The diversity of life forms in angiosperms has been identified as an important factor that enabled flowering plants to become dominant in terrestrial habitats (Ricklefs & Renner 1994, Tiffney & Mazer 1995). Among noteworthy terrestrial life forms are the geophytes, plants with underground perennating organs like bulbs, corms, tubers or rhizomes that lose their aerial parts annually (Hamrick & Godt 1996, Parsons 2000, Proches et al. 2005, Proches et al. 2006, Kamenetsky 2013). Perennial belowground elements allow plants to survive periods of severe climate conditions (Dafni et al. 1981, Parsons 2000, Kamenetsky 2013). Furthermore, it is known that species of geophytes in different lineages of angiosperms have increased their genome size, facilitating the production of larger cells in the underground perennating organs, which is advantageous for fast growth in seasonal habitats (Vesely et al. 2012).
Geophytes are often conspicuous components of vegetation after burning (Doussi & Thanos 2002, Verboom et al. 2002, Tyler & Borchert 2003, Koniak et al. 2009). This life form is more common in monocots, in families like Iridaceae, Orchidaceae, Hyacinthaceae, Amaryllidaceae and Anthericaceae and only occurs in very few dicot taxa (Meerow 2013). There has been great interest in plants with this life form because many geophyte monocot species in genera like Allium, Hyacinthus, Iris, Narcissus and Tulipa are widely cultivated as ornamentals (Kamenetsky 2013).
Regions with a Mediterranean climate sustain outstanding geophyte diversity and this has been documented for California, Chile, the Mediterranean Basin, Cape Province in South Africa, Australia and Turkey (Rundel 1996, Parsons 2000, Goldblatt & Manning 2002, Parsons & Hopper 2003, Çelik et al. 2004, Proches et al. 2006). Different genera dominate in certain regions: Allium and Calochortus in California (Rundel 1996) and Disa, Gladiolus and Moraea in South Africa (Proches et al. 2006), for example. A comparison of California, Chile, South Africa, Mediterranean Basin and Australia showed that Orchidaceae has the highest number of monocot geophytes, followed by Amaryllidaceae (Parsons 2000). Endemic geophytes vary as well and the most remarkable example is in Chile, where 70 % of the geophyte flora is endemic (Hoffmann et al. 1998). In South Africa, geophyte diversity has been found to be dependent on climate, mainly on the amount and reliability of precipitation (Proches et al. 2005). In the New World, geophytes have been recorded from regions with a Mediterranean climate such as California, the northern region of Baja California and central Chile (Rundel 1996, Hoffman et al. 1998).
Furthermore, while the most studied regions for geophyte diversity are Mediterranean, this functional group of plants is present as well in areas with seasonal climate and have been accounted in mountainous regions like in the north of Iran where 124 species of geophytes were recorded as one of the dominant life forms (Naqinezhad et al. 2014); or in the north of Venezuela in which geophytes are in zones with seven months of rain (Wikander 1984). In Burkina Faso a few geophytes have remained in modified dry landscapes (Madsen et al. 2008); or in the Pyrenees an elevated number of 250 geophyte species have been scored (Grau et al. 2012). In Peru, in Prepuna plant communities more than 50 geophytes have been documented, among them are some neotropical genera such like Nothoscordum and Sisyrinchium (Montesinos et al. 2012). In Mexico there are also areas that have a marked seasonal climate with suitable areas for geophytes (Peel et al. 2007, Kottek et al. 2006). Moreover, Mexico is crossed by several mountain chains and has ample differences in climate types that might provide a considerable number of terrestrial ecosystems where geophytes can establish (Marshall & Liebherr 2000).
In this paper we identify geophyte diversity in the biogeographic regions of Mexico. According to Morrone (2014) the Mexican Transition Zone is one of the biogeographical regions in the country, comprising the provinces: Sierra Madre Oriental, Sierra Madre Occidental, Trans-Mexican Volcanic Belt, Sierra Madre del Sur and Chiapas Highlands. These mountain areas have been identified as biodiversity hotspots for temperate taxa (Myers et al. 2000). Among them, the Trans-Mexican Volcanic Belt is a complex mountain chain formed by thousands of volcanic structures that originated in four episodes (Gómez-Tuena et al., 2007). This chain crosses Mexico from east to west and its influence on the Mexican highland biodiversity has been amply discussed, either harboring high biodiversity or promoting its diversification (reviewed in Mastretta-Yanes et al. 2015). Elevated diversity of several angiosperm groups of Mexico has been identified in the Trans-Mexican Volcanic Belt. For example, the highest diversity of Asteraceae, the largest angiosperm family in Mexico is found mainly in the Trans-Mexican Volcanic Belt (Cruz-Cárdenas et al. 2013, Vargas-Amado et al. 2013). The Brazilian subregion in the Mesoamerican Dominion is an additional biogeographic area identified for Mexico and comprises lowland vegetation in the Pacific slopes, in the Yucatan Peninsula and in the Balsas Basin (Morrone 2014). This biogeographic area also provides suitable habitats for geophytes.
With regard to diversity and endemism of geophytes in Mexico, it is known that most species of tribe Tigridieae, a geophyte clade in the Iridaceae are distributed in the Trans-Mexican Volcanic Belt (Munguía-Lino et al. 2015). The Milla clade (Asparagaceae) comprises six geophyte genera endemic to Mexico and it was suggested that a number of areas in the Trans-Mexican Volcanic Belt acted as refugia to this group (Gándara et al. 2009, 2014). In the orchid geophyte genus Bletia, many Mexican endemic species are distributed in the Trans-Mexican Volcanic Belt and in the Sierra Madre Occidental (Sosa et al. 2016).
Here we focus on monocots because the majority of geophytes have been recorded for this lineage and because the monocot flora is well known for Mexico, with approximately 4500 species recorded, of which approximately half are endemic, including groups such as Polianthes, Milla, Bletia, Tigridia, Echeandia, Malaxis, and Schiedeella with geophyte life forms (Espejo-Serna 2012). We concentrate on monocot geophytes to gain new insights into diversity patterns for the flora of Mexico, not only in species from the single region of Mediterranean climate in Mexico, in Baja California, in the neartic California Floristic Province, but also in the rest of the biogeographic provinces identified for the country. We expect the highest diversity of geophytes in the Trans-Mexican Volcanic Belt and in the Sierra Madre del Sur provinces, where seasonal climate has been reported, similar to Mediterranean climate.
The objectives of this paper are: 1) to document monocot geophyte diversity in Mexico, 2) to identify the biogeographic areas with the highest geophyte diversity, and 3) discuss their conservation.
Material and methods
Checklist. We compiled a checklist of the Mexican monocots geophyte species based on checklists, floras and monographs (Correll 1941, Williams 1946, Henrich & Goldblatt 1987, Rzedowski & Calderón de Rzedowski 1985, Salazar 1990, Lascurain-Rangel 1995, López-Ferrari & EspejoSerna 2002, Croat & Carlsen 2003, Espejo-Serna & López-Ferrari 2003, Rodríguez et al. 2003, Rodríguez & Ortiz-Catedral 2003a,b, Espejo-Serna et al. 2005, Rodríguez & Ortiz-Catedral 2005, 2006, 2013, Téllez-Valdés & Geeta 2007, Alvarado-Cárdenas & García-Mendoza 2008, Espejo-Serna et al. 2009, Gándara et al. 2009, Espejo-Serna et al. 2010, Flagg et al. 2010, Sánchez-Ken 2010, Salazar et al. 2011, Barba-González et al. 2012, Tapia-Campos et al. 2012, 2013, Gándara et al. 2014) and in databases such as “eMonocot” (http://e-monocot.org/). Plant families follow the APG III classification (http://www.mobot.org/MOBOT/research/APweb/).
Database. Distribution records were obtained from the literature cited above and complemented with databases such as the Mexican Biodiversity Database (REMIB) (www.conabio.org), TROPICOS (www.tropicos.org) and the Global Biodiversity Information Facility (www.gbif.org). Records from Bletia and Hexalectris (Orchidaceae), Behria, Bessera, Jaimehintonia, Dandya, Milla and Petronymphe (Asparagaceae) were based on our collections (Sosa 2009, Sosa et al. 2016, Gándara et al. 2014). A total of 2593 records were compiled. The distribution of every species was mapped using ArcView (ESRI 1999) in 1 Km2 quadrats in which we divided the Mexican territory. Quadrats were then superposed in the biogeographic provinces to identify the most diverse.
Climate variables. To identify the most significant environmental variables related to distribution of geophytes in Mexico, 19 bioclimatic variables derived from temperature and precipitation data were obtained from WorldClim 1.4 (Hijmans et al. 2005) at a resolution of 1 km2. The bioclimatic variables were extracted using the software R programing languaje using the packages Raster (Hijmans et al. 2016) and rgdal (Bivand et al. 2016) considering the 2593 records of our database. A correlation analysis was performed to eliminate correlated environmental variables using the program PAST v3.06 (Hammer et al. 2011) and only the fifteen least correlated variables (Pearson ≤ 0.8 based on all sample locations) were utilized. A Principal Component Analysis was subsequently performed with PAST v.3.06 (Hammer et al. 2011) to establish the bioclimatic variables with the highest component scores that explain most variation in the PC axes.
Results
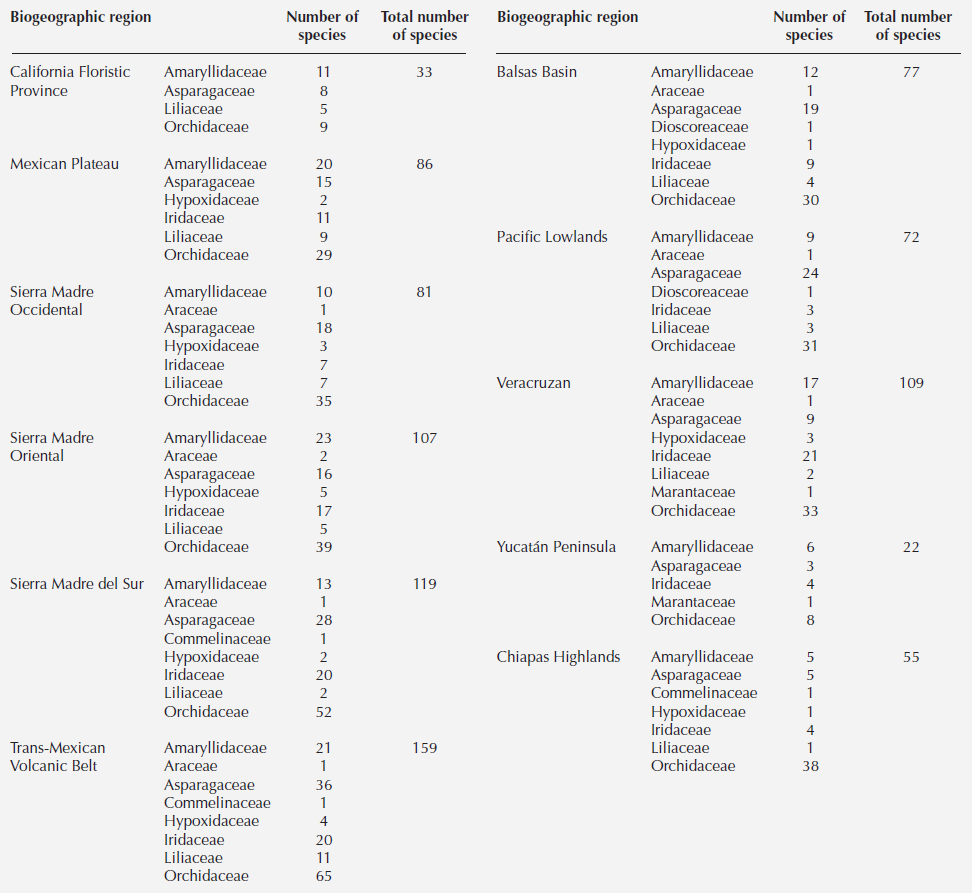
Diversity. The Mexican monocot geophyte checklist comprised 476 species belonging to ten families and 76 genera, the majority in the order Asparagales (Table 1). The families with the largest number of species were the Orchidaceae (181 species), Asparagaceae (114 species), Amaryllidaceae (106 species) and Iridaceae (92 species); the rest included less than 50 species in families such as Araceae, Hypoxidaceae, Commelinaceae, Dioscoreaceae, Liliaceae and Marantaceae (Table 1). The genus with most species was Echeandia (66 species) followed by Tigridia (39 species), Hymenocallis and Zephyranthes (32 species each), Allium (28 species) and Calochortus (23 species) (Table 1). Ten genera, each with only a few species, are endemic (Ainea, Behria, Bessera, Colima, Dandya, Fosteria, Jaimehintonia, Petronymphe, Prochnyanthes, Polianthes) to Mexico, as are 344 species of geophytes. The greatest diversity of geophytes lies in the Trans-Mexican Volcanic Belt (170 species), followed by the Sierra Madre del Sur (119 species) (Table 2, Figure 1).

Figure 1 Highest diversity of Mexican monocot geophytes in biogeographic provinces. Representative monocotiledonous geophytes: from top to bottom and right to left: Tigridia pavonia, Echeandia flavescens, Hexalectris spicata, Bletia roezlii, Milla biflora, Bessera elegans.
Of the 476 monocot geophyte species, 34 are classified as being at some risk of extinction (SEMARNAT 2010), with Orchidaceae and Iridaceae having the majority of at-risk species (10 and 9 species respectively) (Table 3).
Table 3 Geophyte species currently assigned to some risk of extinction category based on NOM-059-SEMARNAT (2010). P: in danger of extinction, A: threatened, Pr: special protection.

Climate variables. From the 19 climate variables our analysis of correlation identified four correlated variables (1, 2, 7, 11) that were not considered for the subsequent PCA analysis. PC1 and PC2 explained 81 % of variability, and based on their scores, the most significant climate variables were: BIO4 Temperature seasonality (standard deviation × 100), BIO12 Annual Precipitation and BIO 16 Precipitation of Wettest Quarter (Table 4).
Table 4 Significant climate variables related to distribution of Mexican monocotiledonous geophytes identified by the PCA. PCA scores of axes PC1 and PC2 represented 81% of the total variation. BIO4 = Temperature seasonality (standard deviation × 100). BIO12 = Annual Precipitation. BIO16 = Precipitation of the wettest quarter.

Discussion
Our records document the noteworthy diversity of geophytes in the Mexican flora; species with this life form represent 2.0 % of the approximately 23,500 vascular plants and 10.4 % of the 4,500 species of monocots. Amaryllidaceae, Asparagaceae, Iridaceae and Orchidaceae are the lineages with the highest diversity of this life form. Two genera stand out owing to their elevated number of species: Echeandia and Tigridia, both distributed in the New World and with their greatest diversity in Mexico (Rodríguez & Ortiz-Catedral 2013, Munguía-Lino et al. 2015).
Of the ten Mexican endemic geophyte genera, five belong to Asparagaceae (Behria, Bessera, Dandya, Jaimehintonia, Petronymphe) and are members of the Milla clade. These taxa have restricted distributions, remarkable distinct floral morphology, few species and are distributed from Arizona to Guatemala, originating in the California Floristic Province (Gándara et al. 2014). Prochnyanthes is a monotypic genus (P. mexicana) and belongs as well to Asparagaceae. The four remaining genera belong to Iridaceae and the best known is Polianthes that includes P. tuberosa, a cultivated species since prehispanic times, used as an ornamental and its extracts used in perfumery; it is currently cultivated worldwide (Trueblood 1973, Waithaka et al. 2001). The remaining genera are monotypic: Ainea, Fosteria and Colima and closely related to Tigridia (Ravenna 1979, Molseed 2008, Rodríguez & Ortiz-Catedral 2003b).
The Trans-Mexican Volcanic Belt province has the highest diversity of geophytes, and the combination of its complex geological history, topography, enormous variation in elevation and climate, along with its geographical position in the central part of Mexico provides suitable habitats for multiple organisms (Marshall & Liebherr 2000). Our results show that the highest number of monocot geophytes lie in this mountain chain, specifically in regions of dry pine-oak forests. This coincides with the richness of species reported for tribe Tigrideae in Mexico, to which most of our reported Iridaceae belong (Munguía-Lino et al. 2015). This also agrees with the findings of Marshall & Liebherr (2000) who identified the Trans-Mexican Volcanic Belt as a biogeographic region with a high degree of endemism in insects, fishes, reptiles and plants. Furthermore, Mastretta-Yanes et al. (2015) concluded that volcanic activity in the Pleistocene leading to simultaneous climate and topographic changes in the Trans-Mexican Volcanic Belt promoted speciation events as well as long-term continuity of biodiversity. Further research to verify whether this area comprising the three grids acted as a refugium for geophytes should include paleoclimate modeling to identify suitable climates in time and include additional taxa with different plant life forms from the diverse lineages reported for this area.
The other biogeographic province with elevated monocot geophyte diversity is the Sierra Madre del Sur. Geophytes were recorded in seasonally dry tropical forests in the Balsas River Basin and in the Chiapas Depression, and in semi-arid shrubby vegetation in the Tehuacán Valley. These zones coincide with areas of endemism reported for Mexican plants (Sosa & de-Nova 2012). One of the quadrats with 50 monocot geophyte species corresponds to the Tehuacán Valley, which was established as a protected area because of its plant diversity and endemism (Méndez-Larios et al. 2005). For the Balsas River Basin a high degree of diversity and endemism of woody species of Leguminosae, Bursera (Burseraceae) and Cordia (Boraginaceae) have been documented (Pineda-García et al. 2007). Seasonally dry tropical forests in the Neotropics are a metacommunity of tropical forests that grow on fertile soils in areas with erratic precipitation regimes and with elevated endemisms (Pennington et al. 2009).
Winter rainfall was suggested to be the most important climate variable for explaining the diversity of geophytes in Mediterranean-like regions, such as South Africa (Proches et al. 2005). In Mexico, the Mediterranean climate only occurs in the north of the Baja California Peninsula (Kassam et al. 2012) (see Figure 1). However, our results suggest that the bioclimatic variables temperature seasonality, annual precipitation and precipitation of the wettest quarter resulted significant for distribution of monocotiledonous geophytes in Mexico. Geophytes are thus able to survive in contrasting habitats with scarce to abundant annual precipitation, with large variation in precipitation of the wettest quarter and remarkably, temperature seasonality is high denoting that temperature is not stable during the year. These values correspond to seasonal habitats, which most plant life forms are not able to survive but geophytes remain underground (by perennating organs like bulbs, corms, tubers or rhizomes) in harsh climate conditions and sprout when conditions are favorable.
The two biogeographic provinces with the greatest diversity and endemism of geophytes, the Trans-Mexican Volcanic Belt and the Sierra Madre del Sur, lie within the Mesoamerican Biodiversity Hotspot (Myers et al. 2000). Our results suggest that many of the endemic species of monocot geophytes are not located in protected areas and several of them are on the Mexican Red List (Figure 2). Although areas with high diversity of geophytes are located in biosphere reserves such as “Sierra Gorda”, “Tehuacán-Cuicatlán” “Sierra de Huautla” and “Sierra de Manantlán” or in protected areas for flflora and fauna like the “Corredor Biológico Chihunautzin” and “Pico de Tancítaro” some regions with high diversity are dispersed in small unprotected zones, mainly in the east end of the Trans-Mexican Volcanic Belt and in the north area of the Balsas Basin (Figure 2). A number of conservation strategies have been proposed for this biodiversity hotspot at different geographic scales, and for different ecosystems and taxa (Harvey et al. 2008, DeClerck et al. 2010, Golicher et al. 2012). All of them agree that it is not possible to manage such large areas parks or reserves, and suggest that it is desirable to adopt an integrative management style that involves human land use while ensuring the continuity of the biodiversity. Specifically for geophytes, it has been suggested that promoting their cultivation by horticultural societies might protect germplasm because many of these species are widely used as ornamentals and others might also be suitable for this type of use (Hoffman et al. 1998).

Figure 2 Distribution of geophytes in the context of protected areas in Mexico. The protected areas shown in this map were based on “Areas Nacionales Protegidas” of the Mexican Government http://sig.conanp.gob.mx/website/pagsig/anp/nal/inde.
Clearly areas with Mediterranean climate such like California (252 species, Parsons 2000), Chile (250 species, Hoffman et al. 1998), Israel (217 species, Fragman & Shmida 1996), SouthWestern Australia (496 species, Parsons & Hooper 2003) and Victoria, Australia (287 species, Parsons 2000) harbor more geophyte species in smaller areas compared to Mexico. Moreover, the Cape Flora of South Africa with 2098 species (Proches et al. 2006) is remarkable for geophyte diversity. However, our climate preferences analyses suggest that geophytes are able to grow in different climates located in areas with marked temperature seasonality and variation of annual precipitation.
Further phylogeographic research should be conducted to identify genetic signatures that might establish whether areas in the Trans-Mexican Volcanic Belt or in the Sierra Madre del Sur acted as refugia for sgeophyte species. In addition, detailed analyses should be conducted to understand whether there are differences in climate preferences in the main groups of geophytes such like Amaryllidaceae, Asparagaceae, Iridaceae or Orchidaceae.
Conclusions
Our records identified an elevated number of geophytes in Mexico, approximately 500 species, representing almost 10 % of the monocots listed for the country. Amaryllidaceae, Asparagaceae, Iridaceae and Orchidaceae are the lineages of this life form with the highest diversity. Echeandia and Tigridia are the most diverse, and ten other small genera are endemic. Two biogeographic provinces, the Trans-Mexican Volcanic Belt and the Sierra Madre del Sur, have the highest diversity and degree of endemism. Areas sustaining a high degree of geophyte diversity and endemism are located in the Mesoamerican Biodiversity Hotspot, in unprotected and threatened areas. Integrative management of the unprotected areas where these plants have been recorded and promoting their cultivation are probably good strategies for their conservation. Our analyses found that the most significant bioclimatic variables for geophyte distribution were: temperature seasonality, annual precipitation and precipitation of the wettest quarter, indicating that even if geophytes are distributed over an ample range of climates they have their seasonality in common.











 nueva página del texto (beta)
nueva página del texto (beta)