The tree species Fagus grandifolia Ehrh. has one of the broadest geographic distributions in North America, ranging from the far north to the subtropical zone, up to the southern end of the boreal zone of the Appalachian Mountains (Fang & Lechowicz 2006, Bradshaw et al. 2010). In contrast, in Mexico there are only 12 small isolated populations of the infra-specific taxon Fagus grandifolia Ehrh. subsp. mexicana (Martínez) A. E. Murray. They are restricted to the Sierra Madre Oriental in the states of Hidalgo, Nuevo León, Puebla, San Luis Potosí, Tamaulipas and Veracruz (Rodríguez-Ramírez et al. 2013, Rodríguez-Ramírez 2014).
The beech forests of Mexico, whose canopy is dominated by Fagus grandifolia subsp. mexicana, represent a particular type of plant association both of North American beech forests and of Mexican montane cloud forests (MCF). Because of their relict distribution and the specific environmental characteristics of the sites where they grow, they are highly vulnerable to climate change and anthropogenic disturbance (Williams-Linera et al. 2003, Rowden et al. 2004, Fang & Lechowicz 2006, Vargas-Rodríguez et al. 2010). This taxon is currently listed in the Mexican standard NOM-059 (SEMARNAT 2010) in the “endangered” category.
As Fagus grandifolia forests cover vast areas in Canada and the United States, it is not surprising that there are many studies of their floristic composition, structure, population dynamics, environmental conditions and forest management, among other aspects. Our knowledge of the beech forests of Mexico is much more sparse (Peters 1997, Williams-Linera et al. 2003, Hoagland 2006, Busby et al. 2009).
An analysis of the structure of sizes, diameters and ages of trees enables the recent history of the population to be reconstructed and the population dynamics and stability to be described (Dance-Caballero & Marlleux-Orjeda 1975, Leak 1975, Whipple & Dix 1979, Veblen 1992, Busby et al. 2009, Boyce 2012, Ferreyra-Aranda 2010). These analyses are important for species included in any risk category because they help to establish strategies for their use, management and conservation (Schmitt & Windisch 2006, Mehltreter 2010). If in addition to these parameters, age is estimated by tree-ring analysis, the contemporary history of the forest can be reconstructed in greater detail and with greater certainty (Rozas 2002). Moreover, the importance value of plant species is also a fundamental parameter, as it enables us to determine the plant community’s stage of succession, assuming that the mature phase is known (Namikawa et al. 2010, Bianchi et al. 2011).
There have been several studies on the structure and species composition of beech forests in Mexico (Williams-Linera et al. 2000, Williams-Linera et al. 2003, López-López 2003, Godínez-Ibarra et al. 2007, Montiel-Oscura 2011, Rodríguez-Ramírez 2014), but there have been only two published studies for Fagus grandifolia subsp. mexicana at the population level. Of these, one determined the age structure and its relationship with climate variables in three very small patches of fewer than 500 individuals each, located within the crater of the Acatlán volcano in Veracruz (Williams-Linera et al. 2000); and the other analyzed allozyme variation and population size at seven locations in the Sierra Madre Oriental (Montiel-Oscura et al. 2013).
Most populations of Fagus grandifolia subsp. mexicana are currently at risk of extinction, mainly due to disturbance caused by human activity (Williams-Linera et al. 2003, Rowden et al. 2004, Rodríguez-Ramírez et al. 2013). The restricted distribution of this taxon, its small population size, and the limited biological information available about it increase its level of vulnerability. The objectives of the present study were to (1) determine the population structure of F. grandifolia subsp. mexicana at three sites in the state of Hidalgo, Mexico, based on the diameter and height of individuals; (2) to estimate to what degree diameter and/or height are related to age of the beech trees and thus whether these parameters can be used to indirectly determine the age structure of populations; and (3) to determine species composition and structure at the community level. The intention is to generate basic and comprehensive information on the current state of Mexican beech forests, which can be useful in other studies to reconstruct the recent history of these forests and to establish conservation and management strategies in the future.
Materials and Methods
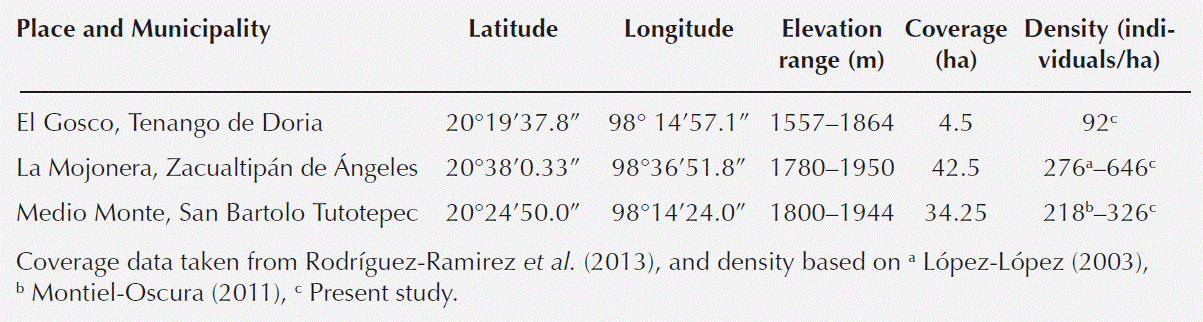
Study Area. The study was carried out at three sites with beech (Fagus grandifolia subsp. mexicana) forest in the state of Hidalgo, Mexico; El Gosco (Tenango de Doria), La Mojonera (Zacualtipán de Ángeles) and Medio Monte (San Bartolo Tutotepec). These forests grow at elevations ranging from 1,557 to 1,987 m (Table 1, Figure 1). The climate is C(fm); temperate humid with rainfall throughout the year, typical of a mountain orobiome (Peters 1995), with frequent fog and an annual average temperature of 12.7 ºC (García 1988). Total annual rainfall is high (1,200 to 2,050 mm), and the landscape is rugged, with abundant steep slopes (Rodríguez-Ramírez et al. 2013).
Figure 1 Map of MCF distribution in the state of Hidalgo and location of beech forests at the study sites
Table 1 General characteristics of the study sites with Fagus grandifolia subsp. mexicana populations
Structural analysis of the beech forests. Five permanent 20 × 50 m sampling plots (0.1 hectare each) were selected at Medio Monte, five at La Mojonera and three at El Gosco in order to determine the forest structure at each site based on the diameters and densities of the trees and shrubs (Rozas 2002, Bianchi et al. 2011). Fewer plots were selected at El Gosco because the site was smaller and the forest more disturbed (Table 1).
The sample plots were situated at least 40 m from open areas to reduce the edge effect as much as possible (Gutiérrez et al. 2009). All trees and shrubs with trunks > 1.5 m tall and > 2.5 cm diameter at breast height (DBH at 1.3 m) were enumerated. The relative importance value (RIV) of each species was estimated using the values obtained for basal area [BA = π(DBH/2)2] and density (D) (Matteucci & Colma 1982) by means of the formula RIV = (relative density + relative basal area)/2. Density values were extrapolated for each species and expressed as the number of individuals/ha. Similarity in floristic composition between sites, based on the presence or absence of species, was calculated by means of the Bray-Curtis index. The analysis was carried out using the PAST v. 3.03 program (Hammer et al. 2001).
The species were identified using specific dichotomous keys for each genus, and by experts for the complex taxonomic groups (e.g., Quercus). All specimens were compared with material from the HGOM (Universidad Autónoma del Estado de Hidalgo) and MEXU (Biology Institute at the Universidad Nacional Autónoma de México) herbariums.
Population structure. The data on the vegetation structure were also used to define the population structure of Fagus, based on the DBH and total height of individuals (estimated by eye as accurately as possible). In addition, the height from the base to the apex or terminal branch of the main stem was measured for all Fagus seedlings (< 41 cm high) and small and large saplings (> 41 cm and < 2.5 cm DBH) in the sample plots.
The number of height and diameter classes was determined using Sturges’ rule: k = 1 + 3.322 (log10 n), where k = number of groups and n = number of individuals (Schmidt et al., 2009). In order to carry out a comparative analysis of the population structure between sites, the same number and width of height and diameter interval classes were chosen for all three sites, using the site with the highest number of individuals as a reference (Pérez-Paredes et al. 2014). The width of the class intervals (AI) was estimated by the formula AI = R/k, where R = rank (Sánchez-Rodríguez et al. 2003).
Regression analysis was used to estimate the allometric relationship between the height and diameter of individuals; of the models generated (one linear and several nonlinear), the model with the highest coefficient of determination (r 2) was chosen (Urban et al. 2010). To determine whether the structure of sizes (height) and/or diameters of individuals was suitable for use as an indicator of the age structure of beech populations, the degree of association between age and height, and age and diameter was estimated for 12 individuals from each site (see next paragraph) through a simple correlation analysis. All calculations were carried out using the STATISTICA version 7.0 program (StatSoft, 2004).
Sampling and counting rings. At each site, 12 healthy beech trees were chosen (trees with no external evidence of rot or disease in the bark) for the extraction of core samples. In each of the selected individuals (three per diameter class: 18-36, 36.1-55, 55.1-72 and 72.1-91 cm DBH), two core samples were taken at a height between 1.3 and 1.5 m using a Pressler drill. The samples were exposed to the sun to facilitate drying, then mounted on wooden rails and polished with sandpaper of successive grain sizes, from 120 (rough) to 1,200 (fine). Polishing is necessary to highlight the growth rings and facilitate counting (Stokes & Smiley 1968).
Estimating age of individuals. Age estimates of the sampled trees were obtained following the method of Applequist (1958) which consists in counting the total number of rings from the bark to the pith, plus the missing rings extrapolated by overlapping a predesigned ring diagram. For samples that did not include pith, the number of missing rings was calculated as recommended by Villanueva-Díaz et al. (2003): the rings of the first 5 cm of the interior part of the sample were counted, since in theory they would be the most similar in width to the rings in the missing part. With these data and the radius of each individual, the number of years in the missing section was estimated by extrapolation.
Results
Population structure by height and diameter classes. An inverse relationship was observed between diameter and average height with respect to density by site. La Mojonera and Medio Monte had densities of 646 and 326 ind/ha, respectively. Average diameter (18 and 17.26 cm) and height (10.6 and 10.6 m) were similar for the two sites. In contrast, El Gosco had only 92 individuals/ha, with an average diameter of 27.79 cm and an average height of 13.58 m (Table 2).
Table 2 Structural attributes of the dominant tree species at the three beech forest sites in the state of Hidalgo
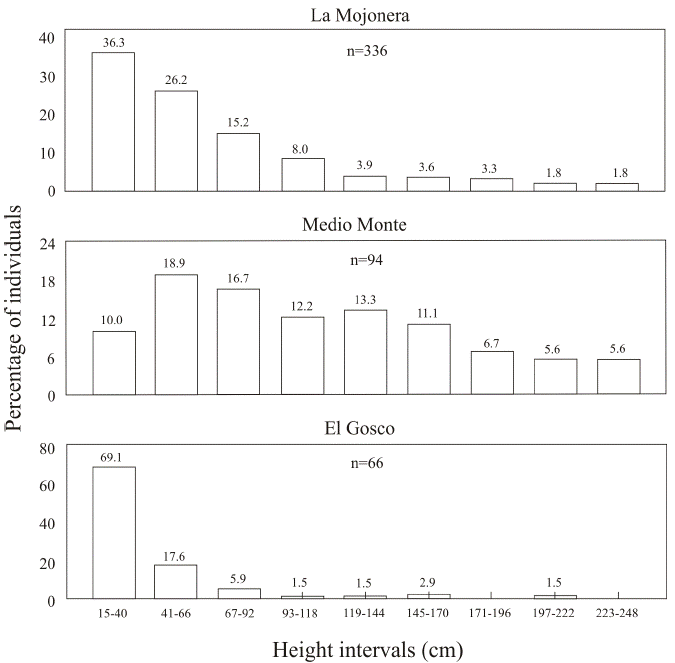
The structure of sizes (heights) was also similar between the populations at the La Mojonera and Medio Monte sites; most individuals (59-62 %) were in the smaller height classes (juvenile plants measuring less than 11 m), while fewer trees (26 %) were of intermediate height (11.1-20.6 m) and only a few individuals (12-15 %) were 20.7 m or taller. In contrast, the distribution of heights at the El Gosco site was heterogeneous. The percentage of individuals with sizes between 1.5 and 11.0 m (38.5 %) was low compared with that of the other two locations (more than 59 % of individuals), and most individuals (46.1 %) measured between 11.1 and 20.6 m; 15.4 % were between 20.7 and 23.8 m and no individuals over 23.9 m were observed within the sample plots (Figure 2).
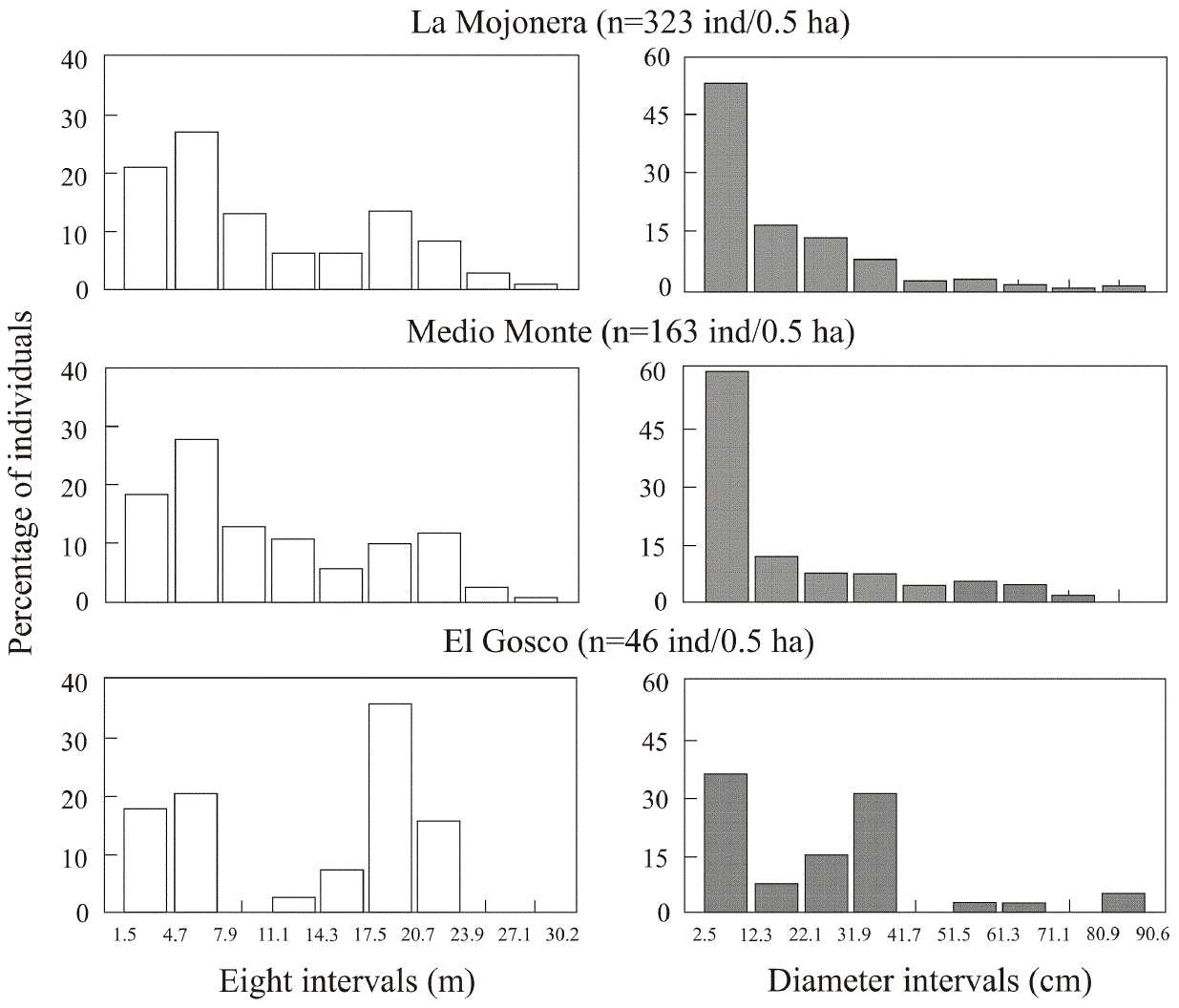
Figure 2 Percentage of Fagus grandifolia subsp. mexicana individuals by height and diameter class at the three study sites
Moreover, the distribution of diameter classes in the populations at the La Mojonera and Medio Monte sites followed a similar pattern; an inverted “J” (Figure 2): at both sites more than 77 % of individuals were of small diameter (2.5 to 31.8 cm), 13.3-16.0 % had medium diameter (31.9-61.2 cm) and fewer than 6.2 % had diameters greater than 61.3 cm. At the El Gosco site, a different pattern was observed, with a smaller number of individuals in small diameter classes (58.9 %) and a higher percentage (33.3 %) with intermediate diameters (Figure 2).
The relationship between tree height and diameter was similar in the three populations and can be described by means of the respective allometric equations which describe a logarithmic function. The values of the coefficients of determination (r 2) ranged from 0.64 to 0.88 (Figure 3) and were statistically significant (P < 0.01, N = 503). The same pattern was observed in the three populations: in the early stages the rate of growth in height and diameter of the individuals was proportional (linear), but after they reached a certain size (for example 16 to 20 m high) the ratio changed, with growth in diameter remaining relatively constant, while growth in height decreased asymptotically (Figure 3).
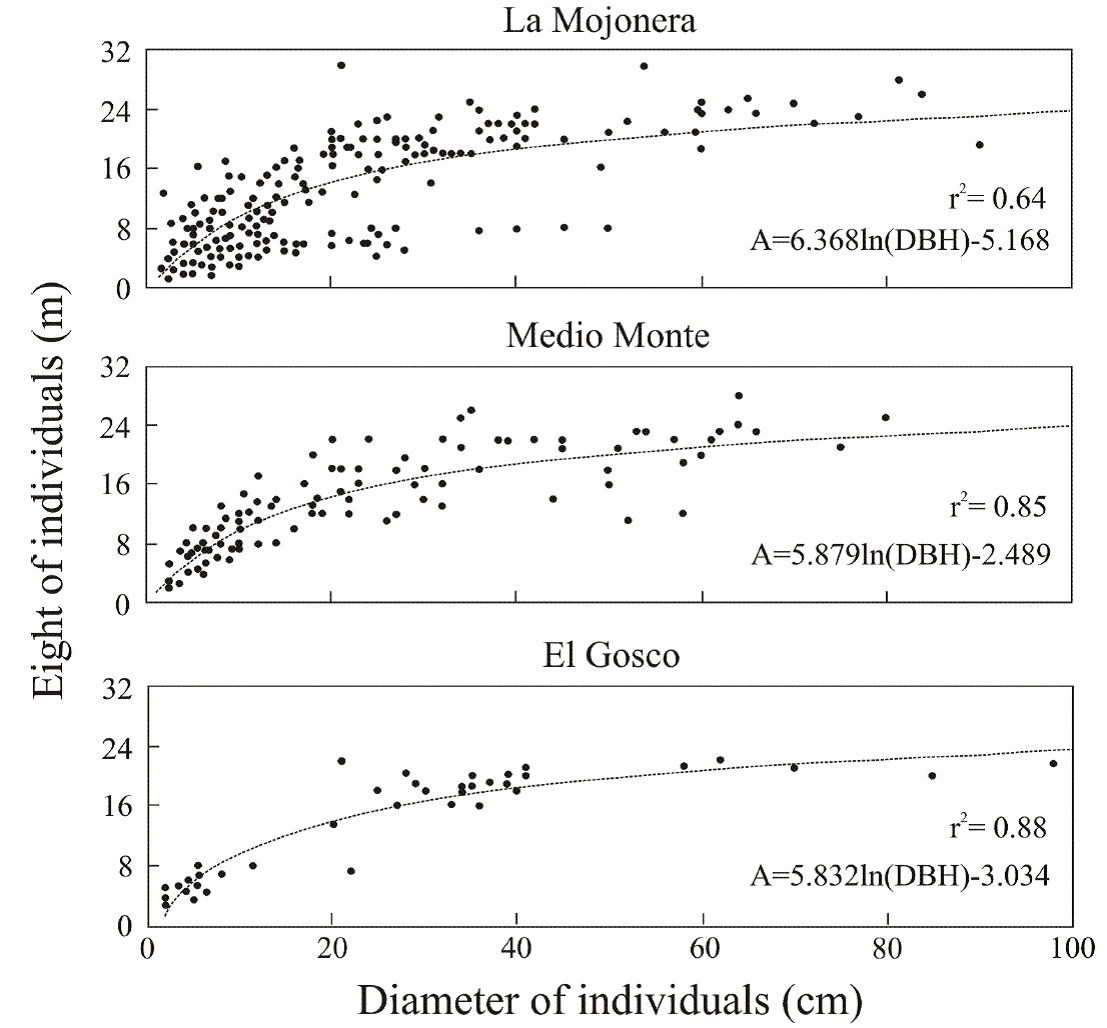
Figure 3 Relationship between normal diameter and height of individuals at each beech forest study site. N= 39 at El Gosco, 302 at La Mojonera and 162 at Medio Monte. In all cases, P <0.01
Relationship between tree age, diameter and height. The oldest beech trees were observed at the La Mojonera site; the longest-lived specimen was approximately 235 years old. This tree was 22 m tall and 98.3 cm in diameter; two other individuals were 205 and 218 years old. At the Medio Monte site, some trees over one hundred years old were found. The longest-lived was 171 years old, 21 meters tall and 68.6 cm in diameter. At the El Gosco site, some trees more than 100 years old were also recorded. The oldest individual was 149 years old (Figure 4).
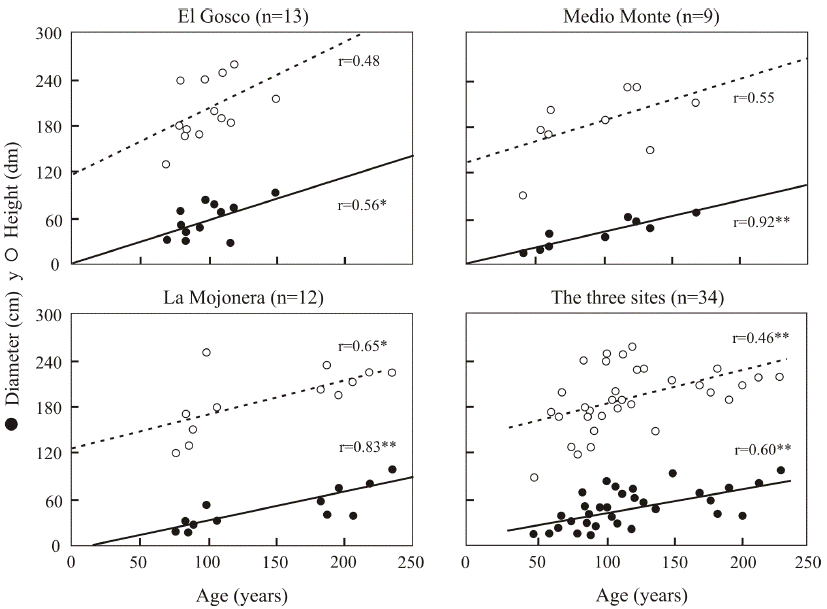
Figure 4 Age-height ratio (dotted line) and age-diameter ratio (solid line) of beech trees by site and all sites combined. *P<0.05, **P<0.01.
The relationship between height and age of individuals was statistically significant in only one of the populations (La Mojonera). The correlation between diameter and tree age was, however, statistically significant (P < 0.01) in all three populations (Figure 4). These results suggest that the structure of diameters (Figure 2) can be regarded as an adequate predictor of the age structure of the beech populations in this study.
Size of seedlings and saplings. A different pattern was observed at each site. At the El Gosco site, most individuals (69 %) were seedlings (< 41 cm), whereas small saplings (< 41 cm and < 170 cm), and taller juveniles (> 171 cm) were practically absent. In contrast, at the La Mojonera and Medio Monte sites, the number of seedlings was lower (36.3 and 10 % respectively), the proportion of small saplings was higher (56.9 and 72.2 % respectively) and the number of tall juveniles was low (Figure 5). It should be noted that the establishment pulse of the individuals classified as seedlings in this study comes from the seed year which occurred synchronously in all three populations in the first half of 2012.
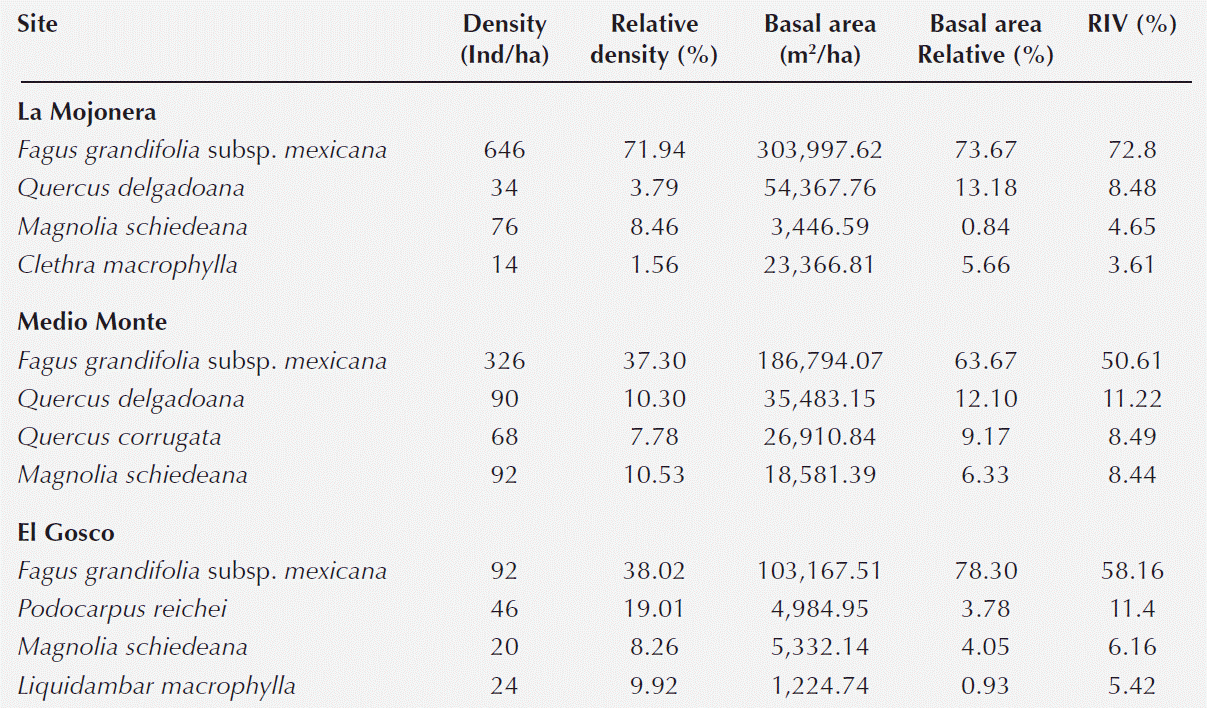
Structure and composition of woody species in the beech forests. In the beech forests in this study, 26 families, 29 genera and 42 species of shrubs and trees (Appendix 1) were identified. According to the estimated RIV, the dominant species at all three sites was Fagus grandifolia subsp. mexicana. All other species had low structural values (Table 1, Appendix 1).
The RIV of Fagus grandifolia subsp. mexicana was highest at the La Mojonera site. Of the six tree species with the next-highest RIV after Fagus, only Magnolia schiedeana was observed at all three sites. Quercus delgadoana was found at two sites, while Liquidambar macrophylla and Podocarpus reichei were found only at the El Gosco site (Table 2).
Floristic composition was similar at all three sites; the values of the Bray-Curtis index ranged from 0.64 to 0.68. Species richness was highest at the Medio Monte site, with 24 species of trees and shrubs, and lower at El Gosco and La Mojonera, which had similar values (19 and 18 species respectively).
Discussion
The results of this study show that the size structure of beech tree populations differed between the study sites. This can be explained by the fact that that in plant populations in general, individuals vary greatly in height as a result of asymmetric competition for light and/or because the distribution of other resources is uneven (Delgado et al. 2005, Cole & Ewel 2006). There are several studies that emphasize the importance of disturbances (e.g. wind, fire, logging) on plant population structure and dynamics. In fact, the temperate forests of North America, including Mexico, have experienced a complex history of natural and anthropogenic disturbances (Rzedowski 1978, Cowell & Hayes 2007, Busby et al. 2008, Bradshaw et al. 2010, Jardel-Peláez et al. 2014).
In the mature beech forests of La Mojonera and Medio Monte, signs of human disturbance were scarce or rare. Light gaps were due mainly to small scale natural disturbances (large fallen branches, death of adult trees due to senescence, uprooting and/or trunks broken by storms). As a result, the two sites had similar conditions, with a mosaic of patches in different stages of natural regeneration (Peters & Platt 1996, Packham et al. 2012, Jardel-Peláez et al., 2014, Rodríguez-Ramírez 2014), which may be related to the prevalence of beech trees in the smaller height classes.
In contrast, at the El Gosco site, there was clear evidence of disturbance by recent human activity such as uncontrolled logging of beech and oak (Quercus spp.) trees, harvesting of wood, many roads, at least one house in the forest and several nearby, and grazing by goats. At this site, the size structure was different, with a predominance of trees exceeding 17 m in height. It is likely that the increase in the number and size of clearings at this site in the past decade due to tree felling is related to the survival of a large number of beech seedlings that germinated in the 2012 seed year (Rodríguez-Ramírez et al. 2013) and of small saplings whose seeds germinated in the 2005 seed year (Godínez-Ibarra et al. 2007). A similar explanation has been given for other beech forests in Mexico and in North America subject to different sources of high or frequent disturbance (Peters 1995, Williams-Linera et al. 2003, Busby et al. 2008).
Moreover, preferential logging of beech saplings (data provided by the local field guides) may be the reason for the small number of individuals in the height range of 7.9 to 11.0 m and the predominance of mature trees. The high frequency and intensity of disturbance at the El Gosco site make it unsuitable for survival and establishment of Fagus grandifolia subsp. mexicana seedlings, so it is important to develop management and conservation strategies.
It is thus necessary to ensure seed production, reduce population pressure and ensure the survival of Fagus, while at the same time continuing to extract forest resources (e.g. removing old and diseased trees). Moreover, removing trees at a “moderate” rate or intensity promotes the establishment of species with different resource use strategies (the intermediate disturbance hypothesis, sensu Connell 1978), especially seedlings of species typical of the cloud forest canopy, such as Liquidambar macrophylla, Quercus spp. and Pinus spp., which have a wide range of uses in Mexico. The usefulness of management strategies of this type has been demonstrated in temperate forests (including Fagus spp.) in various regions around the world (Peters 1997, Grau et al. 2010, von Oheimb et al. 2007).
Seedlings and saplings of Fagus species of Asia, the Americas and Europe (Peters 1995, 1997, Petritan et al. 2009, Packham et al. 2012, Hukusima et al. 2013) grow slowly and can be part of the understory, living under low light levels, for long periods of time (decades) (suppression period), but grow quickly (release period) when gaps form in the canopy (Williams-Linera et al. 2000, Williams-Linera et al. 2003, Busby et al. 2009, Bradshaw et al. 2010, Boyce 2012). Consistent with this observation, in the present study we found that Fagus undergoes regeneration in small naturally-occurring gaps; however, at the El Gosco site, the largest increases in biomass (height and diameter) (Delgado et al. 2005) could be related to the opening up of large spaces (Rodríguez-Ramirez et al. 2013, personal observation) and other sources of disturbance. Although the three study sites are relatively close to each other, differing site conditions (including disturbance) and competitive pressures (e.g. different density values for each stand) affect the population structure and allometric diameter-height relationships; as proposed by Urban et al. (2010) to account for local differences in allometric models in different populations of Fagus sylvatica in Europe.
Peters & Platt (1996) note that Fagus grandifolia has a greater growth rate in diameter than in height, as predicted by biomechanical models in various species of trees (Delgado et al. 2005, Dodonov et al. 2011). Although the growth rate in diameter is slow when the tree forms part of the understory, it appears to be higher than that of other species in the canopy of the Mexican cloud forest. This more “steady” increase in diameter than in height could explain in part why diameter-age correlations were higher than height-age values, as is the case in Appalachian beech populations in the southeastern United States, and in Fagus sylvatica populations in Europe (Lorimer 1980, Urban et al. 2010).
López-López (2003) classified beech trees with diameter greater than 50 cm as old. Applied to the results of this study, these would be individuals over 80 years old. However, as noted above, the diameter-age relationship can be altered by the heterogeneity in environmental conditions that occurs in forests, especially differential penetration of light. This shows that (1) the true age of individuals can only be estimated by counting growth rings, a technique with little or no margin of error (Fritts 1976, Villanueva-Díaz et al. 2003); and (2) it is important to estimate the age of a representative number of trees growing in different conditions to be able to determine with certainty whether variation in diameter (or some other allometric characteristic) is suitable for use as an indicator of the age of individuals (Lorimer 1980, Bosch et al. 1992, Rozas 2002).
In this study, the three populations were determined to be “dynamic;” that is, having a predominance of juvenile individuals, based on the observed structure of diameters. According to a number of authors, a dynamic population structure in tree species means that they have a high potential for regeneration and for growing under environmental conditions favorable for the establishment of their seedlings (Lorimer & Krug 1983, Godínez-Ibarra & López-Mata 2002, Busby et al. 2008, Boyce 2012, Pérez-Paredes et al. 2014). In the case of Fagus grandifolia subsp. mexicana, Williams-Linera et al. (2003) and Montiel-Oscura (2011) state that the La Mojonera, Tutotepec (Hidalgo) and Acatlán (Veracruz) populations have a large number of juveniles, so they are stable enough to maintain themselves unless they are affected by climate change or other severe disturbances, as is the case at El Gosco.
The ages of Fagus grandifolia subsp. mexicana trees at the three study sites ranged from 40 to 235 years old. The oldest individuals were in the La Mojonera forest, where the longest-standing old-growth core dated back to 1777, and the youngest to 1972. Other studies have found similar results with regard to the age of tree populations in mature forests. In the United States, Lorimer (1980) dated beech trees over 280 years old and with diameters up to 110 cm. Busby et al. (2008) estimated the age of Fagus grandifolia populations in northern New England in order to characterize the establishment of this species, finding trees with ages from 26 to 204. Williams-Linera et al. (2000) found a narrower range of ages, from 70 to 120, in a Fagus population located in the Acatlán volcano in Veracruz, which was attributed to a severe disturbance in the past that had caused forest regeneration.
At the community level, the richness of woody species at the three study sites was similar to that reported by Williams-Linera et al. (2003) for other beech forests in Mexico and also similar to those recorded in other studies of montane cloud forest in Hidalgo and the state of Mexico (Alcántara & Luna-Vega 2001, Williams-Linera et al. 2003). Moreover, the RIV estimates showed that Fagus grandifolia subsp. mexicana dominated the vegetation structure of the forest canopy. The degree of dominance was slightly higher at the La Mojonera site than in the other two forests analyzed, as also noted previously by Godínez-Ibarra et al. (2007). In the lower forest layers, the species with highest RIV at all three sites was Magnolia schiedeana, which could be considered as a feature or distinctive characteristic of Mexican beech forests (Peters 1997, Williams-Linera et al. 2000, Alcántara & Luna-Vega 2001, Williams-Linera et al. 2003, Godínez-Ibarra et al. 2007).
Although the El Gosco and Medio Monte sites are geographically close to each other, the species composition in the canopy showed low similarity between the two sites. It is likely that differences between the areas of the two forests and the degree of fragmentation and disturbance caused by human activity (Rodríguez-Ramírez et al. 2013, Ortiz-Quijano et al. 2015) are playing an important role in species establishment and/or replacement and therefore in altering vegetation structure. This is particularly evident at the El Gosco site, where illegal logging of Quercus and Fagus individuals and harvesting of tree ferns (Cyathea fulva and Alsophila firma) have altered the conformation of the canopy and understory, causing Liquidambar macrophylla and Podocarpus reichei to increase in importance. The latter two species are considered to be indicators of disturbance in the Mexican cloud forest, being remnants of the first cohort of trees that grow in clearings (Sosa 1978, Rodríguez-Ramírez et al. 2013, Rodríguez-Ramírez 2014, Jardel-Peláez et al. 2014).
At the Medio Monte site, there is no clear evidence of disturbance caused by human activity (Ortiz-Quijano et al. 2015, present study). Twenty-four tree and shrub species were identified in this forest, which is the largest species richness recorded to date for any beech forest in Mexico (Williams-Linera et al. 2003). The most common species in the canopy were Quercus corrugata, Q. delgadoana and Magnolia schiedeana. Unlike the El Gosco site, several oak species coexist in the Medio Monte forest; Quercus delgadoana, Q. corrugata, Q. xalapensis, Q. lancifolia and Q. aff. trinitatis, most with relatively low importance values.
According to the results of several local level studies in Mexico (Peters 1995, Peters 1997 Williams-Linera et al. 2003, Godínez-Ibarra et al. 2007, Montiel-Oscura 2011) and in various regions of Canada and the United States (Peters 1997, Hoagland 2006), it is common to find species of Befaria, Magnolia, Ostrya, Podocarpus, Quercus and Turpinia in the canopy of pristine Fagus grandifolia forests. It has been suggested that beech forests anywhere in the world (North America, Asia and Europe) where a Fagus species completely dominates the canopy are the product of a high intensity or frequency of disturbance, whether natural or anthropic (Peters 1997, Waltert et al. 2002, Busby 2008, 2009, Bradshaw et al. 2010, Hukusima et al. 2013, Jardel-Peláez et al. 2014). Thus knowledge about species composition and structural heterogeneity of a forest may provide important information about its dynamics (Bianchi et al. 2011).
In addition to Fagus grandifolia subsp. mexicana, at least five of the tree species identified in this study are listed in a risk category by Mexican legislation or have a restricted distribution (SEMARNAT 2010). Magnolia schiedeana, for example, is included in the “threatened” category and it is likely that the populations with the highest density of individuals of this species in the state of Hidalgo grow under beech forest canopies (study in progress). Quercus delgadoana, one of the species with the highest RIV at the La Mojonera and Medio Monte sites, is endemic to the cloud forest of the Sierra Madre Oriental and its distribution is closely associated with beech forests (Valencia et al. 2011). The case of Podocarpus reichei is similar. This species, collected only at El Gosco, is also endemic to Mexico and has a discontinuous distribution in the mountainous regions of several states of Mexico. It is currently classified as vulnerable due to logging and conversion of its habitat to agricultural land (Zamudio 2002).
The objectives of this study were designed to contribute to an understanding of basic aspects of the population structure of Fagus grandifolia subsp. mexicana and the structure of beech forests. The information obtained indicates that the populations at the La Mojonera and Medio Monte sites are stable under current conditions and are likely to continue so if no drastic changes in the environmental conditions occur, as they did at El Gosco, where there is a high risk of the Fagus population and the forest itself disappearing in the near future if control measures are not undertaken. The results are important because they can serve as a basis for designing and carrying out specific studies on various aspects; for example, reconstructing the recent history of these forests to generate simulation models of their dynamics and stability; to propose strategies for the use, management and conservation of beech trees and the other species with which they coexist. These and other potential studies will involve cooperation among different disciplines, specialists and stakeholders.











 nueva página del texto (beta)
nueva página del texto (beta)