangered species in specific areas, which allows evaluating protected areas about the richness and uniqueness of their flora (Rzedowski 2006).
The objective of this study was to record the flora of herbaceous angiosperms in central Veracruz, Mexico, along gradients of elevation and human forest use intensity along the Cofre de Perote mountain. The study was conducted to gather information about the floristic composition, elevational ranges and geographical distribution of the species, as well as to compare species richness and similarity between elevational belts and forest types. In this way, we provide more detailed information about patterns of species richness and distribution, which presents another step towards defining priority areas for conservation of this complex vegetation mosaic.
Materials and methods
Study area. The study was conducted at eight study sites along an elevational gradient of ca. 82 km between 40 and 3,520 m a.s.l. on the eastern slopes of the Cofre de Perote, an extinct volcano of 4,282 m elevation in the central part of the state of Veracruz, Mexico (Figure 1). This region is located at the junction of the Trans-Mexican volcanic belt and the Sierra Madre Oriental, a mountainous area between 19° 25’ 5.7’’ and 19° 36’ 54’’ N, and 96° 22’ 36’’ and 97° 09’ 36.9’’ W. According to Lauer (1972), five climate zones can be found in the study area in combination with six forest types as classified by Miranda & Hernández-Xolocotzi (1963) (Table 1).

Figure 1 Location of the eight study sites along gradients of elevation and forest disturbance in central Veracruz, Mexico. Study sites: 1. La Mancha (50 m); 2. Palmarejo (650 m); 3. Chavarrillo (1,000 m); 4. Los Capulines (1,600 m); 5. El Zapotal (2,100 m); 6. El Encinal (2,500 m); 7. Los Pescados (3,100 m); 8. El Conejo (3,500 m). The limits of the Neotropics according to Löwenberg-Neto (2014) are shown in light green.
Table 1 Overview of the study sites along gradients of elevation and forest disturbance in central Veracruz, Mexico. Forest type: TSDF = tropical semi-humid deciduous forest, TOF = tropical oak forest, HMF= humid montane forest, POF = pine-oak forest, PF = pine forest, and FF = fir forest. Mean annual temperature and mean annual precipitation and number of recorded species within the four habitats with different forest use intensities (OG = old-growth, DE = degraded, SE = secondary, AZ = azonal).
| Study site | Elevational range (m) |
Forest type |
Number of plots |
Temp. (°C) |
Prec. (mm) |
OG | DE | SE | AZ |
|---|---|---|---|---|---|---|---|---|---|
| La Mancha | 30-50 | TSDF | 15 | 26 | 1,221 | 4 | 6 | 8 | - |
| Palmarejo | 610-670 | TSDF | 20 | 23 | 938 | 12 | 35 | 11 | 20 |
| Chavarrillo | 900-1,010 | TOF | 15 | 21 | 1,552 | 11 | 13 | 19 | - |
| Los Capulines | 1,470-1,650 | HMF | 20 | 18 | 1,598 | 24 | 21 | 20 | 31 |
| El Zapotal | 2,020-2,230 | HMF | 15 | 14 | 3,004 | 16 | 18 | 20 | - |
| El Encinal | 2,470-2,600 | POF | 20 | 12 | 1,142 | 47 | 41 | 38 | 35 |
| Los Pescados | 3,070-3,160 | PF | 15 | 10 | 821 | 22 | 26 | 37 | - |
| El Conejo | 3,480-3,540 | FF | 15 | 8 | 829 | 9 | 13 | 10 | - |
Tierra caliente (0-1,250 m).- In this climate zone, we selected three study sites located in two forest types (Figure 1, Table 1): the tropical semi-humid deciduous forest (TSDF) is found in the localities of La Mancha at 50 m and Palmarejo at 650 m (Castillo-Campos & Travieso-Bello 2006) and characterized by the trees Brosimum alicastrum Sw., Cedrela odorata L., Bursera simaruba (L.) Sarg. and Ficus obtusifolia Kunth. Canopy trees lose leaves mostly during the prolonged period of drought (October to May). The tropical oak forest (TOF) in the locality of Chavarrillo at 1,000 m is typically dominated by one to three oak species (Quercus oleoides Schltdl. & Cham., Q. laurina Bonpl. and/or Q. peduncularis Née), whereas other tree species are scarce. The period of leaf fall lasts about four months and it is related with the dry season (February to May).
Tierra templada (1,250-2,200 m).- In this climate zone, two study sites within one forest type were chosen (Figure 1, Table 1): the humid montane forest (HMF), which is found in the localities of Los Capulines at 1,500 m and El Zapotal at 2,100 m. One of the most important ecological factors that characterizes this kind of forest is the frequent occurrence of fog (bosque de niebla or cloud forests; Zamora-Crescencio & Castillo-Campos 1997). In general, this community includes a mix of lower montane forest genera (e.g., Quercus L. and Liquidambar L.) with tropical lowland forest families (Acanthaceae, Rubiaceae and Myrsinaceae). The period of leaf fall lasts about four months and is related to the dry season (February to May).
Tierra fría I (2,200-2,700 m).- In this climate zone, one study site within one forest type was chosen (Figure 1, Table 1): the pine-oak forest (POF), which is found in the locality of El Encinal at 2,500 m. This forest type comprises a community whose dominant trees belong to the genera Quercus and Pinus L. Typically, in the afternoons fog occurs (Narave-Flores 1985, Castillo-Campos 2011), which makes that the temperature and humidity stay constant.
Tierra fría II (2,700-3,200 m).- In this climate zone, one study site within one forest type was chosen (Figure 1, Table 1): the pine forest (PF), which is found in the locality of Los Pescados at 3,100 m. This forest type is dominated by several species of the genus Pinus L. (P. montezumae D. Don in Lamb., P. patula Schltdl. & Cham., P. pseudostrobus Lindl., P. teocote Schltdl. & Cham.) causing a high canopy openness.
Tierra helada (3,200-4,282 m).- In this climate zone, one study site within one forest type was chosen (Figure 1, Table 1): the fir forest (FF), which is found in the locality of El Conejo at 3,500 m. This forest type is a mono-specific Abies religiosa (Kunth) Schltdl. & Cham. community with sparse canopy openness.
Along the complete elevational gradient, mean annual precipitation (MAP) ranges from 813 to 3,004 mm, being highest in humid montane forest at 2,100 m and lowest in coniferous forests above 3,000 m, whereas mean average temperature (MAT) ranges from 9 to 26 °C (SMN 2016) (Table 1). The elevational vertical temperature gradient follows a negative linear pattern with MAT decreasing by 0.55 °C every 100 m (r2 = 0.96, p < 0.001).
Sampling and botanical records. Field sampling was conducted between February 2012 and January 2014 at eight sites within elevational belts of about 500 m each (Figure 1, Table 1). In order to simplify hereafter we will refer to every site as categorical unit (50, 650, 1,000, 1,500, 2,100, 2,500, 3,100, 3,500 m).
We studied terrestrial herbaceous angiosperms (excluding epiphytes), whose life form was defined as plants that have no persistent woody stem above ground or plants that are only slightly woody, rooted on the forest floor and have short height (Moreno 1984, Poulsen 1996). Ferns were not included in this study because their diversity patterns were already described in the work of Carvajal-Hernández & Krömer (2015). Presence-absence was recorded for all species in each elevational belt within 15 to 20 plots of 20 × 20 m. The plot size of 400 m2 was selected in order to have a representative study area of the forest fragments, which is small enough to keep abiotic factors and ecological physiognomy uniform within the plot (Kessler & Bach 1999). The total number of plots for the entire study was 135, resulting in a total study area of 54,000 m2. For our study, we defined four types of habitat with different forest use intensities following Newbold et al. (2015): old-growth, degraded, secondary and azonal forest (Table 2).
Table 2 Classification of habitats with different forest use intensities according to the main physiognomic characteristic, the gap fraction in the canopy, dominance of canopy trees, percentage of shrubs and the presence of lianas (sensu Newbold et al. 2015).
| Habitat | Characteristic | Gaps (%) | Forest use intensity |
Canopy trees |
Shrub (%) |
Lianas |
|---|---|---|---|---|---|---|
| Old-growth | No obvious forest use, dominance of mature trees |
< 10 | Low | High | < 30 | No |
| Degraded | Selective logging, grazing and understory removal |
11-25 | Medium | Low | 30-50 | Low |
| Secondary | Re-growth after clear-cut | > 25 | High | Very low | > 50 | High |
| Azonal | Grows in riparian forest and humid ravines |
< 10 | Low | High | < 30 | No |
To avoid edge effects, our plots were established at least 50 m away from the nearest forest edge. An equal number of plots was studied for every forest type, i.e. five were established in each of the following habitats with different use intensities: i) old-growth, ii) degraded and iii) secondary forest. Only in the sites of 650, 1,500 and 2,500 m we were able to add five plots in vi) existing azonal vegetation, causing the uneven numbers of plots per elevational belt (Table 1).
Taxonomic determination. In each study site (but not in every plot), all terrestrial herbaceous angiosperms species were collected mostly in triplicate and deposited at the following herbaria: Herbario Nacional de México, Instituto de Biología, UNAM, (MEXU, including all unicates), Instituto de Ecología, A.C., (XAL), Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, IPN (CIIDIR) and/or at the local herbarium of the Facultad de Biología, Universidad Veracruzana (XALU). Collection and processing of botanical specimens were made according to the proposal of Lot & Chiang-Cabrera (1986). Botanical determinations were realized by use of the relevant taxonomic literature (Flora de Veracruz and Flora fanerogámica del Valle de México), by comparison with specimens deposited at MEXU and XAL, and consultation of experts in different plant families (see Acknowledgements). Also, morpho-species which are clearly different were incorporated in the floristic list (Krömer et al. 2013). It was not possible to identify all specimens to species or genus level for two main reasons: i) most of the studied plant groups are not well known and their identification is difficult due to a lack of relevant literature, ii) some individuals were found sterile. Taxa were classified according to the classification of the Angiosperm Phylogeny Group (APG; Bremer et al. 2009).
Data analyses. We used the package “vegan v2.3-4” (Oksanen et al. 2016) in R statistical language v3.2.3 (R Core Team 2014) to calculate the number of unobserved species with the Bootstrap function, which is based on presence-absence data and takes into account rare, unique and duplicated species. This species richness estimator is reliable because it has a sensibility to species aggregation in the initial stage of the sampling when the species distribution is random.
Based on their geographical distribution area, each species was placed in one of the following categories (sensu Rzedowski 1991): i) endemic to Mexico, ii) endemic to the southern United States and Mexico (Megamexico 1), iii) endemic to Mexico and Central America (Megamexico 2), iv) endemic to the southern United States and Central America (Megamexico 3), and v) introduced species (Gómez-Pompa et al. 2010, Espejo-Serna 2012).
Finally, to compare our results with other studies, we calculated the taxonomic diversity index (TDI; Magurran 2004) for the total number of species and for the three most important families: Asteraceae, Poaceae and Orchidaceae, with the following equation:
where S is the total species number and A is the entire studied area in m2.
Results
In 135 plots along the elevational transect, we recorded 264 (morpho-)species of terrestrial herbaceous angiosperms from 152 genera and 54 families (Appendix). Of all recorded species, 201 (76 %) were identified to species level, 42 (16 %) to genus level and 21 (8 %) to family level. Monocots contributed 45 % of the species and 28 % of the families, and dicots 55 % of the species and 72 % of the families. Table 3 summarizes the most species-rich taxa at family and genus level. The observed species richness varied between 79 and 90 % of the predicted values by the estimator Bootstrap at every elevational belt (Figure 2).
Table 3 Most representative families and genera of herbaceous angiosperms along gradients of elevation and forest disturbance in central Veracruz, Mexico.
| Family | Species number |
Percentage (%) |
Genera | Species number |
Percentage (%) |
|---|---|---|---|---|---|
| Poaceae | 36 | 14 | Peperomia | 10 | 4 |
| Asteraceae | 31 | 12 | Salvia | 8 | 3 |
| Orchidaceae | 27 | 10 | Begonia | 6 | 2 |
| Cyperaceae | 17 | 6 | Senecio | 6 | 2 |
| Lamiaceae | 13 | 5 | Cyperus | 5 | 2 |
| Araceae | 12 | 4 | Anthurium | 5 | 2 |
| Piperaceae | 10 | 4 | Carex | 5 | 2 |
| Commelinaceae | 9 | 3 | Ageratina | 4 | 2 |
| Rubiaceae | 8 | 3 | Arenaria | 4 | 2 |
| Other families | 101 | 38 | Other genera | 211 | 80 |
| Total | 264 | 100 | Total | 264 | 100 |
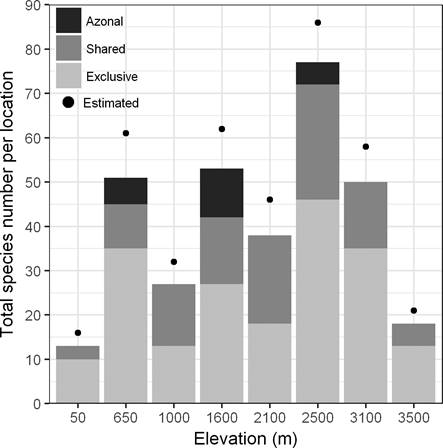
Figure 2 Observed and estimated (Bootstrap species richness estimator) species richness of all species together per elevational belt. We present the number of exclusive species at each study site, the number of species shared with other sites, and the species that are exclusive for azonal habitats.
In order to compare overall species richness between the eight study sites, we used the values excluding and including species of azonal vegetation. In the first case, the highest number of species was found in the site of 2,500 (71), followed by 3,100 m (48) and 650 m (43); in the second case, the site with the highest number of species was 2,500 m (76), followed by 1,600 m (52) and 3,100 m (48) (Figure 2). All habitats of the 2,500 m site taken separately had the highest species richness of the elevational gradient (Table 1), whereas all habitats of 50 m had the lowest richness. The secondary forest in most of the sites had the highest number of species, whereas old-growth forests had the lowest number of species in most of the sites. In the sites with azonal vegetation, this habitat had more species than old-growth forests, and except for 2,500 m, even more than secondary forests (Table 1). TDI was 0.51 for all species and between 0.31 and 0.033 for the three most important families (Table 4).
Table 4 Comparative species richness of herbaceous angiosperms along elevational gradients reported in some studies from México, Brazil and Ecuador. TDI = taxonomic diversity index (calculated for the total species number and for the most important families Ast= Asteraceae, Poa = Poaceae and Orc = Orchidaceae).
| Region, Country | Elevation (m) | Latitude | Species number | Area (ha) | TDI | Ast | TDI | Poa | TDI | Orc | TDI | Authors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manaus, Brazil | 70-150 | 2° 37’ S | 24 | 0.09 | 0.47 | 0 | - | 2 | 0.10 | 0 | - | Costa (2004) |
| Cuyabeno, Ecuador | 250-300 | 0° 00’ S | 70 | 1.00 | 0.46 | 0 | - | 8 | 0.23 | 0 | - | Poulsen et al. (2006) |
| Los Tuxtlas, Veracruz, Mexico | 140-1,670 | 18° 43’ N | 50 | 2.96 | 0.38 | 0 | - | 0 | - | 17 | 0.28 | Krömer et al. (2013) |
| Jalcomulco, Veracruz, Mexico | 350-900 | 19° 21’ N | 60 | 0.67 | 0.46 | 2 | 0.08 | 4 | 0.16 | 2 | 0.08 | Palacios-Wassenaar et al. (2014) |
| Central Veracruz, Mexico | 1,800-2,000 | 19° 29’ N | 139 | 0.02 | 0.92 | 2 | 0.13 | 4 | 0.26 | 2 | 0.13 | García-Franco et al. (2008) |
| Sierra de Manantlán, Jalisco, Mexico | 1,500-2,500 | 19° 30’ N | 181 | 4.30 | 0.49 | ND | ND | ND | ND | ND | ND | Vázquez & Givnish (1998) |
| Central Veracruz, Mexico | 50-3,500 | 19° 31’ N | 264 | 4.80 | 0.52 | 31 | 0.32 | 36 | 0.33 | 27 | 0.31 | This study |
| Central Veracruz, Mexico | 400-900 | 19° 37’ N | 300 | 1.20 | 0.61 | 42 | 0.40 | 53 | 0.42 | 2 | 0.07 | Castillo-Campos (2007) |
| Pacific coast of Mexico | 400-2,860 | 19° 45’ | 1,793 | 140,000 | 0.36 | 333 | 0.28 | 221 | 0.26 | 181 | 0.25 | Vázquez et al. (1995) |
| Sierra de Zapalinamé, Coahuila, Mexico | 1,590-3,140 | 25° 25’ N | 171 | 3.30 | 0.49 | 61 | 0.40 | 27 | 0.32 | 0 | - | Encina-Dominguez et al. (2007) |
Geographical distribution. Most of the study sites shared only low numbers of species (Figure 2). The highest number of exclusive species was found at 2,500 m, followed by 650 m and 3,100 m. Concerning biogeography, 70 % of the taxa showed a Neotropical affinity and we recorded 31 species endemic to Mexico, including two species (Begonia multistaminea Burt-Utley and Sedum obcordatum R.T.Clausen) endemic to Veracruz (see Appendix). Furthermore, 20 species were endemic to Mexico and Central America and two to the South of United States of America and Mexico. Three species are listed in Official Mexican Law (SEMARNAT 2010), two of these are threatened and endemic to Mexico (Anthurium podophyllum (Schltdl. & Cham.) Kunth and Peperomia subblanda C. DC.), and another was under special protection (Monotropa hypopitys L.). Additionally, we found 14 introduced species to Mexico that were mostly recorded in secondary and degraded forests (Table 5 and Appendix).
Table 5 Number of species under geographic distribution and life strategy recorded along gradients of elevation and forest disturbance in central Veracruz, Mexico. OG = old-growth forest, DE = degraded forest, SE = secondary forest, AZ = azonal forest. Total numbers of species in each category are also shown.
| Distributional/life strategy category | OG | DE | SE | AZ | Total |
|---|---|---|---|---|---|
| Under special protection | 1 | 2 | 2 | - | 3 |
| Endemic to Veracruz | 1 | - | - | - | 1 |
| Endemic to Mexico | 11 | 15 | 20 | 9 | 31 |
| South of United States of America and Mexico | 1 | 2 | 1 | - | 2 |
| Mexico and Central America | 9 | 12 | 11 | 9 | 20 |
| Introduced | 5 | 6 | 9 | 2 | 14 |
| Ruderal | 13 | 19 | 27 | 11 | 41 |
| Generalist | 65 | 92 | 73 | 56 | 152 |
Discussion
General taxa richness. A comparison with previous studies on terrestrial angiosperms and other plant groups in state of Veracruz shows that we recorded a high number of species in our study. Although our sampling area was limited (5.4 ha), the total number of species recorded was higher than those reported by Cházaro-Basáñez (1992) who focused on a floristic description of the different forest types within the upper part of the same elevational gradient. Cházaro-Basáñez reported only 12 herbs in the humid montane forest, 17 in the pine-oak forest, two in the pine forest and 14 in the fir forest. Carvajal-Hernández & Krömer (2015) found 155 species of ferns and lycophytes in the same plots of our elevational gradient of which 82 were terrestrial species. Several studies from central Veracruz reported a lower number of terrestrial herbs, e.g., Palacios-Wasenaar et al. (2014) recorded 230 species of vascular plants of which 60 (26 %) were herbs (Table 5). García-Franco et al. (2008) found 258 vascular plant species in similar forests, of which 139 (54 %) were herbs. Novelo-Retana (1978) recorded 238 species of vascular plants of which 67 (28 %) were herbs. Zamora & Castillo-Campos (1997) recorded 390 species of vascular plants of which 225 (58 %) were herbs.
In contrast, a higher number of herbaceous species has been reported in some studies from central Veracruz. The relatively high number of herb species might be explained by the large environmental gradient covered in our study and will be discussed in the next paragraphs. For example, Castillo-Campos et al. (2007) recorded 580 species of vascular plants of which 369 (64 %) were herbs, and Narave-Flores (1985) recorded 853 species of vascular plants of which 557 (65 %) were herbs. For Southern Veracruz, Ibarra-Manríquez & Sinaca-Colin (1987) recorded 991 species of vascular plants of which 536 (54 %) were herbs. However, all these studies were realized in much bigger sampling areas than the present study.
Due to the limited number of similar transect studies in the study area we were only able to compare our results with the following studies realized in Southern Veracruz by Krömer et al. (2013), which however included mainly terrestrial ferns and only a few orchids and bromeliads, Western Mexico (Jalisco) by Vázquez & Givnish (1998) and Vázquez et al. (1995), and Northern Mexico (Coahuila) by Encina-Domínguez et al. (2007). Furthermore, we compared our results with the species numbers of terrestrial angiosperms found along two elevational gradients of Brazil and Ecuador (Table 4).
In most of the cases, our study site shows a higher number of species than the other locations. The TDI also shows that excluding the works from central Veracruz, our study has a higher species per area value than the other studies (Table 4). These differences among the geographical areas can be explained by environmental factors, such as latitudinal influence, precipitation, temperature, elevation and soil nutrients (Vázquez & Givnish 1998, Cicuzza et al. 2013). The TDI indicates different patterns for the three most important families, e.g., there is an increase of the values of Asteraceae with elevation (Table 4), which is different from the family pattern shown in Mexico (Villaseñor et al. 2005). In the case of the Poaceae, the index shows that at lower latitudes this family is an important component of the flora, whereas in central Veracruz the family has similar values than the Asteraceae, and at the highest latitude there was a decrease in the value. In the case of the Orchidaceae, our study shows the highest value compared to the other locations, which demonstrates that the forest fragments in central Veracruz harbor a high number of orchids (Castañeda-Zárate et al. 2012).
On the other hand, species richness in our study was much lower compared to the numbers presented by Castillo-Campos et al. (2007), which is due to the fact that their work was realized in tropical deciduous forest which is recognized as vegetation type with high diversity of herbaceous angiosperms, as well as a more concentrated and exhaustive sampling effort in only one vegetation type. In general, the tropical deciduous forests occur in environments with highlight incidence during the dry season (Chiarucci 1994). Besides, the limitations imposed by the bedrock, such as lack of organic matter in the soil, restrict the establishment of other plant groups (e.g., trees). Therefore, the herbaceous layer is facilitated by excluding competitors due to the physiological and functional traits that are characteristic for this plant group (Castillo-Campos et al. 2007).
Patterns of richness along the elevational gradient. We found a not very pronounced hump-shaped pattern in the overall species distribution along the elevational gradient (Figure 2), which is a pattern found in different groups of vascular plants along tropical elevational gradients, such as ferns (Salazar et al. 2015), terrestrial herbs (Willinghöfer et al. 2012) and shrubs (Chawla et al. 2008). Rahbek (1995) suggested that the distribution of plants in tropical areas is affected by the high variation of environmental factors that can change substantially in small regions, and this causes differences in the form of distributional patterns. We found that the sea level site was less species-rich compared with the other sites. The following sites (from 650 until 2,100 m) have an intermediate species richness (Figure 2). This is probably due to heterogeneity in their landscape in comparison to other areas of the state, such as the coastal plain, caused by the heterogeneous structure of the physiographic discontinuity generated by the union of two regions: Coastal plain of the Gulf of Mexico and Trans-Mexican Volcanic Belt (Narave-Flores 1985, Torres-Cantú 2013).
The highest species richness was found at 2,500 m, which has been also reported from Ecuador for all endemic vascular plant species, endemic species of Acanthaceae, Asteraceae, Lamiaceae, Piperaceae and Scrophulariaceae (Kessler 2002), and for liverworts in the Northern Andes (Wolf 1993). This pattern is based on a contact of different species assemblages within the transition between two climate zones (Lauer 1972, Wolf 1993) and a high level of humidity due to cloud condensation (Rahbek 1995, Hemp 2006). The richness tends to decrease at higher elevations because productivity and temperature decrease with elevation (Currie et al. 2004, Hawkins et al. 2007); both factors affect the competition and growth of plants (Vázquez & Givnish 1998). Furthermore, the kind of dominant tree species (Pinus spp. and Abies religiosa) at the highest sites (3,000 and 3,500 m) has an influence on the herbaceous community because the coniferous litter changes the soil properties (Whittaker 1975, van Wesenbeeck et al. 2003).
Forest use intensity effect. The degraded and secondary forests of the 50, 650, 2,100, 3,100 and 3,500 m sites had higher species richness, compared to the old-growth forests. Furthermore, we found introduced and generalist species most frequently in secondary and degraded forest due to the changes in abiotic factors, such as a drier microclimate, change in soil nutrients and higher light incidence (Köster et al. 2009) that allow them to outcompete native species due to specific arrangements of traits (Schultz & Dibble 2012) (Table 1, Appendix). Similarly, Firn et al. (2011) reported that some herbaceous angiosperms are related to human forest use intensity, which allows the establishment of ruderal species. These species increase the richness in anthropogenically influenced habitats, although native biodiversity is affected negatively by introduced plant species. This indicates that modifications in the structure of the old-growth forest affect the species composition of herbaceous angiosperms because changes in abiotic factors due to forest use intensity may increase the richness, especially of Poaceae and Orchidaceae in degraded habitats, whereas Asteraceae increase in secondary habitats. This is due to the ability of ruderal species to survive or even being favored in drier microclimates (Givnish 1995, Pons & Poorter 2014) with more light in the understory due to the more open canopy of degraded forests (Grime 1977, Lavorel et al. 2011). Consequently, in North American forests, a higher richness of terrestrial herbs was found in the degraded forest with open canopy gaps compared to mature forests with closed canopies (Meekins & McCarthy 2001).
However, the richness of species decreases in the degraded and secondary forests of the 1,600 and 2,500 m sites compared to the old-growth humid montane forest. This similar pattern was found for ferns (Carvajal-Hernández et al. 2014, Carvajal-Hernández & Krömer 2015) and in general for vascular epiphytes (Krömer & Gradstein 2003, Köster et al. 2009). This loss of species is due to the adaptation of many native species to temperate climate with high humidity (Parry et al. 2007). Furthermore, the changes in the structure of soil due to the forest use intensity leads to a loss of microbial organisms that favor the establishment of some species (Camenzind et al. 2014). On the other hand, it is widely documented that fragmentation has a negative effect on species richness in lowland forest, especially on understory plants (Magrach et al. 2014). For example, in the south of Veracruz, Zambrano et al. (2014) found that seeds of understory plants could be affected by altered microclimatic conditions in the fragmented landscape. These species seem to be adapted to moderate conditions of humidity and temperature which, respectively, decrease and increase with forest use intensity (Dale et al. 2001). Peperomia magnoliifolia (Jacq.) A. Dietr. serves as an example in the 650 m site, Begonia multistaminea and P. cobana C. DC. in the 1,600 m site, where these are commonly found in habitats of high humidity and shadow (old-growth forest), but probably cannot tolerate high levels of radiation and low humidity and thus are rare in degraded and secondary forests (Ali 2013, Mathieu et al. 2015).
It was hypothesized that intermediate forest use intensity leads to higher species richness (Connell 1978, Warren et al. 2007) and plantcommunity endemism (Kessler 2001). The mosaic vegetation pattern in our study area is an important shelter for the endemic flora of the region. Since the level of forest use intensity was similar in all sites, the different effects can only be attributed to feedbacks between the specific plant community and the changes in environmental factors, such as microclimate or soil nutrients.
In azonal vegetation (riparian forests), except for the 2,500 m site, the richness was higher than in old-growth forests, which might be due to stable moist environmental conditions and higher soil moisture. In the case of the 650 m site, the species richness recorded in azonal vegetation was almost twice of that observed in the old-growth tropical semi-deciduous forest. This interpretation is consistent with results found by Poulsen & Balslev (1991) in the Amazonian rain forest, who recorded the highest richness of herbs along rivers, which was explained by a mix of species from the border zone to the moist zone next to their study plot and the edaphic and topographic heterogeneity. In the case of terrestrial ferns, Carvajal-Hernández & Krömer (2015) found the same pattern suggesting that fern richness is favored in areas with the influence of water and high humidity. These results confirm the value of the azonal vegetation as reservoirs of biodiversity.
Introduced species. Within the set of introduced species, there is a subgroup known as invasive alien or invasive species, which includes those that survive, are established and reproduce uncontrollably outside their natural environment, causing serious damage to biodiversity, economy, agriculture and public health (CONABIO 2016). We found several introduced species recognized as invasive, e.g. Commelina diffusa Burm. f. is a species that can withstand flooding and infests cultivated lands, roadsides, pastures and wastelands, which is problematic primarily in young crops, but can also cause a problem in mature crops in Mexico due to its sprawling behavior (Boyette et al. 2015). Oeceoclades maculata (Lindl.) Lindl. is competing for the same microhabitat and may displace other native terrestrial orchids (Moreno-Molina & Beutelspacher 2014). Hedychium coronarium J. Koenig has a negative influence on the recruitment of plants from the plant community, with consequences for the biodiversity of invaded areas (de Castro et al. 2016). Foeniculum vulgare Mill. is particularly aggressive in abandoned agricultural fields and grazed areas (Power et al. 2014). Rumex acetosella L. might interfere with secondary succession processes and gap colonization dynamics of native species, and it has the ability to competitively exclude native tussock grasses (Franzese & Ghermandi 2014).
Geographical distribution. In general, the inventoried species show a phytogeographical affinity with southern latitudes, which can be seen by the high number of taxa also occurring in Central and South America. Nevertheless, many endemic taxa of central Mexico have also been encountered. In this context, Rzedowski (2006) suggests that for the flora of Tierra caliente (from sea level until ca. 1,400 m) the southern Neotropical affinity dominates over the boreal affinity. In addition, in Tierra templada, the most important elements have a southern origin with less boreal elements. In the cooler zones (Tierra fría and Tierra helada), the most important floristic elements are equally of southern and boreal affinity with some being endemic species from North America, such as Ageratina pazcuarensis (Kunth) R.M. King & H. and Festuca rosei Piper, whereas others, such as Carex melanosperma Liebm., Corallorhiza maculata (Raf.) Raf. and Muhlenbergia macroura (Kunth) Hitchc. are species endemic to Central America.
Our results show that species richness patterns of herbaceous angiosperms of forest vegetation in central Veracruz are determined by the large environmental gradient of the region. Moreover, degraded and secondary forests exhibit high species richness depending on the elevational belt, which is probably due to the ability of species in several families that compete better under high light conditions. The high richness and turnover of species, including many endemic elements, highlights the importance of this region for plant conservation; however, this area is also highly threatened by land use changes and shows very high deforestation rates (Ellis & Martínez 2010).
Castillo-Campos et al. (2008) proposed to create a system of many protected reserves distributed throughout the state in order to protect this kind of landscape and its flora under the plan of “archipelago reserves” described by Halffter (2005), where all landscape units are connected by small protected areas. In addition, we suggest that an environmental heterogeneity formed by mature, disturbed and secondary forests is acceptable (and unavoidable) and can even increase species richness. This is an opportunity to develop a sustainable management concept to protect and promote species richness and to take into account the need of the local population for forest ecosystem services, such as timber, water, landslide protection, recreation and tourism. This could be an alternative to the current concept of a protected area, such as a national park, that is only focused on protecting alpha diversity without consideration of species turnover rates (Castillo-Campos et al. 2008). Thus, it is necessary to create a conservation and management plan for the study area, which requires taking into account more taxonomic groups, the existing proportions of different habitat types, as well as studies on the socio-economic conditions across the elevational gradient.











 nueva página del texto (beta)
nueva página del texto (beta)


